Abstract
Context: Congenital heart disease is a leading cause of death in the first year, with an incidence of 1.5 million worldwide. It can be treated with bypass surgery, but due to the limited availability of autologous grafts, there has been research into developing a completely tissue-engineered vascular graft. Our group has developed a small diameter, biodegradable nanocomposite graft which is non-thrombogenic and biostable. Objectives: This study looks at the effects of the growth factors, TGF-β1 and BMP-4 on bone marrow-derived stem cell (BMSC) morphology on a POSS PCL scaffold. Materials and methods: BMSCs were seeded onto a new nanocomposite of POSS (polyhedral oligomeric silsesquioxane) and PCL (poly[caprolactone-urea]urethane) and TGF-β1 and BMP-4 were used to induce differentiation of the cells to smooth muscle cells. The distribution of the cells was examined using confocal and electron microscopies and the phenotype of the cells was assessed using immunohistochemistry. Results: It was found that growth factor induction led to a decrease in cell growth on POSS PCL as compared to that of the control surface, and confocal microscopic analysis showed less cytoskeleton reorganization of these cells. After immunohistochemistry analysis, the BMSCs showed no differentiation to smooth muscle cells. Discussion: Growth factor induction on the static scaffold discs led to a change in morphology, with less spreading of the cells, a lower proliferation rate and no differentiation into SMCs. These findings can be attributed to the POSS PCL being manufactured by a coagulation technique, resulting in a structure with low stiffness.
Background
Clinical need
Congenital heart disease (CoHD) has an incidence of between 4 and 9 per 1000 in the UK with 1.5 million new cases worldwide. It is the leading cause of death due to congenital abnormalities with a mortality of 70% in the first year (CitationKliegman et al. 2012).
Commercially used grafts are Dacron and expanded polytetrafluoroethylene (ePTFE). While they have had success in in large calibre situations, these grafts demonstrate low patency rates of 20–30% at 4–5 years for less than 6 mm grafts. Graft failure can occur due to a compliance mismatch between the graft and the native vessel, which leads to anastomotic intimal hyperplasia and also thrombosis. In particular with children, the prosthetic conduit may become relatively narrow compared with normal blood vessels as children mature, which could result in limb ischaemia or affect growth and development (CitationGong and Niklason 2008, CitationMavroudis et al. 1999).
We looked at using a new biodegradable nanocomposite material, which has been developed by Seifalian et al.; a POSS-modified poly[carbonate urea-urethanes] which has the desired anti-thrombogenic potential, promotes effective endothelialisation and possesses good compliance characteristics. This study explores the effect of soluble growth factors, TGF-β1 and BMP-4 on the metabolism, morphology, and differentiation of Bone marrow-derived mesenchymal stem cells (BMSCs) into SMCs on POSS PCL. The morphology of the cells was analysed through actin imaging and differentiation assessed by looking at SMC markers.
Materials and methods
Scaffold production
To produce a porous polymer, coagulated sheets of POSS PCL were created by adding an aromatic POSS PCL solution to sodium bicarbonate (40 μm) and tween 20 surfactant in the ratio 58:40:2. The sample was mixed in a Thinky Mixer ARE-250 (Intertronics, Oxfordshire, UK) for 3 min at 2000 rpm and degassed for 2 min at 1000 rpm. The resulting polymer was then poured on stainless steel moulds and after 20 min, the polymer was immersed in deionized water, which was replaced daily for 3 days to ensure washing off of the DMAC.
Using a tabletop circular cutter, 2 cm2-sized discs were cut out and sterilized in 25 ml vials using an autoclave machine for subsequent use in cell culture.
Cell culture
BMSCs from patients at the Royal Free Hospital with ethical approval were used for these experiments and plated on 75 cm2 cell culture flasks (T 75) (Corning, Sigma-Aldrich Co.). The cells were routinely cultured once a week. At 70% confluence, the cells were washed with PBS and harvested by trypsin EDTA (1 mg/ml, Invitrogen Ltd.). The cells were cultured with Dulbecco's modified Eagle's medium (DMEM, Sigma-Aldrich Co.) supplemented with 10% foetal bovine serum (FBS) and 1% penicillin streptomycin (both Invitrogen Ltd).
Cell seeding
The scaffold discs were placed in a 24-well plate. Cells were collected and resuspended in the normal medium at a density of 5 × 104 cells/cm2 by using a micropipette.
Proliferation assay
Cell proliferation on scaffolds was assessed using an Alamar blue TM (AB) assay (Invitrogen). At 3, 10 and 21 days the basal medium was replaced with 1 ml of 10% AB in cell culture DMEM media. Following incubation for 4 h, two 100 μl samples from each well were added to a FluoroNunc™ black 96-well plate. The fluorescence was detected using a fluorescent plate reader (Fluoroskan Ascent FL fluorometer, Thermo Labsystems, UK) at 620 nm emission with 530 nm excitation.
