Abstract
Objective.The aim of this study was to assess treatment-related changes in prostate-specific antigen (PSA), total and bone alkaline phosphatase (total ALP, bone ALP), and changes on conventional bone scans in patients with metastatic castration-resistant prostate cancer (mCRPC) with bone metastases who received six cycles of radium-223 (Ra-223). Materials and methods. Changes in PSA, total ALP and bone ALP (≥30% increase or decrease), and changes on bone scans were assessed before and after six monthly cycles of Ra-223 therapy (50 kBq/kg body weight) in 14 patients with mCRPC with bone metastases and four patients on placebo. Results.Post-treatment PSA increased by at least 30% in 11 out of 14 patients and remained stable in three. Total ALP and bone ALP decreased in six and nine patients, respectively. In 10 out of 12 evaluable patients the uptake on post-treatment bone scan was reduced in lesions with high pretreatment uptake, in 11 patients accompanied by the development of new or expanded bone lesions. FACBC position emission tomography/computed tomography scans confirmed the growth of new or expanded bone metastases in two patients. Conclusions.These observations support the notion that Ra-223 kills tumour cells in metastases surrounded by highly proliferating osteoblasts, consistent with the reported survival benefit. The radiation effect in small tumour deposits not surrounded by increased osteoblast activity seems, however, insufficient, thus allowing continuous tumour growth. Long-lasting PSA reductions are the exception rather than the rule during Ra-223 treatment, whereas alkaline phosphatases decrease more frequently. To improve the overall anticancer effect, Ra-223 might be a valuable component of combination treatment.
Introduction
Radium-223 (Ra-223) has emerged as a life-prolonging therapeutic option for metastatic castration-resistant prostate cancer (mCRPC) with bone metastases but no or only minor soft-tissue involvement [Citation1]. The radioactive isotope Ra-223 is absorbed to the surface of newly formed inorganic bone matrix, particularly to hydroxyapatite. Ra-223 emits α-particles with a tissue range of only less than 0.1 mm (corresponding to about 10 cells). Ra-223 accumulates particularly in skeletal sites with high osteoblastic activity, for example osteosclerotic bone metastases from prostate cancer [Citation2]. Such sites are visualized on conventional bone scans by increased uptake of 99mTc-labelled bisphosphonates (HDP). The activity of osteoblasts is also reflected by increased serum bone alkaline phosphatase (bone ALP), a component of total alkaline phosphatase (total ALP) [Citation3].
At week 12 of the Alsympca trial, 47% of the patients in the Ra-223 arm had their baseline total ALP reduced by at least 25%, while a reduction of prostate-specific antigen (PSA) of at least 25% was observed in only 16% of the patients [Citation1]. This discrepancy between PSA and total ALP calls for further analyses of these biochemical measures, including other markers of bone metabolism and imaging techniques as well.
In this study, the changes in PSA and markers of bone metabolism were analysed in patients with mCRPC who underwent six monthly cycles of Ra-223 therapy, and the results were compared to those in the placebo arm of the Alsympca trial. Observations from longitudinal bone scans are presented, supported by preliminary findings obtained by anti-1-amino-3-[18F]fluorocyclobutane-1-carboxylic acid (FACBC) positron emission tomography (PET)/computed tomography (CT) examinations.
Materials and methods
Patients
Oslo University Hospital (OUH) Radium Hospital participated in the Alsympca trial [Citation1] and in the subsequent early access programme (EAP; Eudract no. 2012-000075-16). All patients had progressing skeletal metastases after treatment with docetaxel, but no visceral involvement. Patients could present with lymph-node metastases, sized 3 cm or smaller in the Alsympca trial [Citation1], or 6 cm or smaller in the EAP. They could have received palliative localized external beam radiotherapy within 3 months preceding inclusion in the study. Patients were eligible for the present study if they had received six monthly infusions of Ra-223 (50 kBq/kg body weight; Alpharadin group) or physiological saline water (placebo group). Localized palliative radiotherapy was the only permitted additional oncological treatment.
