Abstract
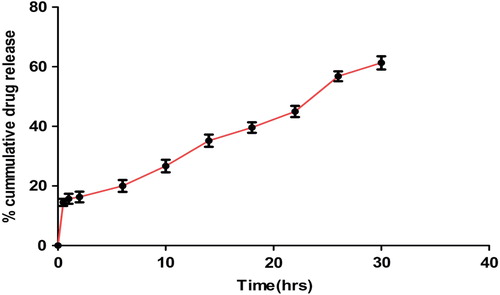
The purpose of this research is to describe the utility of nanofibers as a gastroretentive dosage form and improve the solubility of diacerein (DIA) by using the above approach. Poly L-(lactic acid) (PLLA) nanofibers loaded with DIA were prepared using the electrospinning technique. The nanofibers developed were characterized for morphology, tensile strength, floating behavior, drug entrapment, drug solubility, mucoadhesive detachment force, and drug release profile. The results demonstrated that the drug-loaded nanofibers were smooth, discrete, & non-woven. Analysis by X-ray crystallography (XRD) revealed that the drug in the nanofiber was present in the amorphous state, which largely contributes to higher drug solubility in the nanofibers developed. The buoyancy study demonstrated that the nanofibers developed exhibited zero lag time. Approximately 61.3% of drug was released in 30 h, thus facilitating the slow release of the drug from the nanofiber system. Finally, we concluded that the electrospun nanofibers developed offer a promising gastroretentive drug delivery system to deliver DIA with improved solubility.
Introduction
Oral formulations for sustained and controlled drug release are developed as an attempt to release the drug slowly into the gastrointestinal tract (GIT) and maintain an effective drug concentration for a prolonged period of time (CitationChaudhary et al. 2014). Gastroretentive drug delivery systems (GRDDS) are beneficial with respect to their bioavailability (CitationGarg 2014), therapeutic efficacy (CitationGoyal et al. 2013a), possible reduction of the dose (CitationGarg and Goyal 2012), and improved drug solubility (CitationGoyal et al. 2013b), with the solubility being lower in a high pH alkaline environment (CitationGarg and Gupta 2008, CitationRohilla et al. 2014b). GRDDS have a number of advantages compared to the conventional drug delivery tools (CitationGarg et al. 2013, CitationSharma et al. 2014a). Therefore, it is necessary to design a system that permits longer gastric residence, which will extend the time within which drug absorption can occur (CitationGarg et al. 2012b, CitationSharma et al. 2014b). In the past, a number of approaches, including altered density formulations (CitationGarg et al. 2011b, CitationSharma et al. 2014c), bioadhesive formulations (CitationGarg et al. 2011a, CitationSingh et al. 2014a), swelling systems (CitationGarg et al. 2014b, CitationSingh et al. 2014b), ion exchange resins (CitationGarg et al. 2014c), porous hydrogels (CitationGarg et al. 2014a), osmoregulated formulations (CitationGarg et al. 2012a), and effervescent formulations (CitationGarg and Goyal 2014a) have been developed, to improve the gastric residence time of the drug (CitationGarg and Goyal 2014b, CitationSingh et al. 2012a). However, the aforesaid technologies offer limited functional features, which have been exploited to develop an effective gastroretentive drug delivery strategy (CitationGoyal et al. 2014a, CitationSingh et al. 2012b). Further, with the conventional approach, the objective of GRDDS was only partially achieved due to its inability to improve drug solubility (CitationHussain et al. 2014) and to establish a precise correlation between the rates of dissolution (CitationJohal et al. 2014) and absorption (CitationGoyal et al. 2014b, CitationSingh et al. 2014c).
Out of the techniques developed, the use of nanofibers is advantageous, since a nanofiber seems to possess superior gastroretentive properties. Nanofibers, due to their unique properties, offer a number of functional features to control the floating behavior of the carrier system (CitationJoshi et al. 2014). The properties of nanofibers which are particularly useful in determining the floating behavior are the surface to volume ratio (CitationKalia et al. 2014), specific density (CitationKataria et al. 2014), adsorption characteristics (CitationKaur et al. 2014a), fiber diameter (CitationKaur et al. 2014b), porosity (CitationKaur et al. 2014c), surface hydrophilicity (CitationKaur et al. 2014d), etc.
