Abstract
Our objective was to describe racial and ethnic differences of amyotrophic lateral sclerosis (ALS) in distinct geographic locations around the United States (U.S.). ALS cases for the period 2009–2011 were identified using active case surveillance in three states and eight metropolitan areas. Of the 5883 unique ALS cases identified, 74.8% were white, 9.3% were African-American/black, 3.6% were Asian, 12.0% were an unknown race, and 0.3% were marked as some other race. For ethnicity, 77.5% were defined as non-Hispanic, 10.8% Hispanic, and 11.7% were of unknown ethnicity. The overall crude average annual incidence rate was 1.52 per 100,000 person-years and the rate differed by race and ethnicity. The overall age-adjusted average annual incidence rate was 1.44 per 100,000 person-years and the age-adjusted average incidence rates also differed by race and ethnicity. Racial differences were also found in payer type, time from symptom onset to diagnosis, reported El Escorial criteria, and age at diagnosis.
In conclusion, calculated incidence rates demonstrate that ALS occurs less frequently in African-American/blacks and Asians compared to whites, and less frequently in Hispanics compared to non-Hispanics in the U.S. A more precise understanding of racial and ethnic variations in ALS may help to reveal candidates for further studies of disease etiology and disease progression.
Introduction
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease, is a progressive neuromuscular disease that typically leads to death within three to five years (Citation1). While the etiology of ALS is poorly understood, data from disease registries and epidemiologic studies have provided insight into many of the population based characteristics of the disease. Annual incidence rates worldwide tend to range from 1–2 per 100,000 person-years and prevalence rates are reported as 4–6 per 100,000 persons (Citation2–6). ALS is more prevalent in males than females and typically affects those in the later years of life (Citation7,Citation8).
Less is known about how ALS affects people of different racial and ethnic backgrounds. Some studies suggest that ALS rates are higher among non-Hispanic Caucasians (whites) in Western countries compared with those of African, Asian, and Hispanic descent (minorities) (Citation9–13). However, this postulation is difficult to gauge because of the limited number of minority cases identified and the homogeneous nature of the various ALS registries and epidemiologic studies. Even data from the well-established population based ALS registries in Europe, and to a lesser degree, the patchwork of smaller-scale ALS studies from around the United States (U.S.) provide far less sensitivity to compare minority populations. Studies in China show an incidence rate among Asians significantly lower than that of the overall U.S. average (Citation14,Citation15). Recent studies comparing whites to African-American/blacks found a younger age of disease onset and diagnosis among African-American/blacks (Citation16). Examining racial and ethnic variations in ALS may help further studies on topics such as disease etiology and progression among different subpopulations.
In October 2010, the U.S. government launched its first and only population based countrywide ALS registry (Citation17). This National ALS Registry is allowing researchers to quantify the prevalence of ALS among cases identified within the Registry and describe the demographic characteristics of persons with ALS identified by the Registry. As a separate initiative, surveillance activities were conducted in three states and eight metropolitan areas of the U.S. to help evaluate the completeness of the Registry and to determine how ALS impacts different subpopulations. To examine race and ethnicity, the 11 project areas were selected to over-represent minority populations compared with the racial and ethnic distribution of the U.S. population as a whole.
The primary objective of this paper is to describe the racial and ethnic differences among ALS cases from three states and eight metropolitan areas in the U.S. using the largest combined cohort of clinically reviewed cases to date. Findings from this and other similar studies may provide important insights into the pathogenesis of ALS.
Materials and methods
Project sites and management
Three states (Florida, New Jersey, and Texas) and eight metropolitan areas (Atlanta, Baltimore, Chicago, Detroit, Las Vegas, Los Angeles, Philadelphia, and San Francisco) participated. The state and metropolitan project areas comprise 17.1% and 10.0% of the U.S. population, respectively (Citation18). These 11 areas are 64.4% white, 16.0% African-American/black, and 6.7% Asian; 28.3% of the population is Hispanic. Compared with the U.S. population, Asians, African-American/blacks, and Hispanics were over-represented. All state and metropolitan area projects followed a standard, ATSDR-approved protocol. Details regarding site selection, project management, and data collection materials and methods are described elsewhere (Citation19).
Data collection
Neurologists were contacted to determine if they diagnosed and/or provided care for ALS patients residing in each project's catchment area and those that did were asked to submit case reports for eligible cases. Eligible cases were under the physician's care at any point between 1 January 2009 and 31 December 2011, met one of the El Escorial criteria (Citation20), and had a residential address in one of the project's catchment areas at some point in 2009–2011.
