Abstract
The effect of a small gap sleeve suture at the distal anastomosis of a nerve graft on promoting nerve regeneration and functional recovery was investigated by experimental observation. The model of common peroneal nerve defect in Sprague–Dawley (SD) rats was established, and an autologous sural nerve graft repair was performed. The small gap sleeve suture was applied at the distal anastomosis in the first stage for the experimental group. The results showed that the number of regenerated nerve fibers and the recovery of nerve function for the rats in the experimental group were superior to those in the control group.
Introduction
Peripheral nerve injury is common in surgery, especially in orthopedics, with no satisfying solution for its treatment. Since the first time that Staniforth and Fisher (Citation1978) successfully repaired a long nerve defect with a nerve autograft, an autologous nerve graft to repair a nerve defect has been in use, and it has become the gold standard for repairing peripheral nerve defects (Mackinnon and Dellon Citation1988). In recent years, the research on a variety of nerve substitutes has been rapidly progressing (de Ruiter et al. Citation2009, den Dunnen and Meek Citation2001, Heath and Rutkowski Citation1998, Matsumoto et al. Citation2000, Nicoli Aldini et al. Citation2000, Vasconcelos and Gay-Escoda Citation2000); however, none of the substitutes are clinically accepted. Currently, the common clinical treatment for repairing nerve defects is still a nerve graft, but its therapeutic effect is not ideal. Jiang et al. confirmed that nerve regeneration after repair with the nerve sleeve gap bridging technique is superior to regeneration with traditional epineurium neurorrhaphy (Jiang et al. Citation2006). On this basis, our lab has invented an absorbable biological sleeve for repairing peripheral nerve defects (Patent number: 01136314.2). A series of animal experiments for the suture repair of peripheral nerve defects with a small gap sleeve confirmed that this method is superior to the traditional direct epineurium neurorrhaphy for nerve regeneration and functional recovery of the injured nerve (Jiang et al. Citation2008, Zhang et al. Citation2008a, Citation2008b). However, the application of a small gap sleeve suture in nerve grafts has not previously been reported in the literature.
There are two anastomoses of a nerve graft in the repair of a peripheral nerve defect. The regenerated nerve can reach the proximal anastomosis in a relatively short period of time after nerve transplantation. However, the distal anastomosis requires a longer time for the nerve regeneration, and scar tissue may form during that period of time, thereby introducing factors that can inhibit its continued growth. In addition, the proximal end of the distal anastomosis is generally a thinner nerve, while its distal end is a thicker nerve; additionally, it is usually difficult to match the donor nerve to the receptor nerve in its nature and diameter. Therefore, the proximal anastomosis cannot play a role in selective regeneration, while selective regeneration and multiplied amplification are possible in the distal anastomosis. Thus, the distal anastomosis is an important factor affecting nerve regeneration and functional recovery.
In this study, a rat model of the common peroneal nerve defect was established, and in situ transplantation served as an idealized model of a nerve graft. The repair of a sural nerve graft was performed, and the effect of a small gap sleeve suture at the distal anastomosis in the first stage nerve graft on promoting regeneration and functional recovery was investigated.
Materials and methods
Experimental animals and materials
All animals were managed according to the guidelines of Peking University People's Hospital. Experimental protocols were approved by the Institute Animal Care and Use Committee of Peking University People's Hospital. To prepare the model of the right common peroneal nerve defect, 24 healthy adult male Sprague–Dawley (SD) rats were randomly divided into 3 groups, with 8 in each group. They underwent either an in situ transplantation at the resection segment of the common peroneal nerve (the in situ graft group), an autogenous free sural nerve graft with a direct suture for epineurium neurorrhaphy at both anastomoses (the adventitial suture group), or an autogenous free sural nerve graft with a direct suture for epineurium neurorrhaphy at the proximal anastomosis and a small gap sleeve suture at the distal anastomosis (the sleeve suture group). The bridging material was a deacetylated chitin biological tube (jointly developed by the People's Hospital of Peking University and China Textile Academy; Patent number: 01136314.2).