For the past few years, there has been an increasing trend in the development and fabrication of ultrafine polymer fibers for biomedical (CitationKaur et al. 2014e) and biotechnological applications (CitationKaur et al. 2014f). In the last 10 years, electrospinning has been gaining widespread interest for applications in tissue engineering (CitationKaur et al. 2014g) and drug delivery (CitationKaur et al. 2014h). The extremely high surface area to volume ratio as well as the high and interconnected porosity of electrospun meshes explain the ultra-low density (CitationMalik et al. 2014), 100% drug payload capacity, (CitationMarwah et al. 2014) and mass transfer properties (CitationModgill et al. 2014). Moreover, electrospun mats composed of randomly oriented fibers with diameters down to the nanometer scale are suitable to control the release behavior of the encapsulated drug (CitationMorie et al. 2014, CitationPabreja et al. 2014).
Poly L-(lactic acid) (PLLA) is a biodegradable polymer and has been widely investigated for its applicability in drug delivery (CitationWade and Weller 1994). Recent studies investigating the use of electrospun PLLA as a scaffold for tissue engineering applications have shown the possibility of producing PLLA fibers with diameters ranging from a few micrometers down to hundreds of nanometers. The objective of this study is further extended to utilize the polyanionic behavior of the polymer to control the drug release behavior in gastric pH.
Besides the poor solubility, the poor bioavailability of the drug diacerein (DIA) is associated with a narrow absorption window. Moreover, DIA, upon direct contact with the colon, causes severe diarrhea via activation of chloride secretion by excitation of the submucosal neurons and the release of Ach. Considering the above features, DIA could be a good candidate for GRDDS. According to the above objective, the present work was designed to formulate DIA-loaded PLA nanofibers. The formulation developed was characterized for morphology, physical state of the drug, floating behavior, and release behavior, to establish the utility of electrospun nanofibers as GRDDS.
Materials and methods
Materials
Diacerein (DIA) was obtained from Ravenbhel Healthcare Pvt. Ltd. (Jammu, India) as a gift sample. Poly-L-lactic acid (PLLA) with an average molecular weight of 1,65,000 Da was purchased from Sigma Aldrich (St. Louis, MO, USA). The solvents chloroform and dimethyl formamide (DMF) were purchased from HiMedia Pvt. Ltd. (New Delhi, India). All the other chemicals used were of analytical grade.
Preparation of the DIA-loaded electrospun nanofiber system
PLLA solution at a concentration of 5% w/v was prepared at room temperature by dissolving the polymer in chloroform and DMF in the ratio of 4/1 under gentle stirring for 10–15 min. Subsequently, DIA was added into the polymer solution with a concentration of 1 mg/ml, and the mixture was stirred gently at room temperature to ensure a complete dissolution of the drug and eventually obtain homogeneous polymer/drug solutions for electrospinning (e-nano, IIT, Kanpur, India). For electrospinning, 5 mL of the polymer/drug solution was placed in a 5 mL syringe fitted with a stainless-steel blunt needle of 22G, and the injection rate was adjusted to 0.04 ml/hr. The needle tip of the syringe was connected to a high voltage power supply with the applied voltage of 17 kV. Randomly oriented nanofibers were simply collected by a flat collector wrapped with aluminum foil which was kept at a distance of 15 cm from the needle tip (CitationRohilla et al. 2014a).
Characterizations of the DIA-loaded electrospun nanofiber system
The nanofibers were characterized on the basis of various parameters such as scanning electron microscopy, swelling index, solubility study, X-ray crystallography (XRD), floating lag time (FLT) and floating time (FT), tensile strength, mucoadhesion, actual drug content, and in vitro release.
Morphology
A study using a scanning electron microscope (SEM) is generally meant for topographical (morphological) analysis of the fiber sample and is usually done by employing a SEM at an accelerating voltage of 15 KV. The drug-loaded nanofibrous sheet was cut into 1 × 1 cm2, which was placed on a SEM sample mount and sputter coated with platinum for 10 min. The sample was then imaged by SEM (CitationMeng et al. 2011).
Swelling index
To evaluate the swelling behavior, the DIA-loaded electrospun nanofibers of known weight were placed in test tubes containing 10 mL of citrate buffer solutions (pH 1.4) for 24 h and 48 h at 37°C. Then, the water on the nanofiber surfaces was removed with filter paper and the specimens were weighed in the wet condition (CitationMeng et al. 2011). The swelling ratio for each sample was calculated according to the following equation.
Where, ‘md’ is the initial weight of drug-loaded nanofiber & ‘mw’ is the final weight of drug-loaded nanofiber.