Biochemistry
PSA and markers of bone metabolism (total ALP, bone ALP) and carboxy-terminal pyridinoline cross-linked telopeptide (1CTP) [Citation4] were analysed before the start of treatment (time-point 0), before each infusion and 1 month after the last infusion. PSA was analysed using Delfia (Perkin Elmer, Turku, Finland) up to April 2013, and after this date using Cobas 6000 (Roche, Mannheim, Germany). Total ALP was analysed using Kodak Ektachem (Rochester, NY, USA) up to May 2009, and thereafter on Cobas 6000 (Roche). Bone ALP was analysed using Bechman Access up to June 2009, and thereafter with a MicroVue BAP EIA kit (Quidel Corporation, San Diego, CA, USA). These changes in methods were considered insignificant and none of the reference limits was altered. 1CTP was determined using a competitive radioimmunoassay technique with a kit from Orion Diagnostica, Espoo, Finland.
Imaging
All patients had undergone conventional bone scintigraphy before the start of treatment and 4 weeks after the sixth and last injection. The bone scans were preferentially performed at OUH (28 scans) or alternatively at local hospitals (six scans). The bone-seeking agent used was 99mTc-labelled sodium oxidronate (Technescan HDP; Mallinckrodt Medical, Dublin, Ireland), and imaging was started at least 2.5 h after intravenous injection of 750 ± 75 MBq. At OUH, imaging was performed on an ADAC (Adacab, CA, USA) Vertex dual head single-photon emission computed tomography (SPECT) camera with three-quarter-inch crystal using low-energy high-resolution collimators. The images were processed on an ADAC Pegasys workstation and transferred to the institutional picture archiving and communication system (PACS). Bone scans from institutions other than OUH were collected from the respective hospitals. The central review of pretreatment and post-treatment images was based on consensus between two coauthors experienced in the interpretation of bone scans (EH and TVB). For each bone scan, the number of metastatic lesions was counted. The bone scans were scored according to extent of the disease (EOD) classification suggested by Soloway et al. [Citation5]. Comparing the pretreatment and post-treatment bone scans, changes were described by the following two criteria: (i) reduced extent and/or intensity of HDP uptake in metastatic foci with increased uptake before Ra-223 treatment (“reduced uptake”); and (ii) the appearance of new foci of HDP uptake adjacent to or distant from metastatic foci with increased HDP uptake before Ra-223 treatment (“new lesions”).
Skeletal sites irradiated during the 3 months preceding inclusion in the study, and skeletal sites irradiated during the study period were excluded from the evaluation.
Positron emission tomography scan
FACBC PET/CT was performed before and after treatment in two patients. FACBC is a synthetic cyclic amino acid analogue labelled with fluorine-18 used in investigational PET/CT for staging and restaging of prostate cancer. The tracer seems to show more metastatic lesions and higher lesion to background uptake compared to PET/CT using carbon-11 or fluorine-18-labelled choline [Citation6]. Administered activity was 3 MBq/kg, with a minimum of 200 MBq. Imaging started 5 min postinjection, with a minimum 2 min acquisition time per bed position. Low-dose CT without contrast enhancement was performed and PET/CT fused images were generated.
Ethics
The regional ethics committee approved the study and all patients signed a consent document.
Statistical analyses
Changes in PSA, total ALP, bone ALP and 1CTP were defined as an increase or a decrease of at least 30% from baseline. The summarized results are displayed as median (range) using the Wilcoxon signed rank test for comparisons. A p value < 0.05 was regarded as statistically significant.
Results
By February 2014, 14 of the patients had received Ra-223 and four men had been included in the placebo arm of the Alsympca trial (). Nine of the 13 evaluable patients who had received Ra-223 had an EOD grade of 3 (pretreatment bone scan missing in one patient in the Ra-223 group.) Median PSA was 249 μg/l at treatment start (range 3.6–4230 μg/l). The majority of patients had elevated markers of bone metabolism. Localized radiotherapy was given to four patients during Ra-223 treatment and to one patient in the placebo group.
Table 1. Baseline characteristics of 14 patients who received radium-223 (Ra-223) and four patients on placebo treatment.