Experimental methods
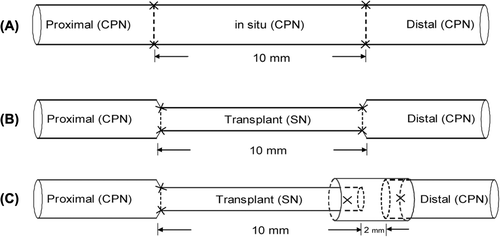
The rats were anesthetized by an intraperitoneal injection of 2% pentobarbital sodium (40 mg/kg). A longitudinal incision was performed along the femoral long axis of the right hind legs, and an 11 to 12-mm segment of the sural nerve was taken below the merger of the nerve fascicle (Millesi Citation1991). To prepare the common peroneal nerve defect model, the nerve was cut at approximately 1 cm below the separation of the common peroneal nerve from the sciatic nerve, and a 10-mm segment of the distal resection was produced. For the in situ graft group, the resection segment of the common peroneal nerve was sutured in situ with an anterograde suture, and both anastomoses were sutured using 11–0 microsutures by adventitial suturing, with 3 stitches for each anastomosis. For the adventitial suture group, the prepared segment of the sural nerve was transplanted into the defect site of the peroneal nerve with an anterograde suture, using the same suture method as that of the in situ graft group for both anastomoses. For the sleeve suture group, the proximal anastomosis of the grafted sural nerve was sutured by an adventitial suture, which was the same as that for the adventitial suture group, and the distal anastomosis was sutured by the small gap sleeve bridging technique using the deacetylated chitin biological tube. While leaving a 2-mm gap, the epineurium was sutured to fix in the wall of the sleeve with 1–2 stitches ().
Figure 1. The diagram of repair model for each group. (A) in situ graft repair; (B) sural nerve graft by adventitial suture; (C) sural nerve graft by an adventitial suture at the proximal anastomosis and a small gap sleeve suture at the distal anastomosis, with a gap of 2 mm. CPN: the common peroneal nerve; SN: sural nerve.
Detection index
At postoperative month 3, the peroneal nerve function index (PFI), the conduction velocity of the regenerated motor nerve, and the maximum tetanic contraction of the tibialis anterior muscle were detected for each group. The regenerated nerve in the transplanted segment and the distal anastomosis were observed with osmium tetroxide staining under a microscope, and the number of nerve fibers was counted. Additionally, the regenerated nerve was generally observed by the naked eye, and nerve regeneration and functional recovery were assessed.
Data analysis
All collected data were statistically analyzed with SPSS19.0 and presented as the mean ± standard deviation (SD). The significance of data in each group was tested by a one-factor analysis of variance (one-way ANOVA). The comparison between groups was tested by the LSD (Least-Significant Difference) method. If P < 0.05, it was considered a significant difference.
Results
The common peroneal nerve function index
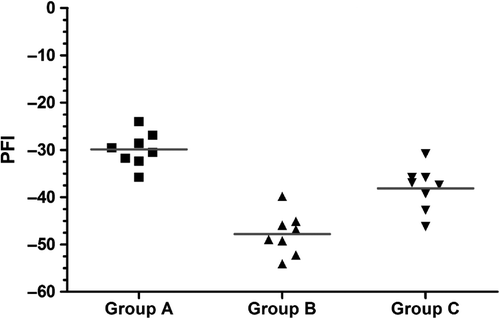
At postoperative month 3, the footprint of each rat was recorded for each group. The left footprint was the normal control, and the right side was the experimental footprint. The parameters of all footprints were detected and fitted into the Bain–Mackinnon–Hunter (BMH) equation (Bain et al. Citation1989) to calculate the common peroneal nerve function index (PFI). The PFIs of each group were ‐29.9 ± 3.58 for the in situ graft group, ‐47.8 ± 4.43 for the adventitial suture group, and ‐38.12 ± 4.67 for the sleeve suture group. The differences among the groups were significant (P < 0.05). The sleeve suture group was superior to the adventitial suture group (P < 0.01) ().