Solubility study
Saturation solubility of the drug and drug-loaded nanofiber was demonstrated in a citrate buffer of pH 1.4. In brief, an excess amount of drug was added to a fixed volume of citrate buffer of pH 1.4, and the mixture was gently stirred at room temperature for 24 h. Aliquots were then taken from the medium and observed under a UV spectrophotometer (UV-1700 Pharma Spec, Shimadzu, Japan) at a wave length of 340 nm. The absorbance value recorded gives the measure of the solubility of both the drug and the drug loaded in the nanofiber (CitationYu et al. 2009b).
X-ray crystallography
XRD is a tool used for identifying the atomic and molecular structure of a crystal. In XRD, a collimated beam of X-rays with a wavelength typically ranging from 0.7 to 2 A° is bombarded onto the sample and the diffraction pattern in the specimen is recorded according to Bragg's law. The intensity of the diffracted X-ray is measured as a function of the diffraction angle 2Ɵ. The diffraction pattern is used to identify the crystalline phases and to measure the structural properties (CitationXu et al. 2006).
According to Bragg's law;
Where ‘d’ is the spacing between atomic planes and ‘ƛ’ is the wavelength of the X-ray.
Floating lag time and floating time
The FLT is a measure of the time taken by the dosage form to float after it is placed in the dissolution medium. In order to calculate the FLT and FT, a nanofiber film with dimensions of approximately 3 × 3 cm2 was cut off and placed into the citrate buffer (pH 1.4), maintained at 37°C. The time between the introduction of the dosage form and its buoyancy on the surface of the fluid medium is called Floating Lag Time (FLT) or Buoyancy Lag Time (BLT), and the time during which the dosage form remains buoyant is called the “Total Floating Time” (TFT), both of which were measured (CitationJaimini et al. 2007).
Tensile strength
Tensile strength of the nanofiber formulation was determined using a Brookfield texture analyzer. A nanofiber film of dimensions 6 × 2 cm2 with an approximate thickness of 1 mm was placed in a vertical position along the axis of the texture analyzer, using clamps. After necessary adjustments, the instrument was run at a load of 7 gram/cm2. The force required to break the fiber was determined (CitationSingh et al. 2014b).
Mucoadhesion
Mucoadhesive strength was determined with the help of the Brookfield texture analyzer. In this test, goat stomach mucosa was attached to an attachment assembly of the instrument. A nanofiber with an area of approximately 2 cm2 was applied on the sticky tape. The instrument was run at a load of 1000 grams, and the weight required to detach the fiber from the mucosa was determined (CitationSingh et al. 2014b, CitationSemalty et al. 2008).
Actual drug content
The drug-loaded nanofiber was dissolved in 5 mL of methanolic DMA solution and the actual amount of DIA was measured using a UV-Vis spectrometer at ƛ = 340 nm. The total amount of drug contained in the sample was back-calculated using the calibration curve of the drug (CitationMeng et al. 2011).
In vitro drug release
The drug-loaded nanofiber was incubated at 37°C in a citrate buffer solution (pH 1.4). Sampling was done at different time intervals to access the nature of drug release from the formulation. The volume of sample taken for analysis each time was replaced with an equal volume of fresh medium. The DIA released in the buffer solution was determined by a UV-Vis spectrophotometer at ƛ = 340 nm. (CitationTaepaiboon et al. 2007, Gagandeep et al. 2014).
Statistical analysis
All data were expressed as mean ± SD.
Results and discussion
Morphology
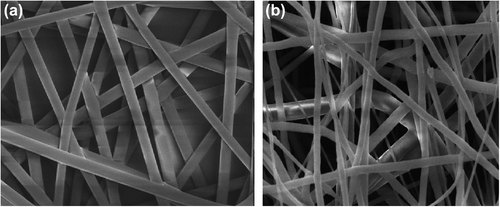
, show the SEM images of DIA-loaded electrospun nanofibers and blank PLLA nanofibers respectively. The average diameter of the drug-loaded nanofiber was found to be 342 nm, and that of the blank nanofiber was found to be 246 nm. The increase in the average fiber diameter in the case of drug-loaded nanofiber could be associated with the slight change in viscosity of the polymer solution after the drug powder was added to it (CitationPillay et al. 2013). It could be also due to complex evaporation of the solvent from the drug-loaded nanofiber (Gagandeep et al. 2014). SEM images revealed that both blank and drug-loaded nanofibers were discrete, non-woven, and smooth. The presence of drug loading was clearly manifested by the physical appearance of the nanofiber, because the color of the nanofiber turns light yellow in the drug-loaded formulation.
