Abstract
Objective. The aim of this study was to review the impact of salvage external beam radiotherapy (EBRT) of postprostatectomy patients with long-term follow-up on biochemical-free recurrence (BFR) and metastatic-free survival, and to describe pathological and clinical predictors of outcome. Materials and methods. In the period 1987–2010, 76 postprostatectomy patients with biochemical and clinical recurrence received salvage EBRT. Patients were treated with conformal EBRT and 68 (90%) received a dose of 70 Gy; eight patients (10%) received a dose of 60–64 Gy. No patients received adjuvant or neoadjuvant androgen deprivation therapy in conjunction with salvage EBRT. Results. The median follow-up time after salvage EBRT was 82 months (range 5–192 months). Seventeen patients (22%) developed biochemical recurrence subsequent to postprostatectomy salvage EBRT during the observation time, and the overall 50 and 75 month actuarial BFR rates after salvage EBRT were 84% and 79%, respectively. Seven patients (9%) developed metastatic disease and two patients died of prostate cancer. Independent predictors of biochemical recurrence were seminal vesicle invasion (SVI) in the prostatectomy specimen (p < 0.05) and prostate-specific antigen doubling time (PSADT) of 6 months or less (p = 0.041) before salvage EBRT. Conclusions. Salvage EBRT provides effective long-term BFR and metastatic-free survival in a selected group of patients with detectable, rising prostate-specific antigen values following radical prostatectomy. SVI and PSADT are prognostic variables for a non-durable response to salvage EBRT and thus predictors of high-risk prostate cancer in patients in whom neoadjuvant and adjuvant androgen deprivation therapy should be considered.
Introduction
Approximately 30,000 patients undergoing radical prostatectomy (RP) for clinically localized prostate cancer in the USA experience recurrence of the disease per year [Citation1,2]. In Norway, 1253 patients underwent radical prostatectomy (RP) in 2010 [Citation3], and approximately 15–40% of these men will experience a relapse of the disease within 5 years [Citation4,5], usually manifested by an elevated prostate-specific antigen (PSA) level.
Three large, randomized studies evaluating the role of adjuvant external beam radiotherapy (EBRT) in patients who had undergone RP showed that adjuvant EBRT reduced the biochemical treatment failure rate [Citation6–8]. In the Southwest Oncology Group (SWOG) study, the reduction in biochemical - free recurrence rate (BFR) was 48% at 5 years [Citation7], and in the European Organisation for Research and Treatment of Cancer (EORTC) study, the reduction was 40% at 5 years [Citation6]. In the SWOG study and in the EORTC study the prescribed radiation doses were 60–64 Gy and 60 Gy, respectively. Adjuvant EBRT for pT3 prostate cancer with postoperatively undetectable PSA significantly reduced the risk of biochemical progression demonstrated in the ARO 96-02/AUO AP 09/95 study [Citation8]. Reports have demonstrated that salvage EBRT provides effective long-term BFR and freedom from metastasis in selected patients presenting with PSA recurrence after RP. Independent predictors of BFR after undergoing salvage EBRT include negative surgical margin status, vascular invasion, presalvage PSA level greater than 0.4 ng/ml, androgen deprivation therapy (ADT), Gleason score (GS) of 7 or higher, seminal vesicle invasion (SVI), pre-EBRT prostate-specific antigen doubling time (PSADT) shorter than 12 months, and presalvage EBRT PSA level [Citation5,9,10].
The purpose of this study was to evaluate the effect of salvage EBRT on BFR and metastasis-free survival in a population-based cohort of patients with a long-term follow-up after retropubic RP, and to identify clinical and pathological factors that characterize those patients most likely to benefit from salvage EBRT. Furthermore, a patient cohort not likely to respond to salvage EBRT could be considered to profit from adjuvant treatment (e.g. immediate ADT). In the future, the new generation of antiandrogen enzalutamide, the androgen biosynthesis inhibitor abiraterone and other drugs are likely to be considered.