General observation for the regenerated nerve
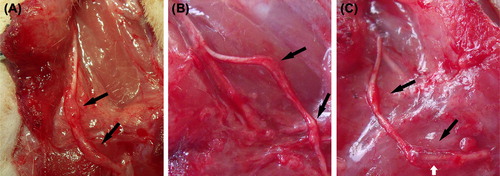
At postoperative month 3, many newly generated tissues with adhesion formations could be observed in the transplanted nerve and its surrounding area. The transplanted nerve grew well in each group, and a thickening was observed. Additionally, the diameter observed by the naked eye achieved or even exceeded that of the normal common peroneal nerve, while hyperplasia at the anastomoses was more obvious, which seemed to form neurofibromas. The chitin tube at the distal anastomosis of the sleeve suture group had not been fully absorbed at postoperative month 3. The newly generated nerve-like tissue due to the sleeve bridging technique could be seen under the surgical microscope with no apparent formation of neurofibromas ().
Figure 3. Observation of the newly regenerated nerve in each group by the naked eye. The transplanted nerve grew well in each group, and a thickening was observed. Many newly generated tissues appear in the surrounding area. The diameter observed by the naked eye achieved or even exceeded that of the normal peroneal nerve; however, hyperplasia at the anastomoses was more obvious (black arrows). It can be seen that the chitin tube at the distal anastomosis of group C has not been fully absorbed, and the newly generated nerve tissue through the sleeve bridging technique can be observed (white arrow). (A) the in situ graft group; (B) the adventitial suture group; (C) the sleeve suture group.
Electrophysiological detection of the regenerated nerve
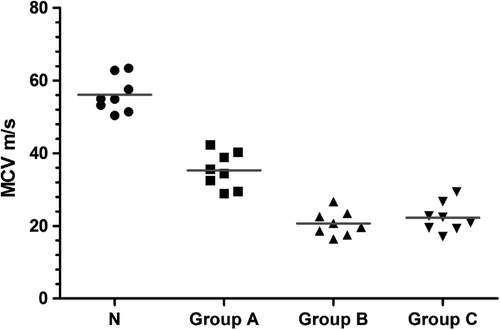
By using the fully exposed right common peroneal nerve and the tibialis anterior muscle, the motor nerve conduction velocity (MCV) after regeneration was measured by the latency difference method using the neuroelectrophysiological instrument. The MCV at the contralateral normal common peroneal nerve was measured by the same method for 8 randomly selected rats in the experiment. The results are the following: the conduction velocity for the normal common peroneal nerve was 55.3 ± 5.8 m/s, and the conduction velocities for the newly generated nerve in each group were 35.3 ± 4.93 m/s for the in situ graft group, 20.69 ± 3.4 m/s for the adventitial suture group, and 22.26 ± 4.07 m/s for the sleeve suture group. There were overall differences among the groups (P < 0.05). A comparison between two groups by the LSD method revealed that the difference between the sleeve suture group and the adventitial suture group was not statistically significant (P = 0.46). The MCVs in each group were lower than that of the normal peroneal nerve ().
Figure 4. The motor nerve conduction velocity for the normal common peroneal nerve and for each group at postoperative month 3. There was no difference between the sleeve suture group and the adventitial suture group (n = 8). MCV: motor nerve conduction velocity; N: the normal peroneal nerve; Group A: the in situ graft group; Group B: the adventitial suture group; Group C: the sleeve suture group.
Detection of the maximum tetanic contraction force recovery for the tibialis anterior muscle
After electrophysiological examination was completed, the bilateral tibialis anterior muscle strength was measured using the PCLAB-UE biomedical signal acquisition system. The values of the maximum tetanic contraction force were measured for both sides. The maximum tetanic contraction forces for the tibialis anterior muscles in each group are shown in . The value of the left side was set at 100%; therefore, the ratio of the right side to the left side represents the degree of recovery. The muscle force percentages of the right side to the left side were 77.26 ± 7.99% for the in situ graft group, 54.61 ± 8.58% for the adventitial suture group, and 67.03 ± 6.13% for the sleeve suture group ().
Figure 5. The degree of tetanic contraction force recovery of the tibialis anterior muscle in each group at postoperative month 3. The values for the adventitial suture group were lower than the sleeve suture group (P < 0.05) (n = 8). Recovery degree: the recovery degree of tetanic contraction force in the tibialis anterior muscle = muscle force on the right side/muscle force on the left side × 100%. Group A: the in situ graft group; Group B: the adventitial suture group; Group C: the sleeve suture group.