The study used a brief standard case reporting form (CRF) created specifically for this project that included questions about patient demographic characteristics as well as questions about date of symptom onset, date of diagnosis, El Escorial criteria classification, and payer type. Predetermined categories on the reporting form for race were: ‘Asian’, ‘African American/black’, ‘White’, ‘Unknown’, and ‘Other’ with an option to specify ‘Other’. Predetermined categories for ethnicity included: ‘Hispanic or Latino’, ‘Non-Hispanic or Latino’, and ‘Unknown’. Forms were completed by the reporting neurologists and/or their designated staff member based on information contained in the medical record. To ensure accuracy of case diagnosis, a symptom-oriented Medical Record Verification Form (MRVF) and electromyogram (EMG) report were requested on a sample of reported cases. The completed MRVFs, along with copies of the redacted EMG reports, if available, were forwarded to the project's consulting neurologist for independent assignment of El Escorial criteria classification (Citation20).
Death certificate data were reviewed in each project area to identify possible ALS cases. The death certificate data were matched to the reported cases and attempts were made to procure case reports for decedents that were not already reported. Compensation was offered for completed forms. This protocol was approved by the Centers for Disease Control and Prevention's Institutional Review Board. No patients were contacted.
Data cleaning, creation of composite records and analysis
Each case report was examined as it was received and case reports for the same person were accepted if reported from different practices. Only one case report for each individual was retained in the final data set. Upon completion of data collection, a composite record was created for cases reported more than one time. The case record with the most complete information was retained and missing information was filled in using the duplicate case report and death certificate data when available. If there were inconsistencies in duplicate case reports the response was changed to ‘Unknown’. The U.S. Census-defined race categories (Citation21) were utilized to categorize cases in which race ‘Other’ was reported. For example, a case reported as ‘Lebanese’ race was placed into the ‘White’ category. At the conclusion of this reassignment process, 18 cases remained as race ‘Other’. Data were analyzed using Microsoft Excel® (Citation22) and SPSS (Citation23). Incident cases were determined by date of diagnosis on the CRF and are expressed in 100,000 person-years. Race and ethnicity specific incidence rates were calculated utilizing race/ethnicity specific populations from the 2010 U.S. Census (Citation21). A Poisson distribution was assumed in the calculation of the 95% confidence intervals (Citation24). Age adjusted rates were standardized to the year 2000 U.S. Standard Population. Time from symptom onset to diagnosis was calculated using incident cases with a known month and year of both symptom onset and diagnosis.
Results
Reported cases and demographic characteristics
Based on the 2010 U.S. Census population and previous estimates of ALS incidence and prevalence (Citation2–6), a total of 6677 cases were expected and 5883 unique cases were collected within the three states and eight metropolitan areas used in this analysis. Of these cases, 4401 (74.8%) were white, 546 (9.3%) were African-American/black, 214 (3.6%) were Asian, 704 (12.0%) were of unknown race, and 18 (0.3%) were categorized as other race (e.g. American Indian, Caribbean, and multiple races). African-American/blacks were more likely to be between 50 and 59 years of age at diagnosis while all other races were more likely to be 60–69 years of age. Over half (55.3%) of the African-American/black cases were diagnosed before age 60 years with a median age of 58 years. For all races, cases were more likely to be male than female, with the greatest difference between genders in the Asian race (59% male vs. 41% female) and the smallest difference in the African-American/black race (51% male vs. 49% female) ().
Table I. Demographic characteristics of all reported prevalent ALS cases by race and ethnicity for the period 1 January 2009 through 31 December 2011 in all 11 project sites (n = 5883).
Ethnicity was defined as Hispanic (10.8%), non-Hispanic (77.5%), or unknown (11.7%). Among Hispanics, the racial background was reported for 60% (383/634) of the population: 374 (59.0%) were white, four (0.6%) were black, four (0.6%) were other race and one (0.2%) was Asian. Over half of the Hispanic cases had a known country of birth (352/634), with the most common country being the U.S. (123/634). Other countries of birth for Hispanic cases included: Central American countries and Caribbean Islands (105), Mexico (72), South American countries (43), European countries (7) and Asian countries (2). Of the non-Hispanic cases, 3113 cases had a known country of birth with the most common being the United States (2753), India (38), China (28), and Germany (21). Hispanics were most likely to be diagnosed between ages 50 and 69 years with a median age at diagnosis of 58 years, while non-Hispanics were most likely to be diagnosed between ages 60 and 69 years, with a median age of 62 years. For both Hispanic and non-Hispanic cases, the number of cases per age group decreased after age 69 years.