Materials and methods
Patients and pathological findings
The study was approved by the regional ethics committee of south-east Norway. In the period from October 1985 to 2011, 386 patients underwent retropubic RP for clinically localized prostate cancer at Sørlandet County Hospital Arendal. Patients were followed up every 3–6 months for 2 years, every 6 months for the next 3 years, and annually thereafter. Eighty-four patients experienced biochemical recurrence of the disease, and 76 of these patients were treated with salvage EBRT. The medical records of all 76 patients were reviewed retrospectively, including PSA values, operative and pathology reports, and radiographic staging studies (). The clinical stage was assigned preoperatively according to the 1992 International Union Against Cancer tumour, node, metastasis (TNM) system [Citation11]. The prostate specimens have recently been re-evaluated by a genitourinary pathologist (LV) without any knowledge of the clinical data [Citation11]. Final histological classification was done according to the International Society of Urological Pathology (ISUP) Consensus on Gleason grading of prostate cancer [Citation12].
Table 1. Patient characteristics and treatment details of definitive prostatectomy.
Postprostatectomy patterns of failure and treatment characteristics
Postprostatectomy PSA and salvage postprostatectomy treatment details are listed in . All patients had detectable PSA levels before initiation of salvage EBRT. PSADT was calculated for all patients, with three consecutive increases in PSA levels at least 6 weeks apart recorded before initiation of salvage EBRT. The relationship between PSA and time was modelled using a log-linear form, with the logarithm of PSA modelled as a linear function of time during the three measurements [Citation13]. PSADT was calculated as the reciprocal of the slope of the regression line [Citation14]. The PSA values used to calculate the PSADT were only those PSA measurements values from the start to the end of three consecutive increases. The median PSADT was 7.8 months (range 2–51 months). According to Trock et al. [Citation15], PSADT dichotomized at 6 months most strongly separated men for whom salvage EBRT was and was not associated with an increase in prostate cancer-specific survival. Hence, PSADT dichotomized at 6 months was chosen in the analyses.
Table 2. Postprostatectomy prostate-specific antigen (PSA) levels and salvage external beam radiation therapy (EBRT) treatment details.
Biochemical failure after RP was defined as a confirmed PSA rise of at least 0.2 ng/ml. A local failure was recorded as a palpable tumour in the prostatic fossa combined with a detectable PSA level and no evidence of distant or regional metastasis on bone scan, X-ray or magnetic resonance imaging (MRI). Until the year 2004, the majority of patients given salvage EBRT were diagnosed with a verified histological recurrence in the prostatic fossa. Later bioptic verification of local recurrence was not mandatory. Therefore, the majority of the patients given salvage EBRT had biochemical failure only. MRI was introduced in 2001; before then, soft-tissue metastases were ruled out by traditional X-ray, ultrasound and computed tomography (CT) scans. In accordance with published data, both a palpable nodule detected by digital rectal examination and a slowly rising serum PSA level can be signs of local recurrence [Citation16]. Patients with a palpable nodule in the prostatic fossa, and those with a low-velocity rising PSA level (i.e. PSADT >12 months), and no evidence of systemic or regional disease, were given salvage EBRT. None of the patients who were delivered salvage EBRT received neoadjuvant or adjuvant ADT as a component of the salvage therapy.
Sixty-eight patients (90%) were treated with a dose of 70 Gy and eight patients (10%) with a dose of 60–64 Gy. Patients were instructed in the standard manner to prepare their bowel and bladder before planning CT and before radiation treatment. All patients underwent simulation and were treated using three-dimensional conformal EBRT techniques; fields encompassed the prostate and seminal vesicle bed plus periprostatic tissues. No attempt was made to comprehensively irradiate the pelvic lymph nodes.
Postirradiation evaluation
Biochemical failure after salvage EBRT was defined as detection of a postsalvage radiation PSA value greater than 0.2 ng/ml followed by a second PSA level higher than the first by any amount, by a continued increase in PSA level after treatment greater than the pretreatment PSA level, or by the initiation of ADT after salvage EBRT [Citation9].