Swelling ratio
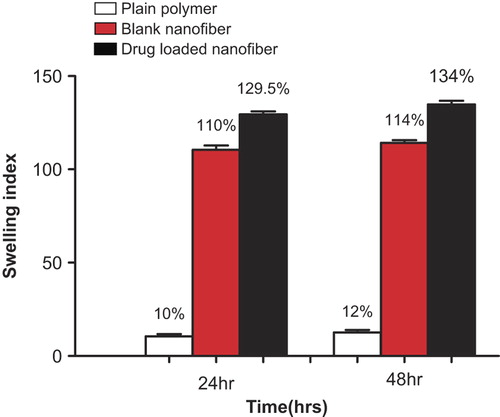
The swelling ratio of a randomly oriented drug-loaded PLLA nanofibrous patch was found to be 110%, while the ratios for blank nanofibers and plain polymer were found to be 129.5% and 12% respectively. Under gastric conditions, the high degree of swelling is due to the large amount of water retained in the interconnected fibrous pores by capillary action. The decrease in swelling in the case of drug-loaded nanofiber is due to a change in fiber morphology and a decrease in the degree of porosity. There is no appreciable change in the swelling index after 48 h. Clearly, this demonstrates the polyanionic behavior of the polymer matrix, under gastric atmosphere. This behavior further indicates the release mechanism of the entrapped drug, which could be primarily reliant on the diffusion mechanism. Further, the swelling index in the case of the plain polymer clearly demonstrated the hydrophobic nature of the polymer. Absolute impermeability to the medium gives an indication of the compact, rigid, and semi-crystalline molecular arrangement of PLLA. The shows the swelling index values of the plain polymer, blank nanofiber, and drug-loaded nanofiber after 24 h and 48 h respectively.
X-ray crystallography
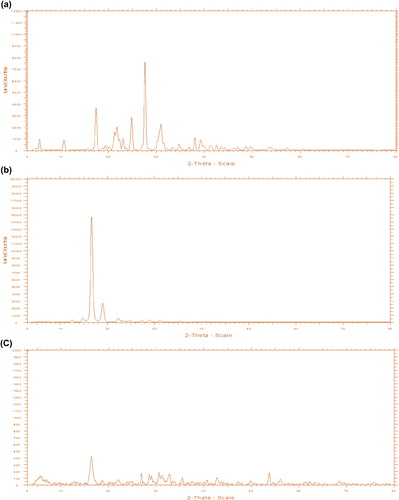
XRD was used to access changes in the level of crystallinity during the electrospinning process. The decrease by 28° in the peak intensities at 2Ɵ in the diffractogram of the drug-loaded nanofiber reveals that the drug loses its crystalline structure in the developed formulation (CitationChew et al. 2006). This might be due to the rapid evaporation of the solvent during the electrospinning process, hindering the formation of the DIA crystal. Moreover, the instantaneous transition from liquid phase to solid phase during electrospinning also causes a decrease in the degree of crystallinity. The diffractogram of drug, polymer, and drug-loaded nanofibers are shown in , & respectively. In addition to the physical state of the drug, XRD studies also unveiled the polymorphic behavior of PLLA in the electrospun nanofiber. There is a significant decrease in the peak intensity of the PLLA nanofiber at 2Ɵ of 16°, which could be due to different molecular arrangement of PLLA in the nanofiber when compared to its native state. The loss of crystallinity is also supported by the swelling study, the results of which showed that permeability was increased 10-fold. The results of both the XRD and swelling study inferred that porosity and loss of crystalline structure of the polymer contribute toward controlling the permeability of the developed nanofiber.
Solubility study
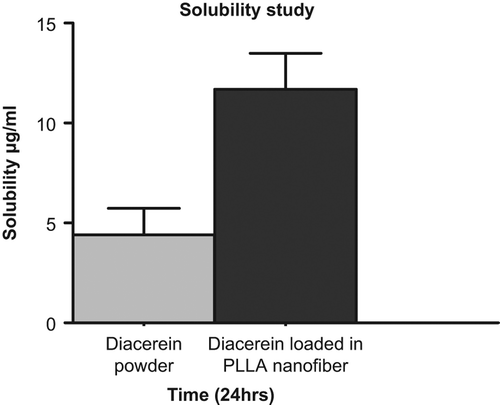
The solubility study indicated that the solubility of the drug was found to be 4 μg/ml, which increased by almost 3-fold (11 μg/ml) in case of a nanofiber system. Furthermore, the molecular reshuffling of PLLA helps in the formation of a nanoslide dispersion with higher affinity for the dissolution medium. Higher medium affinity of PLLA nanofiber was also supported by the swelling studies, where an almost 10-fold increase in the permeability was observed in the nanofiber when compared to that of the plain polymer. This is clearly manifested by better wettability and dispersibility of drug in the electrospun nanofiber (CitationYu et al. 2009b). This increase in the solubility is attributed to loss of crystallinity during the electrospinning process, resulting from instantaneous phase transition from a liquid drug/polymer solution to a solid nanofiber system. Moreover, the increase in the surface area also contributes to increase in the solubility (CitationYu et al. 2009a). shows the solubility study of DIA powder and DIA after being subjected to electrospinning. The loss of crystallinity is supported by the diffractogram (, & ) of drug-loaded nanofibers, in which peak intensity was decreased at 2Ɵ of 28°, confirming the loss in crystalline state of the drug in the nanofiber system (CitationChew et al. 2006).