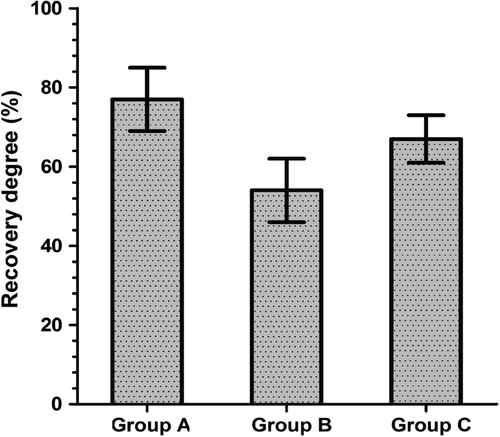
Table I. The maximum tetanic contraction force of the bilateral tibialis anterior muscles in each group (Newton).
Observations and the counts for the normal and regenerated myelinated nerve fibers
At postoperative month 3, the common peroneal nerve graft segment at the experimental side and the distal anastomosis were collected from rats in each group. Meanwhile, the common peroneal nerve and the sural nerve at the normal side were collected from eight randomly selected rats in the three groups. After staining with 1% osmium tetroxide, the nerves at the experimental side were cross-sectioned 5 mm away from the anastomosis and at the middle of the transplanted nerve, with a 2-μm slice thickness. They were observed and photographed under a microscope. The Leica Q550CW analytical system was used to analyze the osmium tetroxide staining results, and the number of myelinated fibers was calculated. The number of regenerated myelinated nerve fibers in the transplanted segment and the distal anastomosis in each group are shown in . The overall difference in the number of nerve fibers in the transplanted segment among the groups was significant (P < 0.01), and the difference in the number of distal regenerated nerve fibers among the groups was significant (P < 0.05). The ratio (R) of the regenerated nerve fibers in the distal anastomosis to that in the transplanted segment was calculated (R = the number of nerve fibers in the distal anastomosis/the number of nerve fibers in the transplanted segment). The R values of the adventitial suture group and the sleeve suture group can be considered as the magnification ratio of regeneration for the regenerated nerve crossing through the distal anastomosis ().
Figure 6. The regeneration ratio of the regenerated nerve crossing through the distal anastomosis in each group at postoperative month 3. The values for the sleeve suture group were significantly higher than those for the adventitial suture group (P < 0.01). Regeneration ratio = the number of nerve fibers in the distal anastomosis/the number of nerve fibers in the transplanted segment (n = 8). Group A: the in situ graft group; Group B: the adventitial suture group; Group C: the sleeve suture group.
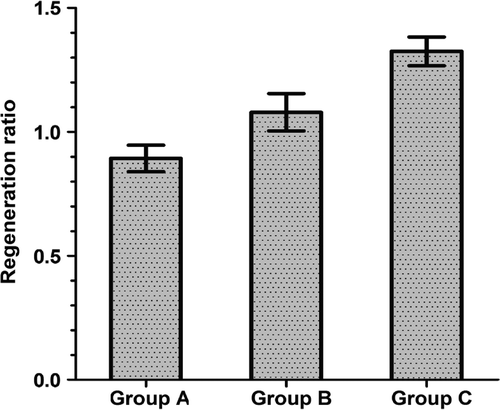
Table II. The number of nerve fibers for the regenerated nerve in each group and the normal nerve.
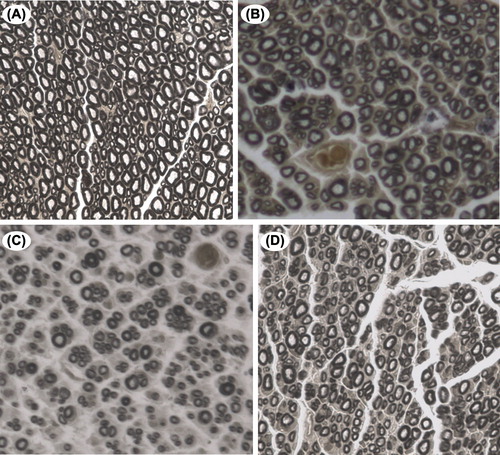
A microscopic observation under high magnification revealed that the regenerated nerve fibers in each group had a lower degree of uniformity in diameter compared with the normal nerve. Additionally, they were disorganized, and connective tissue was generated in the axon. The thickness of myelin sheath of the regenerated nerve fibers was uneven, and necrosis of the myelin remnants was occasionally visible ().