Statistics
Categorical variables were analysed using Fisher’s exact test or the chi-squared test, where appropriate. In univariate survival analyses, event–time distributions were estimated using the Kaplan–Meier method and compared using log-rank tests. Cox proportional hazards regression was used in the multivariate analyses, and log–log plots were used to assess the assumption of proportional hazards. Patients who died of unrelated causes without developing biochemical or clinical failure after undergoing salvage EBRT were treated as censored cases. Two-tailed tests were performed and results were considered significant when p < 0.05. Analyses were carried out using SPSS version 16.0 (SPSS, Chicago, IL, USA) and R version 2.9.1 (R Foundation for Statistical Computing).
Results
All patients had their surgery at the Sørlandet County hospital in Arendal. The median age of the patients at surgery was 61 years (range 44–72 years), and the median PSA level before prostatectomy was 7.6 ng/ml (2.6–36 ng/ml). Patients’ characteristics and treatment details at the time of RP are listed in . The median postprostatectomy PSA level before undergoing salvage EBRT was 1.0 ng/ml (0.22–10.6 ng/ml). The median time from RP to salvage EBRT was 35 months (4–156 months).
In total, 17 patients (22%) developed biochemical recurrence subsequent to postprostatectomy salvage EBRT during the observation time. The overall 50 and 75 month actuarial BFR rates after salvage EBRT were 84% and 79%, respectively. The median follow-up time after salvage EBRT was 82 months (range 5–192 months). The median time to PSA relapse following salvage EBRT was 12 months (2–68 months).
Seven patients (9%) were diagnosed with metastatic disease during the observation period. Two patients (3%) died of prostate cancer during the observation time.
Fifty-one (67%) of the patients who received salvage EBRT subsequent to RP had positive margin status (). Event–time distributions reflecting BFR were estimated using the Kaplan–Meier method and compared using log-rank tests exploring negative margin status versus positive margin status and PSA level 0–1 ng/ml versus PSA greater than 1.0 ng/ml before salvage EBRT. These showed no significant differences (p = 0.54 and p = 0.19, respectively) (). PSADT shorter than 6 months versus PSADT of 6 months or longer and GS, however, demonstrated statistical significance in estimating the event–time distribution using the Kaplan–Meier method and compared using log-rank tests (p = 0.03 and p = 0.003, respectively) (). SVI showed statistical significance in estimating the event–time distribution using the Kaplan–Meier method and compared using the log-rank test (p < 0.05) ().
Figure 1. Proportion free from prostate-specific antigen (PSA) relapse following salvage external beam radiation therapy (EBRT) in relation to (a) surgical margin status, (b) pre-salvage EBRT PSA level 0–1.0 ng/ml or ≥1.0 ng/ml, (c) prostate-specific antigen doubling time (PSADT) 0–6 months or >6 months, (d) Gleason score (GS) 3 + 4 or less or GS 4 + 3 or more, and (e) absence or presence of seminal vesicle invasion (SVI).
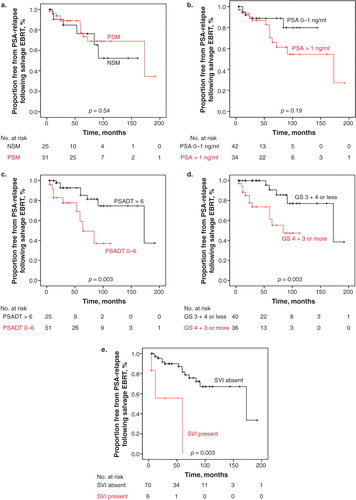
Multivariate survival analyses for risk of biochemical failure following salvage EBRT are presented in . Only analyses explored in univariate statistics, which demonstrated significant differences, were incorporated in the multivariate analyses. Presalvage EBRT PSADT less than 6 months [hazard ratio (HR) 0.3, p = 0.04] and SVI (HR 7.9, p < 0.05) were both significant independent predictors of biochemical failure, while the GS of the specimen was not (HR 2.1, p = 0.29).
Table 3. Multivariate analysis of predictors for biochemical-free recurrence following salvage external beam radiation therapy for prostate cancer.