Cell differentiation
The cells were placed in DMEM-α medium with 10% FBS and 1% Penicillin streptomycin. To induce MSC differentiation to SMCs, TGF-β1 (5 ng/mL) and BMP-4 (2.5 ng/mL) were added to the medium. The control culture was grown in parallel in basal culture medium. Cells were cultured in differentiating media for up to 3 weeks. Media were changed twice weekly throughout the culture period.
Cytoskeleton morphology
Morphology of the cytoskeleton was observed using an actin stain. After fixing the cells in 3.7% formalin, the scaffolds were washed with PBS and dehydrated with acetone. The cells were permeabilized in PBS with 5% bovine serum albumin (BSA) and 0.2% triton X-100. The cells were bound with 50 μg/ml of fluorescently conjugated phalloidin tetramethylrhodamine B isothiocyanate solution (Sigma-Aldrich) in 0.5% BSA and 0.1% triton X-100. Cell morphology was imaged using confocal microscopy at 550 nm emission.
Cell morphology and distribution was also observed using scanning electron microscopy.
Immunofluorescent staining
Immunofluorescent staining for SMC markers, smooth muscle α actin (α-SMA), and smooth muscle myosin heavy chain (SM-MHC). The formaldehyde-fixed cells were incubated with the primary antibodies; mouse monoclonal anti-α-SMA (1:100, C6198, Sigma-Aldrich) and mouse monoclonal anti-SM-MHC (1:50, M7786, Sigma-Aldrich), respectively, for 60 min at room temperature. After three washes with PBS, the secondary antibodies a goat antimouse IgG conjugated with FITC (1:100, Millipore, Billerica, MA) for α-SMA and SM-MHC were added, respectively, and incubated for 45 min at room temperature. Cell nuclei were stained with propidium iodide (PI).
Results
Metabolism assay – effect of growth factors
Cells on the TCP show good growth and at all time points, the TCP with growth factors showed significantly greater differences of 246%, 720% and 2312%, respectively as compared to TCP without growth factors at the same time points ().
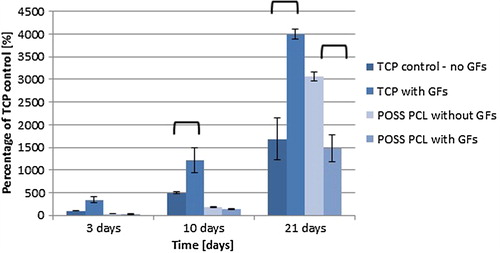
Therefore, growth factors have a positive growth effect on TCP and a negative growth effect on POSS-PCL.
Confocal micrsocopy
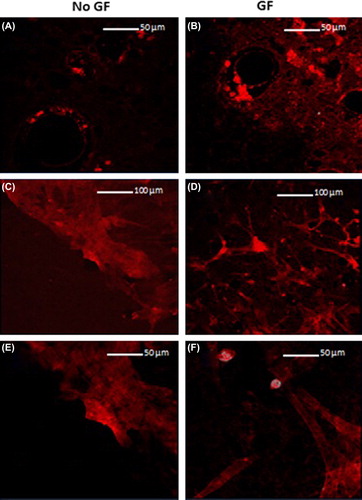
shows that at 3 days, the actin is aggregated around the pores and the individual fibres cannot be seen. After 21 days, the cells are far more spread out and adhere primarily around the pores. The cells without growth factor stimulation (C and E) show significantly greater spreading of the cytoskeleton.
SEM imaging
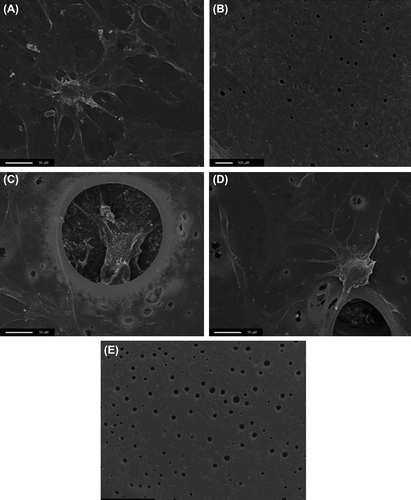
In , C and D show that the cells aggregate in and around the pores. B shows that BMSCs without growth factors demonstrate greater growth than E (with growth factors), with more cytoplasm seen. It can also be seen that there is some variation in the size of the pores.
Immunohistochemistry analysis
After immunohistochemistry, the cells on POSS PCL did not show differentiation to the SMC phenotype, whereas this was seen on the control tissue culture plate sample. On POSS PCL, neither SM-MHC nor alpha-SMA markers could be seen using confocal microscopy. Only the cell nuclei (stained with propidium iodide) could be identified on the polymer.