Figure 7. The osmium tetroxide staining for the distal anastomosis of the common peroneal nerve in the normal control and the experimental groups. Microscopic observation under high magnification revealed that the regenerated nerve fibers in each group had a lower degree of uniformity in the diameter compared with the normal nerve. Additionally, they were disorganized, and connective tissue was generated in the axon. The thickness of the myelin sheath of the regenerated nerve fiber was uneven, and necrosis of myelin remnants was occasionally visible (osmium tetroxide staining, 400 ×). (A) normal peroneal nerve; (B) the in situ graft group; (C) the adventitial suture group; (D) the sleeve suture group.
The numbers of regenerated nerve fibers in the transplanted segment had no significant difference between the adventitial suture group and the sleeve suture group, which were both greater than the number of normal sural nerve fibers*. The numbers of regenerated nerve fibers in the distal anastomosis was significantly different between the adventitial suture group and the sleeve suture group**, and they were less than the number of normal common peroneal nerve fibers* (*P < 0.05; **P < 0.01, n = 8).
Discussion
In recent years, research on the neural sleeve has progressed rapidly, and some techniques have been applied clinically (Ichihara et al. Citation2008). However, the effect of a nerve sleeve on a nerve graft has not been previously reported in the literature. The current clinical treatment for nerve defects is still mainly a nerve graft. The donor nerve segment is often smaller than the receptor nerve. In this case, during regeneration of the damaged nerve, all or most of the transplanted nerve is believed to accept a grow-in of the proximal nerve. However, when passing through the distal anastomosis, barriers due to the invasion of the surrounding tissues, the foreign body reaction, and hyperplasia of the connective tissue between the nerve stumps may block the growth of the regenerating nerve axons; thus, a part of the regenerated nerve fibers will be lost. In this study, the regenerated nerve observed by the naked eye indicated obvious hyperplasia at the distal anastomosis in the adventitial suture group. In addition to the hyperplasia of scar tissue, neurofibromas were also present at this site (Lane et al. Citation1978). The hyperplasia of scar tissue can block the continued growth of the nerve axons (Atkins et al. Citation2006), resulting in a loss of nerve fibers and a further reduction in the recovery of its function (Menovsky and Beek Citation2003). No apparent proliferation of scar tissue was found in the sleeve suture group, and the continuously regenerated nerve was clearly observed in the sleeve. The experimental results showed that the various indexes of nerve function with the sleeve were higher than those in the direct adventitial suture group, indicating that the use of a small gap sleeve suture could affect the following: block the influence of the surrounding scar tissue between the nerve stumps on nerve regeneration; effectively prevent the formation of scars and neurofibromas at the anastomosis; reduce the escape of the regenerated nerve fibers at this site and maximize the use of the regenerated nerve fibers; and increase the innervation of the distal effector. Thus, the functional recovery of the nerve would be better.
The reaction to a foreign body (suture thread) between the nerve stumps is a major cause for scar formation (Bain et al. Citation1989). For this, Snyder has proposed a repair method using epineurial sleeve technology to achieve the optimum environment for axonal regeneration and to prevent excessive scar formation in the suture area (Snyder Citation1981). Siemionow et al. (Citation2002) and Tetik et al. (Citation2002) confirmed that the use of this sleeve technology can not only enable a good junction for the neural stem, prevent the leakage of axial flow, and reduce the number of foreign bodies in the junction of the stumps but also guide nerve fibers to grow toward its effector, instead of “escaping” to the surrounding fibrous tissue. Their results also showed that the neurological function recovery was faster when using this repair technique, with a larger number of myelinated fibers in the regenerated nerve. This method can also play a role in the transmission of the stimulating factor for nerve regeneration that can promote nerve regeneration (Ayhan et al. Citation2003). The small gap sleeve suture in this study is indeed similar to their abovementioned method. When the small gap sleeve suture was performed, there was a defined space between the epineurium suture point and the plane of the stump so that the suture thread could not enter the gap of the ends. Thus, the “interference” for the nerve stumps could be decreased, and the foreign body reaction at the anastomosis of the stumps could be effectively reduced. In addition, sleeve usage can also play a barrier role in blocking the “violation” by the surrounding tissues at the anastomosis, which is conducive to the formation of the microenvironment required for nerve regeneration.