Discussion
Recurrence of prostate cancer heralded by rising PSA levels in patients undergoing RP presents a difficult dilemma for patients and their urologists. Three randomized controlled trials have shown a benefit of using adjuvant EBRT compared with a wait-and-see approach [Citation6–8]. Improvements in BFR, as well as clinical-free survival, were documented in the studies. Retrospective studies have shown that salvage EBRT in patients who experience recurrence of disease after undergoing RP offers a prolonged BFR of survival. Most of the studies, however, have a postirradiation follow-up of less than 5 years [Citation5,9,10]. Furthermore, the salvage EBRT doses in the majority of these studies are now considered low doses of radiation (66 Gy) [Citation4,5,17].
For patients with recurrent prostate cancer after RP, salvage EBRT remains the only potentially curative treatment [Citation4,9]. Studies have demonstrated better outcomes with salvage EBRT when it is administered at the earliest evidence of disease progression, that is, when the PSA level has just started to increase above detectable levels [Citation4]. However, the use of very low PSA thresholds increases the risk of overtreating patients whose PSA level is detectable owing to residual benign prostatic tissue [Citation1]. The risk of overtreatment can be avoided by confirming a trend of increasing serum PSA levels, rather than simply one or two low-threshold values. Approximately one-third of men with a PSA after RP greater than 0.4 ng/ml in any period following surgery will proceed to the development of clinically evident disease [Citation5].
Trock et al. compared prostate cancer-specific survival among men who received salvage EBRT versus observation in a retrospective cohort of 635 men with biochemical or local recurrence after prostatectomy [Citation15]. With a median follow-up of 6 years after recurrence, salvage EBRT was associated with a significant three-fold improvement in prostate cancer-specific survival, regardless of whether ADT was also included, and the improvement was primarily confined to men with PSADT shorter than 6 months. Moreover, Trock et al. evaluated interactions between salvage EBRT and PSADT dichotomized at cut-off points reported in the literature (3, 6, 9, 10 and 12 months) [Citation18,19]. PSADT dichotomized at 6 months most strongly separated men for whom salvage EBRT was and was not associated with an increase in prostate cancer-specific survival [Citation15]. This effect was observed regardless of surgical margin status or GS. Furthermore, salvage EBRT was associated with an increase in survival only if given sooner than 2 years after recurrence. Initiating salvage EBRT when the PSA level was 2 ng/ml or lower was associated with a survival benefit [Citation15].
Several authors have assessed the ability of different variables to predict the outcome following salvage EBRT; pretreatment serum PSA was a consistent predictor of outcome. In a larger study (285 patients) presented by Goenka et al., a preradiotherapy PSA greater than 0.4 ng/ml was found to have significant predictive power with regard to BFR following salvage EBRT [Citation9]. However, there is no consensus on the exact level of PSA at which salvage EBRT should be delivered. The serum PSA level following salvage RP is a snapshot of a larger continuum; however, PSADT is a dynamic view of serum PSA. PSADT, therefore, would seem to reflect more accurately the biological activity of the prostate cancer disease.
Several reports have described PSADT as a clearly significant and reliable tool to distinguish patients destined to have prolonged and low PSA levels following salvage EBRT from patients destined for progression of prostate cancer [Citation4,5]. Short PSADTs (<3 months) have been strongly associated with generalized disease [Citation20,21]. In the present population-based study with long-term follow-up, a significant relationship was found between PSADT and biochemical response to salvage EBRT. This series of postprostatectomy salvage EBRT patients with long-term follow-up describes PSADT between 2 and 57 months, and it is in this context that the benefits of localized, systemic or no additional therapy can improve our knowledge. All patients who developed metastases following salvage EBRT had PSADT less than 9 months (range 1.9–8.6 months). Since biochemical recurrence is rather high in this group of patients, one would like to argue for concomitant ADT. To the authors’ knowledge, no randomized controlled trials have yet been performed with inclusion of this selected patient population. The approach of randomizing patients directly after RP to adjuvant radiation therapy with or without ADT is explored in the RADICALS study; however, that study has not yet concluded [Citation22].