Floating lag time and floating time
There was no FLT for the drug-loaded nanofiber, because the nanofiber system floated onto the surface the moment it was put into the dissolution medium. The nanofibers floated on the surface of the dissolution medium for more than 3 days. The reason for this is attributed to the extremely high surface area—nanofibers with 100 nm diameter provide a specific area of 1000 sqm/gram (CitationYu et al. 2009a). Increased surface of the nanofiber contributes to the significant reduction of bulk density. As the fiber diameter shrinks to nanoscale, the surface to volume ratio increases, by up to 1,000 times higher than that of the microfiber.
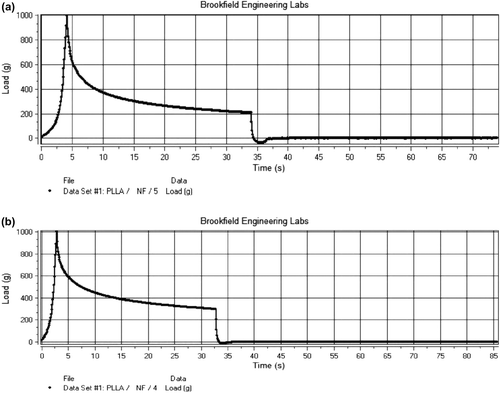
Tensile strength
The & show the tensile strength of blank PLLA nanofiber and drug-loaded nanofiber. The tensile strength of blank PLLA nanofibers was found to be 47 g/cm2, and that of the drug-loaded nanofibers was found to be 29 g/cm2. The high value of tensile strength for blank nanofibers is due to the small fiber diameter, as mechanical strength of fibers is inversely proportional to the diameter of the fiber (CitationChew et al. 2006, CitationBaji et al. 2010). Further, the diameter of the fiber per unit area is inversely proportional to diameter of the fiber. Thus as the diameter of the fiber increases, the fiber density decreases proportionally, which is associated with a decrease in mechanical strength.
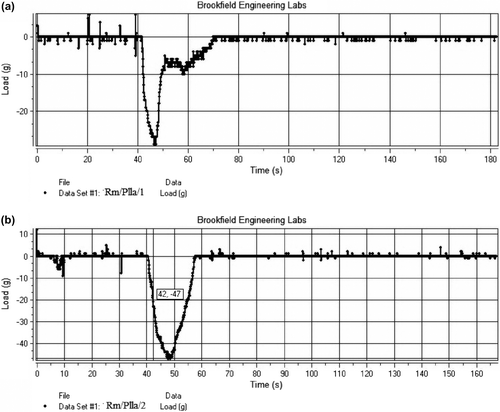
Mucoadhesive strength
The mucoadhesive strength of the blank nanofiber was found to be 38 g/cm2, and that of the drug-loaded nanofiber was found to be 14 g/cm2. , show the mucoadhesive strength of the blank PLLA nanofiber and the drug-loaded nanofiber. The decrease in mucoadhesive force in the case of drug-loaded nanofiber is attributed to a decrease in the specific surface area resulting from an increase in the diameter of the fiber and in fiber density (CitationSingh et al. 2014b).
Actual drug content
The actual drug content generally signifies the amount of the drug present in the formulation. The drug content of the nanofiber patch was found to be 89.47%. Less than 100% of drug payload is considered to be a result of the fact that all nanofibers have not been collected on to the collector plate during the electrospinning process (CitationSingh et al. 2014b).