Reported El Escorial classification
In total, 4846 (82.4%) cases were reported as ‘definite’, ‘probable’, or ‘probable laboratory- supported’ ALS; 754 (12.8%) were reported as ‘possible’ ALS; and 283 (4.8%) were reported as ‘not classifiable’ according to the El Escorial criteria (Citation17). Asians were the most likely to have a ‘possible’ ALS diagnosis (18.2% of Asians vs. 13.6% of African-American/black vs. 12.1% of whites). Whites were more likely to be classified as ‘definite’ or ‘probable’ compared with African-American/blacks and Asians and less likely to be classified as ‘probable laboratory- supported’ or ‘possible’ ().
Table II. Reported El Escorial criteria of all reported prevalent ALS cases by race and ethnicity for the period 1 January 2009 through 31 December 2011 in all 11 project areas (n = 5883).
Incidence
A total of 3819 cases were newly diagnosed during the period 1 January 2009 through 31 December 2011. The overall crude annual incidence rates for 2009, 2010 and 2011 were 1.42 per 100,000 person-years, 1.53 per 100,000 person-years and 1.62 per 100,000 person-years, respectively, and the overall crude average annual incidence rate was 1.52 per 100,000 person-years. The average crude annual incidence rate for whites was 1.79 per 100,000 person-years compared with 0.80 per 100,000 person-years for African-American/blacks and 0.76 per 100,000 person-years for Asians (). The average crude annual incidence rate for non-Hispanics was 1.65 per 100,000 person-years compared with 0.57 per 100,000 person-years for Hispanics ().
Figure 1. Stratified age-adjusted average annual incidence rates for reported ALS cases by race and ethnicity for the period 1 January 2009 through 31 December 2011 in all 11 project areas.
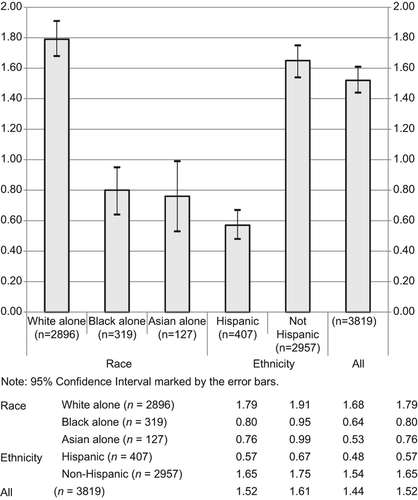
The overall age-adjusted average annual incidence rate was 1.44 per 100,000 person-years. The age-adjusted average incidence rates differed by race (1.48 whites vs. 0.89 for African-American/blacks vs. 0.78 for Asians) and by ethnicity (0.84 for Hispanics vs. 1.36 for non-Hispanics). Age-specific incidence rates increased until age 79 years.
Time from symptom onset to diagnosis
Of the 3819 incident cases, there were 269 cases with missing information for month and/or year of symptom onset and/or month of diagnosis, leaving 3550 cases for this analysis. The overall mean duration of time from symptom onset to diagnosis was 16.9 months, with the lowest mean occurring among African-American/blacks (15.6 months) and the highest among Asians (20.4 months). The median time from symptom onset until diagnosis was 11 months for whites, African-American/blacks, and non-Hispanics; while it was 12 months for Asians and Hispanics. Ninety percent of cases were diagnosed within 36 months of symptom onset ().
Table III. Time from symptom onset to diagnosis for all reported incident ALS cases by race and ethnicity for the period 1 January 2009 through 31 December 2011 in all 11 project areas (n = 3550).
Payer type
Overall, 65.6% of cases identified within the three states and eight metropolitan areas used in this analysis utilized a non-federal payment type such as private insurance or an HMO (by itself or in combination with other payer types). Sixty-two percent of cases had at least one federal payer (Medicaid, Medicare, and Veterans Administration (VA)) including 55.9% of whom utilized Medicare. Asians were least likely to utilize a federal payer (52.8%). The use of self-pay differed by race (12.6% of Asians vs. 5.4% of whites vs. 6.6% of African-American/blacks). A total of 144 cases were exclusively self-pay: 1.9% of whites, 3.5% of African-American/blacks and 6.1% of Asians. A larger percentage of Hispanics had no federal payer compared with non-Hispanics (43.8% vs. 35.0%) ().
Table IV. Payer type for all reported prevalent ALS cases by race and ethnicity for the period 1 January 2009 through 31 December 2011 in all 11 project areas (n = 5883).