Prostate-specific antigen
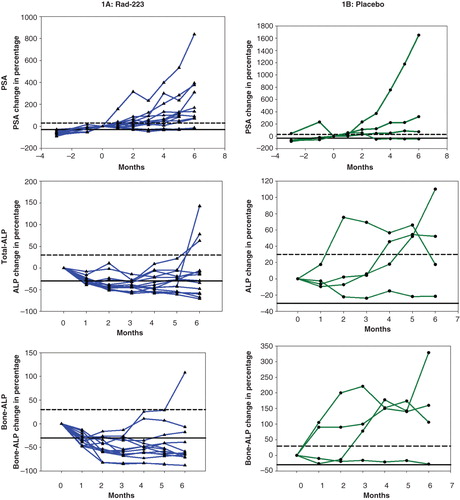
Four weeks after the sixth and last injection of Ra-223, PSA had increased significantly in 11 patients in the Alpharadin group, where the median value was 415 μg/l (range 21–6950 μg/l) (, ), and in three of the four patients in the placebo group. A PSA reduction was observed in two patients without localized radiotherapy, lasting for 2 and 4 months, respectively. In one of the four patients on placebo, PSA decreased by at least 30% (after radiotherapy), remaining at that level throughout the study period (), but this patient had received radiation therapy during the study period.
Table 2. Number of patients that received Ra-223 with changes of PSA, total-ALP and bone-ALP from baseline after three- and six months treatment.
Figure 1. Longitudinal changes (% from baseline, 0) of prostate-specific antigen (PSA), total alkaline phosphatase (total ALP) and bone alkaline phosphatase (bone ALP) during 6 months of therapy with radium-223 (Ra-223) (A: 14 patients) or placebo (B: four patients). (Several parts of individual curves overlap each other.) Dashed horizontal line indicates ≥30% increase from baseline; solid horizontal line indicates ≥30% reduction from baseline.
Alkaline phosphatases
Total ALP decreased significantly during Ra-223 therapy, reaching a median of 92 U/l (range 32–392 U/l) and 149 U/l (range 40–360 U/l) at 3 and 6 months, respectively (, ). Three months after treatment initiation, nine of the 14 patients had decreased total ALP values. Four weeks after the sixth and last injection of Ra-223 only six patients had total ALP values greater than or equal to 30% below the baseline. None of the four patients in the placebo arm had decreased total ALP, and an increase of more than 30% was seen in two patients.
The changes in bone ALP reflected those of total ALP, although with more patients displaying decreased bone ALP. In eight of the 14 patients in the Alpharadin group, 1CTP had increased after six cycles.
Imaging
In 10 of the 12 evaluable patients receiving Ra-223, a reduction in HDP uptake was observed in lesions with high pretreatment HDP uptake, the interpatient number of lesions with reduced uptake varying from two to 39 (); however, new metastatic foci of HDP uptake developed in 11 of these 12 patients. Patient number 13 () displays this scenario, with the development of multiple new lesions adjacent to pretreatment high-intensity lesions or appearing as distant loci, concomitant with multiple uptake-reduced lesions. further illustrates the development, as comparison of this patient’s pretreatment and post-treatment FACBC PET/CT revealed new tumour growth adjacent to a known tumour lesion in Th7. A similar development was observed in C2, with new post-treatment HDP uptake and corresponding increased FACBC tracer uptake on PET/CT.
Table 3. Six month changes on [99mTc]bisphosphonate bone scan.
Figure 2. Whole-body [99mTc]bisphosphonates (HDP) bone scans for patient no. 13: pretreatment (left column A and C) and post-treatment (right column B and D), with anterior views (upper row A and B) and posterior views (lower row C and D). Development of new/expansion of existing lesions (n = 24) and concomitant reduced HDP uptake in pretreatment known lesions. Arrows point to C2 and Th7, further visualized by FACBC positron emission tomography/computed tomography; this examination was not available for the left thigh (broken arrow).