In a smaller study (49 patients) presented by Leventis et al., PSADT was demonstrated to have similar predictive power [Citation23]. Leventis et al. reported a 5 year BFR of 27% for patients with PSADT greater than 11.8 months versus 16% for patients with a shorter PSADT (p = 0.036). Ward et al., in their study of 211 patients undergoing salvage EBRT with a median dose of 64 Gy, report BFR of 48% and 66% at 5 years, for PSADT less than 12 months and greater than 12 months, respectively [Citation5]. Furthermore, clinical metastasis and prostate cancer death were substantially less (p = 0.045 and p = 0.100) at 5 and 10 years following salvage EBRT for patients with a PSADT longer than 12 months (0%, 17% and 0%, 0%, respectively) than for patients with a PSADT of less than 12 months (10%, 29% and 1%, 15%, respectively) in their study.
Positive surgical margins remained an independent risk factor of BFR after RP with less relevant impact in pT3b disease in a 10 year follow-up study published by Rouanne et al. [Citation24]. In the present series the majority, 67% of patients, given salvage EBRT were diagnosed with positive surgical margins. However, the need for immediate versus delayed EBRT remains debatable. Patients with low-risk disease had a similar outcome in terms of biochemical progression-free survival regardless of surgical margin status [Citation25]. A randomized prospective trial in patients with positive surgical margins comparing adjuvant and salvage EBRT is needed before any definitive clinical recommendations can be given.
In the multivariate Cox regression model, PSADT less than 6 months together with SVI were found to be predictors of failure following salvage EBRT. Stephenson et al., in their multi-institutional cohort of patients undergoing salvage EBRT, report that PSADT less than 10 months is a powerful predictor of BFR following salvage EBRT, together with the GS of the specimen, SVI and surgical margin status [Citation4]. One could speculate that a patient cohort not likely to respond to salvage EBRT could be considered to profit from additional treatment, such as immediate ADT.
As a further consequence of the above finding, bone marrow could be analysed for circulating tumour cells (CTCs). Berg et al. have shown that patients given radical EBRT for prostate cancer with a GS of at least 4 + 3 = 7 and a positive bone marrow for CTCs have double the risk of developing biochemical recurrence [Citation26]. Thus, it would be interesting to analyse patients with biochemical recurrence after RP for CTCs to determine whether the presence of CTCs could predict patients at risk of being resistant to salvage EBRT.
A limitation of this study is the absence of a comparison group that did not receive salvage EBRT. The series reported here is limited, with only 76 patients being analysed. However, a strength of the study is that ADT was not administered to patients in a neoadjuvant or adjuvant setting. The results show that patients with a PSADT greater than 6 months following RP have a better outcome in terms of BFR following salvage EBRT than those with a PSADT less than 6 months. Hence, assessment of PSADT following RP should be considered before initiating salvage EBRT.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Stephenson AJ, Shariat SF, Zelefsky MJ, Kattan MW, Butler EB, Teh BS, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA 2004;291:1325–32.
- Moul JW. Prostate specific antigen only progression of prostate cancer. J Urol 2000;163:1632–42.
- Kreftregisteret i Norge 2011. Available from www.kreftregisteret.no.
- Stephenson AJ, Scardino PT, Kattan MW, Pisansky TM, Slawin KM, Klein EA, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol 2007;25:2035–41.
- Ward JF, Zincke H, Bergstrahl EJ, Slezak JM, Blute ML. Prostate specific antigen doubling time subsequent to radical prostatectomy as a prognosticator of outcome following salvage radiotherapy. J Urol 2004;172:2244–8.
- Bolla M, van Poppel H, Colette L, van Cangh P, Vekemans K, Da Pozzo L, et al. Postoperative radiotherapy after radical prostatectomy: a randomized controlled trial (EORTC trial 22911). Lancet 2005;366:572–8.
- Thompson IM, Tangen CM, Paradelo J Lucia MS, Miller G, Troyer D, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA 2006;296:2329–35.