Familial ALS
The case reporting form asked neurologists to report if an immediate family member (parent, sibling, child) was diagnosed with ALS. Overall, 244 (4.1%) cases had an immediate family member diagnosed with ALS, while 5023 (85.4%) did not and 616 (10.5%) were unknown. Familial ALS (FALS) was reported more frequently among whites than other races (4.6% of whites, 2% of African-American/blacks and 2.8% of Asians). Non-Hispanic cases had nearly double the percentage of cases reporting possible FALS than Hispanics (4.4 % vs. 2.7%).
Discussion
This project compiled the largest sample of minorities of any published, clinically reviewed ALS research study to date. We found differences in disease incidence rates by race and ethnicity. The racial distribution of the population in our analysis has a larger percentage of minorities (35.6% non-white) than that of the U.S. population per the 2010 U.S. Census (27.6% non-white). According to the 2010 U.S. Census (Citation18), African-American/blacks make up 13% of the total U.S. population and 16% of the population in the 11 project areas; however, African-Americans/blacks comprised only 9% of our cases.
Similar findings were found for the Asian population, which made up 5% of the U.S. population, but 7% of the project population and less than 4% of our collected cases. Hispanics comprised 16% of the U.S. population, 28% of the project population and 11% of our collected cases. Of those with a known racial background, the Hispanic population consisted predominately of white-Hispanics. We expected to collect case reports by race and ethnicity that were proportionately similar to the U.S. Census data in the respective geographic areas. Contrary to this expectation, the collected cases were still disproportionately white and non-Hispanic, further demonstrating the racial and ethnic differences in disease incidence.
While the overall crude average annual incidence rate for ALS of 1.52 per 100,000 person-years and overall age-adjusted average annual incidence rate of 1.44 per 100,000 person-years are within the 1–2 per 100,000 person-years range reported in the literature (Citation2–6), this project may have found overall crude and age-adjusted incidence rates lower than the actual national incidence rates due to the over-representation of minority groups in our population. Notably, there is a difference among the racial groups for both crude average incidence rates of ALS (1.79 per 100,000 person-years white; 0.80 per 100,000 person-years African-American/black; 0.76 per 100,000 person-years Asian) and age-adjusted average incidence rates (1.48 per 100,000 person-years white, CI 1.42–1.53; 0.89 per 100,000 person-years African-American/black, CI 0.79–0.99; 0.78 per 100,000 person-years Asian, CI 0.64–0.92). There is a statistically significant difference in the age- adjusted average annual incidence rates for whites compared with African-American/blacks and whites compared with Asians.
A difference exists in the crude average incidence rates for non-Hispanics (1.65 per 100,000 person-years) compared with Hispanics (0.57 per 100,000 person-years). A statistically significant difference also exists between non-Hispanics and Hispanics when comparing age-adjusted average incidence rates (1.36 per 100,000 person-years non-Hispanics, CI 1.31–1.41 vs. 0.84 per 100,000 person-years Hispanics, CI 0.75–0.92). Similarly to previously published literature (Citation9–13), whites were found to have the highest incidence rate of ALS compared with other races. African-American/blacks had the second highest incidence rate of ALS followed by Asians; non-Hispanics had a higher incidence rate of ALS than Hispanics. After age adjustments the differences in rates diminished, but there is still a significant white predominance compared with other races. These differences may be due in part to whites traditionally having better access to healthcare and receipt of health services (Citation24,Citation25), thereby increasing their likelihood of being diagnosed versus their non-white counterparts. However, given the severity of symptoms as ALS progresses it is unlikely that a case never seeks treatment.
Age at diagnosis was slightly lower among African-American/black cases than the other racial groups, where the median age at diagnosis was 58 years (). Hispanics had a median age at diagnosis four years less than non-Hispanics (58 years vs. 62 years) (). There is a male predominance in all races and no major differences in the distribution of male and female cases within races ().
All cases were assigned an El Escorial classification (Citation19) by the reporting neurologist. While over half of reported white and African-American/black cases were reported as ‘definite’ ALS, approximately 40% of Asians were reported at that level. A larger percentage of Asian cases were reported as ‘possible’ ALS, than in any other racial group.