In the process of nerve injury repair, whether the functional nerve fascicles can accurately dock or not is a key factor affecting nerve function recovery. It is difficult to achieve accurate docking between the stumps when repairing nerves with the currently available technology. Nerve fibers that are misconnected to the distal trump will not function and will eventually disappear; therefore, part of the effective nerve fibers would be lost upon repairing a nerve injury. Because there are two anastomoses in the nerve transplantation process, the positioning of the nerve fibers will become more difficult, and therefore, the probability of misconnection will be higher. Due to selective regeneration in the regeneration process of a nerve (Lundborg et al. Citation1986), if a gap with a certain space exists between the stumps, the newly generated motor and sensory nerve fibers have the ability to spontaneously and selectively grow toward the corresponding functional fascicles at the distal stump or even the corresponding Bungner's band, finally reaching the target organ. Therefore, nerve regeneration can proceed toward its original innervated effector by selective regeneration (Brushart and Seiler Citation1987, Nachemson Citation1988). The small gap sleeve suture fully utilizes this characteristic, leaving a gap between the nerve stumps to form a nerve regeneration chamber (Lundborg et al. Citation1982). It increases the selective choices for the nerve fiber axon to grow toward the target effector, with a reduced misconnection rate; therefore, its functional recovery would be better. Small gap sleeve sutures can play a role in the selective regeneration of the nerve regeneration process so that the distal effector can obtain a relatively more effective innervation; thus, its functional recovery is better.
Another important feature of peripheral nerve regeneration after an injury is that the number of nascent axonal sprouting can greatly exceed the number of nerve fibers in the proximal stump of the injury plane, which is the “multiplied amplification” phenomenon. For the multiplied amplification in the peripheral nerve, our laboratory has conducted a series of research studies (Jiang et al. Citation2007, Yin et al. Citation2011) to prove that the multiplied amplification of the nerve supports the functional recovery in the receptor innervation area. Due to multiplied amplification in the nerve regeneration process, the number of nerve fibers innervating the distal effector can be increased. In this study, a comparison of the number of nerves between the distal anastomosis and the transplanted segment revealed that the numbers of nerve fibers in the adventitial suture group and the sleeve suture group in the distal anastomosis were higher than those in the transplanted segment; however, the regeneration ratio for the sleeve suture group was higher than that for the adventitial suture group, and the degree of its function recovery was also better. This indicated that the small gap sleeve suture used at the distal anastomosis could fully affect the multiplied amplification in the nerve regeneration process, and this amplification effect was even more obvious when the regenerating nerve penetrated the distal anastomosis, which increased the number of nerve fibers innervating the distal effector. Based on the above findings, we can conclude that, with the same number of transplanted nerve fibers, using a sleeve suture can enable more regenerated nerve fibers to reach the distal end, which can innervate the effector, i.e., using a sleeve suture at the distal anastomosis in the first stage can improve the number of regenerated nerve fibers innervating the distal effectors and improve the nerve function recovery. Additionally, it can be speculated that by using the sleeve suture at the distal anastomosis, the transplanted nerve can achieve the same effect as an in situ transplantation without using a nerve of the same thickness as that of the in situ nerve.