Discussion
The role of TGF-β1 in regulating proliferation and differentiation into SMCs has been demonstrated in previous studies. TGF-β1 has reported to promote vessel maturation by stimulating ECM production and by inducing differentiation of mesenchymal cells to mural cells in mammalian development (CitationHirschi et al. 2003). BMP-4 has also shown to regulate vasculogenesis during embryonic development (CitationHogan 1996).
Wang et al. tissue engineered an elastic vessel using smooth muscle cells (SMCs) differentiated from human adipose-derived stem cells (hASCs) under pulsatile stimulation in a bioreactor. Using TGF-β1 and BMP-4 for 7 days, hASCs differentiated into the SMC phenotype, with SMC markers identified, including smooth muscle alpha actin (α-SMA), calponin, and smooth muscle myosin heavy chain (SM-MHC) (CitationWang et al. 2010).
CitationGong and Niklason (2008) used bone marrow-derived stem cells seeded on a PGA scaffold to create a tissue- engineered graft. After 8 weeks in a biomimetic system, the cells differentiated into SMCs.
In this study, BMSCs were put in a medium with inducing growth factors TGF-β1 and BMP-4 for 3 weeks. Though others reported the differentiation into SMCs, this was not our experience. The decreased proliferation and spreading of the cells after growth factor induction was also unexpected. Previous studies mention that matrix formation is increased by TGF-β1 (CitationWang et al. 2010). Also, BMP-4 has shown to improve survival of ADMSCs with 2.5 ng/ml of BMP-4 inducing a cytoprotective effect (CitationLopez et al. 2011). These findings can be explained by the POSS PCL topographic environment affecting BMSC behaviour and response to the growth factors (CitationDischer et al. 2005). In this study, POSS PCL was manufactured as a coagulated sheet, which resulted in a soft foam-like polymer. A study by Park et al shows that the stiffness of the substrate plays an important role in the differentiation of MSCs in response to TGF-β1. They showed that on soft substrates, there was less spreading, a lower proliferation rate and a lower expression of SMC markers (CitationPark et al. 2011). These findings are in accordance with what we found.
It was proposed that the pores would provide a favourable environment for cell growth. This was supported by SEM imaging, which identified that the cells tended to aggregate around the pores, in accordance with studies highlighting the importance of surface topography in encouraging cell adhesion (CitationDischer et al. 2005).
Without growth factor induction, the polymer supports the growth and spreading of BMSCs; so these cells can be used as a cell source given the right conditions. Future work would involve assessing BMSC differentiation on POSS PCL when manufactured as a cast sheet, which is tougher and so will provide a more favourable environment.
Conclusion
In conclusion, growth factor stimulation led to a decrease in growth of cells on coagulated POSS PCL and decreased cytoskeleton reorganization due to the low substrate stiffness. The influence of growth factors on the morphology suggests that the BMSC behaviour had been altered by the polymer. POSS PCL proves to support the growth of BMSCs with and without growth factors and the porous structure encourages cell adhesion, showing its promise for use as a tissue engineered graft.
Acknowledgements
I would like to thank Dr Achala de Mel and Dr Maria Manikou for their support in the lab, and also Arnold for his support in the polymer lab.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Discher DE, Janmey P, Wang YL. 2005. Tissue cells feel and respond to the stiffness of their substrate. [Review] [92 refs]. Science. 310: 1139–1143.
- Gong Z, Niklason LE. 2008. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). FASEB J. 22:1635–1648.
- Hirschi KK, Burt JM, Hirschi KD, Dai C. 2003. Gap junction communication mediates transforming growth factor-beta activation and endothelial-induced mural cell differentiation. Circul Res. 93:429–437.
- Hogan BL. 1996. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. [Review] [160 refs]. Genes Dev. 10:1580–1594.
- Lopez MAV, Garcia MNV, Entrena A, Lopez SO, Garcia-Arranz M, Garcia-Olmo D, Zapata A. 2011. Low doses of bone morphogenetic protein 4 increase the survival of human adipose- derived stem cells maintaining their stemness and multipotency. Stem Cells Dev. 20:1011–1019. available from: ISI:000291001000008
- Mavroudis C, Backer CL, Duffy CE, Pahl E, Wax DF. 1999. Pediatric coronary artery bypass for Kawasaki congenital, post arterial switch, and iatrogenic lesions. Ann Thorac Surg. 68: 506–512.
- Park JS, Chu JS, Tsou AD, Diop R, Tang Z, Wang A, Li S. 2011. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials. 32: 3921–3930.
- Kliegman RM, Stanton B, St. Geme J, Schor N, Behrman RE. 2012. “Acyanotic heart disease”. In: Kliegman RM, Ed. Nelson Text Book of Paediatrics. 19th ed. Amsterdam: Elsevier, pp. 1549–1605.
- Wang C, Cen L, Yin S, Liu Q, Liu W, Cao Y, Cui L. 2010. A small diameter elastic blood vessel wall prepared under pulsatile conditions from polyglycolic acid mesh and smooth muscle cells differentiated from adipose-derived stem cells. Biomaterials. 31: 621–630.