In vitro drug release
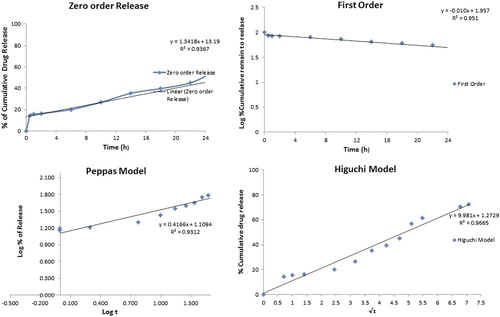
shows the release profiles of DIA from the DIA-loaded randomly oriented PLLA nanofibers. An initial burst release of about 15% during the first hour of study may be attributed to the high surface area of the nanofibers, followed by controlled release of the drug from the core of the nanofibers. The release behavior is also supported by the swelling, indicating that the release mechanism is predominantly governed by a diffusion process. Further, the microporous structure of the nanofiber system restricts the free access of the dissolution medium into the fiber matrix, and thereby causes extended drug release. The graph shows that 61.3% of the drug was released in 30 h. PLLA being an anionic polymer remained unionized at acidic pH for longer periods of time, thus facilitating the slow release of the drug from the nanofiber system (CitationGrainger and El-Sayed 2010). The release profile, according to the Higuchi equation (R2 = 0.966), showed characteristics of diffusion kinetics. In the Peppas model, an exponent value (n = 0.416) also indicated that the drug release through the nanofiber follows a diffusion pattern. Synchronization of the Higuchi and Peppas models shows smooth and uniform release patterns which may follow zero order kinetics ().
Conclusion
DIA-loaded electrospun PLLA nanofibers as a gastroretentive system were successfully produced. Based on the above characterizations and data, it can thus be concluded that nanofiber systems constitute a promising alternative approach in GRDDS, with unique properties that provide multiple functional features to improve gastroretention.
Acknowledgement
The author, Dr. Amit K Goyal, is thankful to the Department of Biotechnology (DBT), New Delhi, for providing financial assistance to carry out research (F. No. 102/IFD/SAN/2577/2011-2012 dated September, 28, 2011).
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Baji A, Mai Y-W, Wong S-C, Abtahi M, Chen P. 2010. Electrospinning of polymer nanofibers: Effects on oriented morphology, structures and tensile properties. Compos Sci Technol. 70:703–718.
- Chaudhary S, Garg T, Murthy RS, Rath G, Goyal AK. 2014. Recent approaches of lipid-based delivery system for lymphatic targeting via oral route. J Drug Target. 1–12.
- Chew SY, Hufnagel TC, Lim CT, Leong KW. 2006. Mechanical properties of single electrospun drug-encapsulated nanofibres. Nanotechnology. 17:3880.
- Gagandeep, Garg T, Malik B, Rath G, Goyal AK. 2014b. Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur J Pharm Sci. 53:10–16.
- Garg R, Gupta G. 2008. Progress in controlled gastroretentive delivery systems. Trop J Pharm Res. 7:1055–1066.
- Garg T. 2014. Current nanotechnological approaches for an effective delivery of bio-active drug molecules in the treatment of acne. Artif Cells Nanomed Biotechnol. 1–8.
- Garg T, Goyal AK. 2012. Iontophoresis: drug delivery system by applying an electrical potential across the skin. Drug Deliv Lett. 2:270–280.
- Garg T, Goyal AK. 2014a. Biomaterial-based scaffolds–current status and future directions. Expert Opin Drug Deliv. 11:767–789.
- Garg T, Goyal AK. 2014b. Liposomes: targeted and controlled delivery system. Drug Deliv Lett. 4:62–71.
- Garg T, Goyal AK, Arora S, Murthy R. 2012a. Development, optimization & evaluation of porous chitosan scaffold formulation of gliclazide for the treatment of type-2 diabetes mellitus. Drug Deliv Lett. 2:251–261.
- Garg T, Kumar A, Rath G, Goyal AK. 2014a. Gastroretentive drug delivery systems for therapeutic management of peptic ulcer. Crit Rev Ther Drug Carrier Syst. 31:531–557.
- Garg T, Rath G, Goyal AK. 2014b. Ancient and advanced approaches for the treatment of an inflammatory autoimmune disease-psoriasis. Crit Rev Ther Drug Carrier Syst. 31:331–364.
- Garg T, Rath G, Goyal AK. 2014c. Comprehensive review on additives of topical dosage forms for drug delivery. Drug Deliv.
- Garg T, Singh O, Arora S, Murthy R. 2011a. Dendrimer—A novel scaffold for drug delivery. Int J Pharm Sci Rev Res. 7:211–220.
- Garg T, Singh O, Arora S, Murthy R. 2011b. Patented microencapsulation techniques and its application. J Pharm Res. 4:2097–2102.