In this study, the number of regenerated nerve fibers in the transplanted segment of the sleeve suture group and the adventitial suture group was greater than the number of normal sural nerve fibers, and they could pass through the distal anastomosis to reach the distal end. Additionally, the number of nerve fibers in the distal segment could increase to more than half of the number of normal common peroneal nerve fibers. The observation of regeneration of the transplanted nerve segment by the naked eye revealed that the nerve in the transplanted segment was significantly thicker, which was preliminarily judged to achieve or even exceed the diameter of the normal peroneal nerve; however, the possibility of hyperplasia of other tissues cannot be ruled out. The number of myelinated nerves in the nerve fascicles was more than the number of its own nerve fibers, indicating that the nerve in the transplanted segment could proliferate and could accommodate more than the number of its own nerve fibers. The sural nerve in the transplanted segment undergoes remodeling during the regeneration process, which can support and “allow” the passing of a greater number of regenerated nerve fibers than the number of its own nerves, indicating that the nerves in the transplanted segment are not only the “channel” for nerve regeneration but also the “bridge.” This result suggested that even if a smaller nerve segment was used to repair the defect of a larger nerve, the original number of nerves in the injured nerve could be achieved. The clinical application of this result will demonstrate the effect of “saving” the donor nerves. When undergoing a graft for repair and using a smaller free nerve as the donor, the donor nerve can more easily obtain nutrition from the surroundings, and the ingrowth of the peripheral vessels and fluid infiltration is also easier. Therefore, survival of the transplanted nerve segment is easier, and there is a reduced probability of decline of the donor nerve due to insufficient nutrition.
Based on the experiment and the above discussion in this study, we can draw the following conclusions: the application of a small gap sleeve suture at the distal anastomosis in the first stage of a free nerve graft for common peroneal nerve defect in a rat can promote the number of the regenerated distal nerves and improve its function recovery; and the transplanted nerve undergoes a remodeling process, which can support the passage of a greater number of regenerated nerve fibers than the number of the original nerve fibers.
Although this study confirmed that a small gap sleeve suture at the distal anastomosis of a nerve graft can promote nerve regeneration and functional recovery, further investigation is needed to explore whether a scar is formed that blocks the regenerated nerve fiber if the small gap sleeve suture is performed to suture the gap of the stumps at the distal anastomosis of a long nerve graft and whether the application of a small gap sleeve suture at the distal anastomosis in the second stage of resection can also enhance nerve regeneration and functional recovery.
Declaration of interest
This study was supported by National Natural Science Foundation of China (31171150, 31271284, 81171146, 30971526, 30801169), Program for New Century Excellent Talents in University (BMU 20110270), Beijing City Science & Technology New Star Classification (A-2008-10) and the National Science & Technology Pillar Program during the 11th Five-Year Plan (2007BAI04B06). The authors alone are responsible for the content and writing of the paper.
References
- Atkins S, Smith KG, Loescher AR, Boissonade FM, O’Kane S, Ferguson MW, Robinson PP. 2006. Scarring impedes regeneration at sites of peripheral nerve repair. Neuroreport. 17:1245–1249.
- Ayhan S, Markal N, Siemionow K, Araneo B, Siemionow M. 2003. Effect of subepineurial dehydroepiandrosterone treatment on healing of transected nerves repaired with the epineurial sleeve technique. Microsurgery. 23:49–55.
- Bain JR, Mackinnon SE, Hunter DA. 1989. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 83:129–138.
- Brushart TM, Seiler WA. 1987. Selective reinnervation of distal motor stumps by peripheral motor axons. Exp Neurol. 97:289–300.
- de Ruiter GC, Spinner RJ, Yaszemski MJ, Windebank AJ, Malessy MJ. 2009. Nerve tubes for peripheral nerve repair. Neurosurg Clin N Am. 20:91–105, vii.
- den Dunnen WF, Meek MF. 2001. Sensory nerve function and auto-mutilation after reconstruction of various gap lengths with nerve guides and autologous nerve grafts. Biomaterials. 22:1171–1176.
- Heath CA, Rutkowski GE. 1998. The development of bioartificial nerve grafts for peripheral-nerve regeneration. Trends Biotechnol. 16:163–168.
- Ichihara S, Inada Y, Nakamura T. 2008. Artificial nerve tubes and their application for repair of peripheral nerve injury: an update of current concepts. Injury. 39:429–39.
- Jiang BG, Yin XF, Zhang DY, Fu ZG, Zhang HB. 2007. Maximum number of collaterals developed by one axon during peripheral nerve regeneration and the influence of that number on reinnervation effects. Eur Neurol. 58:12–20.