![Figure 2. Whole-body [99mTc]bisphosphonates (HDP) bone scans for patient no. 13: pretreatment (left column A and C) and post-treatment (right column B and D), with anterior views (upper row A and B) and posterior views (lower row C and D). Development of new/expansion of existing lesions (n = 24) and concomitant reduced HDP uptake in pretreatment known lesions. Arrows point to C2 and Th7, further visualized by FACBC positron emission tomography/computed tomography; this examination was not available for the left thigh (broken arrow).](/cms/asset/fb240459-764c-44eb-8cc9-69cc326150b1/isju_a_982169_f0002_c.jpg)
Figure 3. [18F]FACBC positron emission tomography/computed tomography fused images of C2 (upper row) and Th7 (lower row) for patient no. 13: pretreatment (left column) and post-treatment (right column). Tumour expansion with increased FACBC uptake (arrows) in areas of no/very weak uptake pretreatment, interpreted as new tumour tissue.
![Figure 3. [18F]FACBC positron emission tomography/computed tomography fused images of C2 (upper row) and Th7 (lower row) for patient no. 13: pretreatment (left column) and post-treatment (right column). Tumour expansion with increased FACBC uptake (arrows) in areas of no/very weak uptake pretreatment, interpreted as new tumour tissue.](/cms/asset/c249f32d-35c4-40c1-a75e-d4d628a5506b/isju_a_982169_f0003_c.jpg)
Lesions with reduced HDP uptake were observed even in the placebo group, although to a substantially lesser degree than in the Alpharadin group.
Discussion
Four weeks after six cycles of Ra-223, a reduction in PSA was observed in none of the 14 patients, while total ALP and bone ALP decreased in six and nine patients, respectively. In general, Ra-223 treatment resulted in reduced intensity and/or volume of most bone lesions with increased HDP uptake on the baseline scan. However, during the 6 months of treatment new foci of increased uptake of HDP emerged. Preliminary, limited experience with FACBC PET/CT examinations supports the observation of mixed responses on conventional bone scan.
The present findings concerning PSA are in agreement with those from the Alsympca study [Citation1], although that trial combined pre- and post-docetaxel patients without separating these patients from those without localized radiotherapy, the later sometimes leading to PSA reduction [Citation7]. However, the significant delay in PSA increase compared to the placebo group may explain the observed survival benefit. The changes in PSA from this large trial, supported by the present observations, seem to be different from those observed in post-docetaxel mCRPC patients receiving abiraterone or enzalutamide [Citation8,9]. These treatments resulted in more marked PSA reductions in at least 30% of the patients. Notably, an increase in PSA during Ra-223 therapy may result from the development or progression of soft-tissue metastases not influenced by Ra-223. Increased PSA during treatment with Ra-223 may also be explained by a contemporary development of lymph-node or visceral metastases, in particular in patients with very advanced disease. As post-treatment abdominal CT was not systematically performed in patients in this study, the proportion of patients with such progression cannot be calculated.
As in the Alsympca trial [Citation1], total ALP decreased rapidly in the present study, reflected by a reduction in bone ALP. However, total ALP and bone ALP tended to rise after four to five cycles. These markers indicate osteoblast activity [Citation3,10], and are particularly increased in the presence of osteosclerotic, osteoclastic metastases. The subsequent decrease in these markers after radionuclide therapy has previously been described during Ra-223 treatment, but also after therapy with strontium-89 and docetaxel [Citation7,11]. A decrease in total ALP during docetaxel therapy was associated with beneficial survival in mCRPC patients, viewed as a surrogate measure of tumour cell death [Citation12]. However, when using a cytotoxic agent and in particular a bone-seeking tracer, radiation-induced cell stunning or kill of highly proliferating osteoblasts surrounding a skeletal metastasis may contribute to the reduction of total ALP or bone ALP.
1CTP is a degradation product from collagen type 1. It reflects the activity of osteoclasts and has been reported to reflect pathological degradation of bone better than degradation seen in physiological bone turnover [Citation13]. Nilsson et al. [Citation11] observed significant decreases in 1CTP during Ra-223 therapy. Kamiya et al. [Citation4] reported that the serum 1CTP level was a more reliable marker than alkaline phosphatases for detecting bone metastases and for predicting the probability of survival in prostate cancer patients with bone metastases. In the present limited patient sample, the authors refrain from any comparison.