- Garg T, Singh O, Arora S, Murthy R. 2012b. Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst. 29:1–63.
- Garg T, Singh S, Goyal AK. 2013. Stimuli-sensitive hydrogels: an excellent carrier for drug and cell delivery. Crit Rev Ther Drug Carrier Syst. 30:369–409.
- Goyal AK, Rath G, Garg T. 2013a. Nanotechnological approaches for genetic immunization. DNA and RNA Nanobiotechnologies In Medicine: Diagnosis And Treatment Of Diseases. Erdmann, Volker A., Barciszewski, hab. Jan (Eds.). Springer-Verlag Berlin Heidelberg, pp. 67–120.
- Goyal G, Garg T, Malik B, Chauhan G, Rath G, Goyal AK. 2013b. Development and characterization of niosomal gel for topical delivery of benzoyl peroxide. Drug Deliv.
- Goyal G, Garg T, Rath G, Goyal AK. 2014a. Current nanotechnological strategies for an effective delivery of drugs in treatment of periodontal disease. Crit Rev Ther Drug Carrier Syst. 31:89–119.
- Goyal G, Garg T, Rath G, Goyal AK. 2014b. Current nanotechnological strategies for treating glaucoma. Crit Rev Ther Drug Carrier Syst. 31:365–405.
- Grainger SJ, El-Sayed MEH. 2010. Stimuli-Sensitive particles for drug delivery. In: Jabbari E, Khademhosseini A, Eds. Biologically-Responsive Hybrid Biomaterials. Singapore: World Scientific, pp. 171–190.
- Hussain T, Garg T, Goyal AK, Rath G. 2014. Biomedical Applications of Nanofiber Scaffolds in Tissue Engineering. J Biomater Tiss Eng. 4:600–623.
- Jaimini M, Rana A, Tanwar Y. 2007. Formulation and evaluation of famotidine floating tablets. Curr Drug Deliv. 4:51–55.
- Johal HS, Garg T, Rath G, Goyal AK. 2014. Advanced topical drug delivery system for the management of vaginal candidiasis. Drug Deliv. 1–14.
- Joshi D, Garg T, Goyal AK, Rath G. 2014. Advanced drug delivery approaches against periodontitis. Drug Deliv. 1–15.
- Kalia V, Garg T, Rath G, Goyal AK. 2014. Development and evaluation of a sublingual film of the antiemetic granisetron hydrochloride. Artif Cells Nanomed Biotechnol. 1–5.
- Kataria K, Sharma A, Garg T, Goyal AK, Rath G. 2014. Novel technology to improve drug loading in polymeric nanofibers. Drug Deliv Lett. 4:79–86.
- Kaur M, Garg T, Narang RK. 2014a. A review of emerging trends in the treatment of tuberculosis. Artif Cells Nanomed Biotechnol. 1–7.
- Kaur M, Garg T, Rath G, Goyal AK. 2014b. Current nanotechnological strategies for effective delivery of bioactive drug molecules in the treatment of tuberculosis. Crit Rev Ther Drug Carrier Syst. 31: 49–88.
- Kaur M, Malik B, Garg T, Rath G, Goyal AK. 2014c. Development and characterization of guar gum nanoparticles for oral immunization against tuberculosis. Drug Deliv.
- Kaur N, Garg T, Goyal AK, Rath G. 2014d. Formulation, optimization and evaluation of curcumin-beta-cyclodextrin-loaded sponge for effective drug delivery in thermal burns chemotherapy. Drug Deliv. 1–10.
- Kaur P, Garg T, Rath G, Murthy RS, Goyal AK. 2014e. Surfactant-based drug delivery systems for treating drug-resistant lung cancer. Drug Deliv. 1–12.
- Kaur R, Garg T, Das Gupta U, Gupta P, Rath G, Goyal AK. 2014f. Preparation and characterization of spray-dried inhalable powders containing nanoaggregates for pulmonary delivery of anti-tubercular drugs. Artif Cells Nanomed Biotechnol. 1–6.
- Kaur R, Garg T, Rath G, Goyal AK. 2014g. Advanced aerosol delivery devices for potential cure of acute and chronic diseases. Crit Rev Ther Drug Carrier Syst. 31:495–530.
- Kaur V, Garg T, Rath G, Goyal AK. 2014h. Therapeutic potential of nanocarrier for overcoming to P-glycoprotein. J Drug Target. 22:859–870.
- Malik R, Garg T, Goyal AK, Rath G. 2014. Polymeric nanofibers: targeted gastro-retentive drug delivery systems. J Drug Target. 23:109–124.