- Jiang B, Zhang P, Yan J, Zhang H. 2008. Dynamic observation of biomechanic properties of sciatic nerve at the suture site in rats following repairing. Artif Cells Blood Substit Immobil Biotechnol. 36:45–50.
- Jiang B, Zhang P, Zhang D, Fu Z, Yin X, Zhang H. 2006. Study on small gap sleeve bridging peripheral nerve injury. Artif Cells Blood Substit Immobil Biotechnol. 34:55–74.
- Lane JM, Bora FW Jr., Pleasure D. 1978. Neuroma scar formation in rats following peripheral nerve transection. J Bone Joint Surg Am. 60:197–203.
- Lundborg G, Dahlin LB, Danielsen N, Gelberman RH, Longo FM, Powell HC, Varon S. 1982. Nerve regeneration in silicone chambers: influence of gap length and of distal stump components. Exp Neurol. 76:361–375.
- Lundborg G, Dahlin LB, Danielsen N, Nachemson AK. 1986. Tissue specificity in nerve regeneration. Scand J Plast Reconstr Surg. 20:279–283.
- Mackinnon S, Dellon A. 1988. Surgery of Peripheral Nerves. New York: Thieme.
- Matsumoto K, Ohnishi K, Kiyotani T, Sekine T, Ueda H, Nakamura T, et al. 2000. Peripheral nerve regeneration across an 80-mm gap bridged by a polyglycolic acid (PGA)-collagen tube filled with laminin-coated collagen fibers: a histological and electrophysiological evaluation of regenerated nerves. Brain Res. 868:315–328.
- Menovsky T, Beek JF. 2003. Carbon dioxide laser-assisted nerve repair: effect of solder and suture material on nerve regeneration in rat sciatic nerve. Microsurgery. 23:109–116.
- Millesi H. 1991. Indications and techniques of nerve grafting. In: Gelberman RH, Ed. Operative Nerve Repair and Reconstruction. Philadelphia: Lippincott, pp. 525–543.
- Nachemson AK. 1988. Axonal regeneration and growth direction in square-shaped mesothelial chambers. Scand J Plast Reconstr Surg Hand Surg. 22:199–206.
- Nicoli Aldini N, Fini M, Rocca M, Giavaresi G, Giardino R. 2000. Guided regeneration with resorbable conduits in experimental peripheral nerve injuries. Int Orthop. 24:121–125.
- Siemionow M, Tetik C, Ozer K, Ayhan S, Siemionow K, Browne E. 2002. Epineural sleeve neurorrhaphy: surgical technique and functional results–a preliminary report. Ann Plast Surg. 48:281–285.
- Snyder CC. 1981. Epineurial repair. Orthop Clin North Am. 12: 267–276.
- Staniforth P, Fisher TR. 1978. The effects of sural nerve excision in autogenous nerve grafting. Hand. 10:187–190.
- Tetik C, Ozer K, Ayhan S, Siemionow K, Browne E, Siemionow M. 2002. Conventional versus epineural sleeve neurorrhaphy technique: functional and histomorphometric analysis. Ann Plast Surg. 49: 397–403.
- Vasconcelos BC, Gay-Escoda C. 2000. Facial nerve repair with expanded polytetrafluoroethylene and collagen conduits: an experimental study in the rabbit. J Oral Maxillofac Surg. 58:1257–1262.
- Yin XF, Kou YH, Wang YH, Zhang P, Zhang DY, Fu ZG, et al. 2011. Can “dor to dor+ rec neurorrhaphy” by biodegradable chitin conduit be a new method for peripheral nerve injury?Artif Cells Blood Substit Immobil Biotechnol. 39:110–115.
- Zhang P, Xue F, Kou Y, Fu Z, Zhang D, Zhang H, Jiang B. 2008a. The experimental study of absorbable chitin conduit for bridging peripheral nerve defect with nerve fasciculi in rats. Artif Cells Blood Substit Immobil Biotechnol. 36:360–371.
- Zhang P, Yin X, Kou Y, Wang Y, Zhang H, Jiang B. 2008b. The electrophysiology analysis of biological conduit sleeve bridging rhesus monkey median nerve injury with small gap. Artif Cells Blood Substit Immobil Biotechnol. 36:457–463.