- Wiegel T, Bottke D, Steiner U, Siegmann A, Golz R, Störkel S, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol 2009;27:2924–30.
- Goenka A, Magsanoc JM, Pei X, Schechter M, Kollmeier M, Cox B, et al. Long-term outcomes after high-dose postprostatectomy salvage radiation treatment. Int J Radiat Oncol Biol Phys 2011;77:1–7.
- Cheung R, Kamat AM, de Crevoisier R, Allen PK, Lee AK, Tucker SL, et al. Outcome of salvage radiotherapy for biochemical failure after radical prostatectomy with or without hormonal therapy. Int J Radiat Oncol Biol Phys 2005;63:134–40.
- Ohori M, Wheeler TN, Scardino PT. The New American Joint Committee on Cancer and International Union Against Cancer. TNM classification of prostate cancer. Clinicipathologic correlations. Cancer 1994;74:104–14.
- Epstein JI, Allsbrook WC, Amin MB, Egevad LL; ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol 2005;29:1228–42.
- Zelefsky MJ, Ben-Porat L, Scher HI, Chan HM, Fearn PA, Fuks ZY, et al. Outcome predictors for the increasing PSA state after definitive external-beam radiotherapy for prostate cancer. J Clin Oncol 2005;23:826–31.
- Memorial Sloan Kettering Cancer Center. PSA doubling time calculator. Available from http://www.mskcc.org/mskcc/html/10088.cfm.
- Trock BJ, Han M, Freedland SJ, Humphreys EB, DeWeese TL, Partin AW, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 2008;299:2760–9.
- Trapasso JG, de Kernion JB, Smith RB, Dorey F. The incidence and significance of detectable levels of serum prostate specific antigen after radical prostatectomy. J Urol 1994;152:1821–5.
- Bernard JR, Buskirk SJ, Heckman MG, Diehl NN, Ko SJ, MacDonald OK, et al. Salvage radiotherapy for rising prostate-specific antigen levels after radical prostatectomy for prostate cancer: dose–response analysis. Int J Radiat Oncol Biol Phys 2010;76:735–40.
- Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999;281:1591–7.
- Maffezzini M, Bossi A, Collette L. Implications of prostate-specific antigen doubling time as indicator of failure after surgery or radiation therapy for prostate cancer. Eur Urol 2007;51:605–13.
- Sandler HM, Dunn RL, McLaughlin PW, Hayman JA, Sullivan MA, Taylor JM. Overall survival after prostate-specific-antigen detected recurrence following conformal radiation therapy. Int J Radiat Oncol Biol Phys 2000;48:629–33.
- D’Amico AV, Moul JW, Carroll PR, Sun L, Lubeck D, Chen MH. Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst 2003;95:1376–83.
- ClinicalTrials.gov. Radiation therapy and androgen deprivation therapy in treating patients who have undergone surgery for prostate cancer (RADICALS). Available from http://clinicaltrials.gov/show/NCT00541047.
- Leventis AK, Shariat SF, Kattan MW, Butler B, Wheeler TM, Slawin KM. Prediction of response to salvage radiation therapy in patients with prostate cancer recurrence after radical prostatectomy. J Clin Oncol 2001;19:1030–9.
- Rouanne M, Rode J, Campeggi A, Allory Y, Vordos D, Hoznek A, et al. Long-term impact of positive surgical margins on biochemical recurrence after radical prostatectomy: ten years follow-up. Scand J Urol 2014;48:131–7.
- Alkhateeb S, Alibhai S, Fleshner N, Finelli A, Jewett M, Zlotta A, et al. Impact of positive surgical margins after radical prostatectomy differs by risk group. J Urol 2010;183:145–50.
- Berg A, Berner A, Lilleby W, Bruland ØS, Fosså SD, Nesland JM, et al. Impact of disseminated tumor cells in bone marrow at diagnosis in patients with nonmetastatic prostate cancer treated by definitive radiotherapy. Int J Cancer 2007;15:1603–9.