- Marwah H, Garg T, Goyal AK, Rath G. 2014. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 1–15.
- Meng Z, Xu X, Zheng W, Zhou H, Li L, Zheng Y, Lou X. 2011. Preparation and characterization of electrospun PLGA/gelatin nanofibers as a potential drug delivery system. Colloids Surf B Biointerfaces. 84:97–102.
- Modgill V, Garg T, Goyal AK, Rath G. 2014. Permeability study of ciprofloxacin from ultra-thin nanofibrous film through various mucosal membranes. Artif Cells Nanomed Biotechnol. 1–6.
- Morie A, Garg T, Goyal AK, Rath G. 2014. Nanofibers as novel drug carrier – an overview. Artif Cells Nanomed Biotechnol. 1–9.
- Pabreja S, Garg T, Rath G, Goyal AK. 2014. Mucosal vaccination against tuberculosis using Ag85A-loaded immunostimulating complexes. Artif Cells Nanomed Biotechnol. 1–8.
- Pillay V, Dott C, Choonara YE, Tyagi C, Tomar L, Kumar P, et al. 2013. A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J Nanomater.
- Rohilla R, Garg T, Bariwal J, Goyal AK, Rath G. 2014a. Development, optimization and characterization of glycyrrhetinic acid-chitosan nanoparticles of atorvastatin for liver targeting. Drug Deliv. 1–8.
- Rohilla R, Garg T, Goyal AK, Rath G. 2014b. Herbal and polymeric approaches for liver-targeting drug delivery: novel strategies and their significance. Drug Deliv. 1–17.
- Semalty M, Semalty A, Kumar G. 2008. Formulation and characterization of mucoadhesive buccal films of glipizide. Indian J Pharm Sci. 70:43–48.
- Sharma A, Garg T, Aman A, Panchal K, Sharma R, Kumar S, Markandeywar T. 2014a. Nanogel-an advanced drug delivery tool: current and future. Artif Cells Nanomed Biotechnol. 1–13.
- Sharma R, Garg T, Goyal AK, Rath G. 2014b. Development, optimization and evaluation of polymeric electrospun nanofiber: a tool for local delivery of fluconazole for management of vaginal candidiasis. Artif Cells Nanomed Biotechnol. 1–8.
- Sharma R, Singh H, Joshi M, Sharma A, Garg T, Goyal AK, Rath G. 2014c. Recent advances in polymeric electrospun nanofibers for drug delivery. Crit Rev Ther Drug Carrier Syst. 31:187–217.
- Singh B, Garg T, Goyal AK, Rath G. 2014a. Recent advancements in the cardiovascular drug carriers. Artif Cells Nanomed Biotechnol. 1–10.
- Singh H, Sharma R, Joshi M, Garg T, Goyal AK, Rath G. 2014b. Transmucosal delivery of Docetaxel by mucoadhesive polymeric nanofibers. Artificial Cells Nanomed Biotechnol. 1–7.
- Singh K, Arora N, Garg T. 2012a. RFID: a trustable security tool in pharmaceutical industry. Am J Pharm Tech Res. 2:113–127.
- Singh K, Arora N, Garg T. 2012b. Superbug: Antimicrobial resistance due to NDM-1. IJIPLS. 2:58–66.
- Singh O, Garg T, Rath G, Goyal AK. 2014c. Microbicides for the treatment of sexually transmitted HIV infections. J Pharmaceutics. 1–18.
- Taepaiboon P, Rungsardthong U, Supaphol P. 2007. Vitamin-loaded electrospun cellulose acetate nanofiber mats as transdermal and dermal therapeutic agents of vitamin A acid and vitamin E. Eur J Pharm Biopharm. 67:387–397.
- Wade A, Weller PJ. 1994. Handbook of pharmaceutical excipients, Washington: American Pharmaceutical association, London: pharmaceutical press.
- Xu X, Yang Q, Wang Y, Yu H, Chen X, Jing X. 2006. Biodegradable electrospun poly (L-lactide) fibers containing antibacterial silver nanoparticles. Euro Polym J. 42:2081–2087.
- Yu D-G, Zhu L-M, White K, Branford-White C. 2009a. Electrospun nanofiber-based drug delivery systems. Health. 1:67.
- Yu D, Zhang X, Shen X, Branford-White C, Zhu LM. 2009b. [The improvement of poorly water-soluble drug solubility through electrospun drug-loaded nanofibers]. Yao Xue Xue Bao = Acta pharmaceutica Sinica. 44:1179–1182.