The comparison of pretreatment and post-treatment bone scans in part explains the observations regarding the changes in PSA, total ALP and bone ALP, since increased tracer uptake on bone scans reflects increased osteoblastic activity and new bone formation. The higher the uptake of HDP on bone scans, the higher the expected uptake of Ra-223. Accumulation in normal bone is probably low [Citation14]. After treatment with Ra-223, reduced uptake is observed in skeletal foci with pretreatment high HDP uptake, corresponding to sites with high osteoblastic activity. Radiation-induced tumour cell death is to be expected at these sites, limited to a narrow range. The post-treatment reduced uptake of HDP may reflect a diminished tumour burden, as well as a direct radiation effect on the osteoblasts (stunning or cell death). Small or microscopic bone metastases surrounded by no or minimal osteoblast activity, and therefore no major uptake of Ra-223, are not sufficiently irradiated and may thus increase in size, resulting in new sites of HDP uptake on the post-treatment bone scans. Small lesions may, however, have high uptake that may not be detected on conventional bone scans because of the limited geometric resolution of the gamma camera. PET/CT systems have much higher geometric resolution than conventional gamma cameras, and PET/CT with the bone-seeking tracer sodium fluoride (NaF) will detect smaller lesions and therefore more lesions than conventional bone scans.
The findings on the bone scans are supported by the FACBC PET/CT examinations in two of the patients. Cook et al. [Citation15] suggested that PET/CT with NaF may reflect the true tumour response earlier and more specifically than bone scans. Importantly, such examinations may indicate that the new sites of HDP uptake reflect tumour growth and not only transient reparative reactions of the bone (“pseudoprogression” [Citation16]”) which, in the present study, is supported by increasing PSA and decreased alkaline phosphatases. Experience from pretreatment and post-treatment FACBC PET/CT examinations in two patients combined with bone scans suggests that small tumour sites without major pretreatment osteoblastic reactions expand during Ra-223 therapy, as reflected by expanded or new post-treatment HDP uptake and visualized by tumour uptake on FACBC PET/CT. The use of FACBC or other more tumour-specific markers will probably replace unspecific tracers such as bisphosphonates or NaF in the future.
Only a few reports are available with observations before and after Ra-223 therapy. Croke et al. [Citation17] described one patient with an overall reduction of pretreatment HDP uptake 1 year after the start of Ra-223 treatment, and this finding essentially supports the present results. However, a closer view of Croke and colleagues’ published pretreatment and post-treatment bone scans shows that even in this patient new lesions may have appeared, not only restricted to the right humerus as mentioned by the authors.
The findings in the present study indicate that Ra-223 accumulates in skeletal sites with increased HDP uptake in bone metastases in patients with mCRPC, and this is followed by reduced osteoblast activity in these areas, reflected by decreases in total ALP and bone ALP. Within these sites the tumour burden probably diminishes, as reflected by the temporary decrease or at least stabilization of PSA in some patients. However, minor bone metastases or micrometastases that are present before treatment, but without increased HDP uptake in the surrounding skeleton, are probably not sufficiently irradiated and may thus progress during Ra-223 therapy. This may, in part, explain the increase in PSA at the end of treatment in the majority of patients. Furthermore, these admittedly limited observations render the future use of Ra-223 as adjuvant monotherapy questionable but support the combination of Ra-223, if possible at higher doses [Citation18], with other systemic active anticancer drugs. Finally, from a practical point of view the authors now inform patients starting Ra-223 monotherapy that a PSA decrease will occur only exceptionally, but that they can hope for transient stabilization of PSA, prolongation of life and reduction of pain.
Limitations of this study are the small sample size, lack of bioptic verification, and the fact that some of the bone scans were stored in a format that made optimal screen contrast adjustment impossible, and reprocessing was not possible because raw data were not available. Furthermore, most patients had relatively advanced bone involvement. A strength of the current project is that this study, to the authors’ knowledge, is the first one to visualize the response of bone metastases during Ra-223 therapy as an explanation of the observed changes of PSA and biomarkers of bone turnover. A general limitation using conventional bone scans is the limited geometric camera resolution.