There are many possible explanations for this finding which go beyond the scope of this project. For example, Asians may have sought diagnosis or treatment earlier than the other racial groups, before the disease has had time to progress to ‘definite’, ‘probable’, or ‘probable laboratory supported’ or Asians do not seek care from a neurologist after the initial diagnosis, perhaps seeking alternative forms of treatment (Citation27–29). Finally, this may be a result of the data collection and cleaning methods utilized in this project (e.g. disease symptoms and thus El Escorial classifications may have progressed after the initial case report was received or when creating composite records for cases reported more than one time, a lower El Escorial criteria classification may have been retained). Further research examining health care seeking behaviors and disease progression among subpopulations may be warranted to explain the differences in reported El Escorial categories by race.
The median and 90th percentiles were utilized to compare racial differences in the time from symptom onset to diagnosis. The median time from symptom onset to diagnosis is longest (12 months) for Asians and Hispanics. Nearly all cases were diagnosed within three years of symptom onset. This measure may reflect the length of time until symptoms progress enough to meet the El Escorial criteria warranting an ALS diagnosis by a physician, it may reflect the patient's delay in seeking treatment until symptoms progress, or it may reflect a recall bias of symptom onset by the patient.
With some caveats, most patients diagnosed with ALS are eligible for Medicare. Over half of the cases (55.9%) utilized Medicare. Rates of Medicare usage were highest among whites (57.6%) and African-American/blacks (50.7%). Asians had the smallest percentage of reported Medicare payer types (46.7%), perhaps supporting the hypothesis that Asians with ALS do not seek additional treatment from neurologists after receiving an ALS diagnosis (Citation28,Citation29). These cases may be reported from the first neurologist visit, when an initial diagnosis is made, and thus the patient has yet to become eligible for Medicare due to an ALS diagnosis as opposed to age. It is also possible that this group is less likely to have had traditional employment for the required amount of time required for these benefits. Further research may provide clarity on the difference in the use of Medicare as a payer type by race.
Payer type is especially important for the National ALS Registry because it relies on federal administrative data sets and a self-registration web portal. Over one-third (37.2%) of all cases in this analysis listed no federal payer (Medicare, Medicaid or VA) and might not be captured in the federal data sets. Asians in particular are more likely to not have a federal payer type (45.8% vs. 36.7% of whites vs. 34.8% of African-American/blacks). Non-Hispanics were more likely to have a federal payer than Hispanics. In total, 6.1% of cases utilized self-pay (2.5% were self-pay only) which could represent uninsured individuals in this population. The self-pay payer type was highest among Asians followed by African-American/blacks and then whites. The cases with no federal payer will need to be captured in the self-registration web portal component of the Registry.
Overall, this analysis is consistent with previous studies (Citation9–13) by demonstrating that there appear to be racial and ethnic differences in ALS rates in the U.S. Specifically, this analysis shows that ALS occurs less frequently in those that are African-American/black, Asian, and Hispanic compared with whites and non-Hispanics in the U.S. It is unclear if this finding is due to methodological issues (e.g. under-ascertainment of minority cases, small sample size) or can be attributed to behavioral factors (e.g. access to adequate health care, diet), socioeconomic status, environmental exposures (e.g. occupational or residential), or genetic factors (e.g. genetic predisposition). However, since this analysis used the largest sample of minorities with ALS to date, the finding is likely due to one, or a combination, of the behavioral, environmental, and genetic factors of ALS. Further research is needed to better understand why these differences occur among racial and ethnic populations.
Acknowledgements
The authors thank the state and local health departments and other organizations that assisted with data collection, and Eric Sorenson from the Mayo Clinic for serving as a consulting neurologist.
This project was funded by McKing Consulting Corporation through two contracts funded by the Agency for Toxic Substances and Disease Registry.
Disclaimer: The conclusions of this article are those of the authors and do not necessarily represent the views of ATSDR, CDC, or the U.S. Department of Health and Human Services.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Logroscino G, Traynor B, Hardiman O, Chio A, Mitchell D, Swingler RJ, et al. Incidence of amyotrophic lateral sclerosis in Europe. J Neurol Neurosurg Psychiatry. 2010;81:385–90.
- Chio A, Mora G, Calvo A, Mazzini L, Bottachi E, Mutani R. Epidemiology of ALS in Italy: a 10-year prospective population based study. Neurology. 2009;72:725–31.
- McGuire V, Longsreth WT, Koepsell TD, van Belle G. Incidence of amyotrophic lateral sclerosis in three counties in western Washington State. Neurology. 1996;47: 571–3.
- Sorenson EJ, Stalker AP, Kurland LT, Windebank AJ. Amyotrophic lateral sclerosis in Olmsted County, Minnesota, 1925–1998. Neurology. 2002;59:280–2.