In conclusion, this study demonstrates a mixed response on bone metastases in patients with mCRPC treated with Ra-223, thus explaining the low proportion of patients with long-lasting PSA response. The frequent decrease in total ALP and bone ALP may be explained by the radiation effect of Ra-223 in sites of high osteoblastic activity reflecting sites of macroscopic skeletal bone metastases. Irradiation of minor skeletal tumour deposits with no or limited HDP uptake seems insufficient, allowing tumour growth. This supports the view of using Ra-223 as part of combination therapy. More studies, preferably with biopsies, are desirable to confirm or disapprove these preliminary observations.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369:213–23.
- Pandit-Taskar N, Larson SM, Carrasquillo JA. Bone-seeking radiopharmaceuticals for treatment of osseous metastases, Part 1: alpha therapy with 223Ra-dichloride. J Nucl Med 2014;55:268–74.
- Lorente JA, Valenzuela H, Morote J, Gelabert A. Serum bone alkaline phosphatase levels enhance the clinical utility of prostate specific antigen in the staging of newly diagnosed prostate cancer patients. Eur J Nucl Med 1999;26:625–32.
- Kamiya N, Suzuki H, Endo T, Yano M, Naoi M, Nishimi D, et al. Clinical usefulness of bone markers in prostate cancer with bone metastasis. Int J Urol 2012;19:968–79.
- Soloway MS, Hardeman SW, Hickey D, Raymond J, Todd B, Soloway S, et al. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer 1988;61:195–202.
- Nanni C, Schiavina R, Brunocilla E, Borghesi M, Ambrosini V, Zanoni L, et al. 18F-FACBC compared with 11C-choline PET/CT in patients with biochemical relapse after radical prostatectomy: a prospective study in 28 patients. Clin Genitourin Cancer 2014;12:106–10.
- Smeland S, Erikstein B, Aas M, Skovlund E, Hess SL, Fossa SD. Role of strontium-89 as adjuvant to palliative external beam radiotherapy is questionable: results of a double-blind randomized study. Int J Radiat Oncol Biol Phys 2003;56:1397–404.
- de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995–2005.
- Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187–97.
- Garnero P. Markers of bone turnover in prostate cancer. Cancer Treat Rev 2001;27:187–92.
- Nilsson S, Franzen L, Parker C, Tyrrell C, Blom R, Tennvall J, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol 2007;8:587–94.
- Sonpavde G, Pond GR, Berry WR, de Wit R, Armstrong AJ, Eisenberger MA, et al. Serum alkaline phosphatase changes predict survival independent of PSA changes in men with castration-resistant prostate cancer and bone metastasis receiving chemotherapy. Urol Oncol 2012;30:607–13.
- Sassi ML, Eriksen H, Risteli L, Niemi S, Mansell J, Gowen M, et al. Immunochemical characterization of assay for carboxyterminal telopeptide of human type I collagen: loss of antigenicity by treatment with cathepsin K. Bone 2000;26:367–73.
- Silberstein EB. Teletherapy and radiopharmaceutical therapy of painful bone metastases. Semin Nucl Med 2005;35:152–8.
- Cook GJr, Parker C, Chua S, Johnson B, Aksnes AK, Lewington VJ. 18F-fluoride PET: changes in uptake as a method to assess response in bone metastases from castrate-resistant prostate cancer patients treated with 223Ra-chloride (Alpharadin). EJNMMI Res 2011;1:4.
- Fossa SD, Heilo A, Lindegaard M, Skinningrud A, Ous S. Clinical significance of routine follow-up examinations in patients with metastatic cancer of the prostate under hormone treatment. Eur Urol 1983;9:262–6.
- Croke J, Leung E, Segal R, Malone S. Clinical benefits of Alpharadin in castrate-chemotherapy-resistant prostate cancer: case report and literature review. BMJ Case Rep 2012;2012:12
- Parker CC, Pascoe S, Chodacki A, O’Sullivan JM, Germa JR, O’Bryan-Tear CG, et al. A randomized, double-blind, dose-finding, multicenter, phase 2 study of radium chloride (Ra 223) in patients with bone metastases and castration-resistant prostate cancer. Eur Urol 2013;63:189–97.