- Chio A, Logrosino G, Traynor BJ, Collins J, Simeone JC, Goldstein LA, et al. Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology. 2013;41:118–30.
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R.How common are the ‘common’ neurologic disorders? Neurology. 2007;68:326–37.
- Beghi E, Logroscino G, Chiò A, Hardiman O, Mitchell D, Swingler R, et al. The epidemiology of ALS and the role of population based registries. Biochim Biophys Acta. 2006;1762:1150–7.
- Mehal JM, Holman RC, Schonberger LB, Sejvar JJ. Amyotrophic lateral sclerosis/motor neuron disease deaths in the United States, 1999–2009. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:346–52.
- Cronin S, Hardiman O, Traynor BJ. Ethnic variation in the incidence of ALS: a systematic review. Neurology. 2007;68:1002–7.
- Gordon PH, Mehal JM, Holman RC, Rowland LP, Rowland AS, Cheek JE. Incidence of amyotrophic lateral sclerosis among American Indians and Alaska natives. JAMA Neurol. 2013;70:476–80.
- Gundogdu B, Al-Lahham T, Kadlubar F, Spencer H, Rudnicki SA. Racial differences in motor neuron disease. Amyotroph Lateral Scler Frontotemporal Degener. 2014; 15:114–8.
- Zaldivar T, Gutierrez J, Lara G, Carbonara M, Logroscino G, Hardiman O. Reduced frequency of ALS in an ethnically mixed population: a population based mortality study. Neurology. 2009;72:1640–5.
- Sejvar JJ, Holman RC, Bresee JS, Kochanek KD, Schonberger LB. Amyotrophic Lateral Sclerosis Mortality in the United States, 1979–2001. Neuroepidemiol. 2005;25: 144–52.
- Fong KY, Yu YL, Chan YW, Kay R, Chan J, Yang Z, et al. Motor Neuron Disease in Hong Kong Chinese: Epidemiology and Clinical Picture. Neuroepidemiology. 1996;15: 239–45.
- Fong GC, Cheng TS, Lam K, Cheng WK, Mok KY, Cheung CM, et al. An epidemiological study of motor neuron disease in Hong Kong. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:164–8.
- Kazamel M, Cutter G, Claussen G, Alsharabatu M, Oh SJ, Lu L, et al. Epidemiological features of amyotrophic lateral sclerosis in a large clinic based African-American population. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:334–7.
- Antao V, Horton DK. The national amyotrophic lateral sclerosis (ALS) registry. J Environ Health. 2012;75:28–30.
- U.S. Census Population estimates. (Online). 2013. Available from: URL:http://www.census.gov/popest/data/national/totals/2013/index.html.
- McKing Consulting Corporation. ALS State Surveillance Final Summary Report Web site. (Online). 2012. Available from: URL:http://wwwn.cdc.gov/als/ALSAPRReports.aspx.
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. World Federation of Neurology Research Group on Motor Neuron Diseases. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9.
- U.S. Census Bureau About Race. (Online). 2013. Available from: URL:http://www.census.gov/topics/population/race/about.html
- Microsoft Excel (computer program). Redmond, Washington: Microsoft; 2010.
- IBM SPSS Statistics for Windows, Version 19.0 (computer program). Armonk, NY: IBM Corp; 2010.
- Gardner MJ, Altman DG. Confidence intervals rather than p-values: estimation rather than hypothesis testing. Br Med J (Clin Res Ed). 1986;292:746–50.
- Lasser K, Himmelstein D, Woolhandler S. Access to Care, Health Status, and Health Disparities in the United States and Canada: Results of a Cross-National Population-Based Survey. Am J Public Health. 2006; 96:1300–7.
- Disparities in Healthcare Quality Among Racial and Ethnic Minority Groups: Selected Findings From the 2010 National Healthcare Quality and Dispar. March 2011. Agency for Healthcare Research and Quality, Rockville, MD. Retrieved from http://www.ahrq.gov/research/findings/nhqrdr/nhqrdr10/minority.html
- Najm W, Reinsch S, Hoehler F, Tobis J. Use of complementary and alternative medicine among the ethnic elderly. Altern Ther Health Med. 2003;9:50–7.
- Ma GX. Barriers to the use of health services by Chinese Americans. J Allied Health. 2000;29:64–70.
- Huang B, Appel HB, Nicdao EG, Lee HJD, Ai AL. Chronic Conditions, Behavioral Health and Use of Health Services Among Asian American Men: The First Nationally Representative Sample. Am J Mens Health. 2013;7:66–76.

