Abstract
Magnetic iron oxide nanoparticles (IONPs) were coated with gelatin type B by means of the two-step desolvation method. Drug loading by adsorption was studied under various conditions such as different temperature, contact time, pH, and initial gemcitabine concentration. Further, Langmuir isotherm curves were constracted and constants were calculated. According to the Langmuir isotherm, the Gibbs free energy of the adsorption process at 25°C was − 4.74 kJ/mol. On the other hand, this value at 37°C was − 7.86 kJ/mol. In vitro drug release was performed at pH levels of 5 and 7.4, with gemcitabine-loaded magnetic gelatin nanoparticles and free gemcitabine, and both the results were subsequently compared.
Introduction
Gelatin is a protein biopolymer which is derived from collagen (CitationDing et al. 2011). There are two types of gelatin obtainable, depending on the pre-treatment procedure, and they are known commercially as type-A gelatin (isoelectric point at pH ∼ 8–9) and type-B gelatin (isoelectric point at pH ∼ 4–5), which are obtained under acidic and alkaline pre-treatment conditions respectively (CitationJongjareonrak et al. 2010). Gelatin is a naturally occurring polymer with relatively low antigenicity, and has been used for decades in parenteral formulations and is an approved plasma expander (CitationGabr et al. 1996, CitationOfokansi et al. 2010).
Gelatin is made of animal connective tissues, and it is a translucent, colorless, brittle, and nearly flavorless solid substance. Gelatin has a unique property which enables it to produce thermally biodegradable gel. When a solution which contains gelatin is cooled, it creates a gel, and when this gel is heated once again, it liquefies. The existence of multifunctional groups such as −NH2 and −COOH in the gelatin chain, makes it an appropriate candidate to bind with drug (CitationGomez-Guillen et al. 2011). It is also commonly used to immobilize drugs and genes to produce controlled-release products for pharmaceutical and medical applications. Its water solubility, biodegradability, biocompatibility and non-toxicity, ease of chemical modification and cross-linking, make gelatin ideal for use in gelatin-based nanoparticles as carrier systems for drug delivery. It can also be a promising candidate for the surface modification of iron oxide (CitationGaihre et al. 2008).
Lung cancer comprises 15% of the new cancer cases every year, and also contributes to 18% of cancer deaths. Although the treatment approaches change depending on the stages and histopathologic types, generally, three types of treatments exist (surgical, radiotherapy or/and chemotherapy) (CitationLippincort 1997). One of the most important difficulties in the pharmacologic treatment of the disease is the mobilization of the active agents which are used for the purpose of therapy, to reach especially the specific targeted area, and to achieve constant drug release (CitationKalevi et al. 2008).
Gemcitabine (2′,2′-difluorodeoxycytidine, dFdC), is an analog of deoxycytidine, with high activity against many different types of solid tumors (CitationHodge et al. 2011). Gemcitabine is used as a single agent or in combination with other anti-cancer molecules, in the treatment of a wide variety of malignancies, including colon, pancreatic, lung, head and neck, ovarian, bladder, and breast cancers , (CitationMartín-Banderas et al. 2013, CitationBarbara et al. 2007). Gemcitabine exhibits certain limitations, such as very short plasma half-life and rapid metabolism to its inactive form by cytidine deaminase. Furthermore, common side effects like systemic toxicity due to high dosage and no specificity with regard to healthy cells, has also limited its antineoplastic properties (CitationArya et al. 2011).
Many drug delivery systems therapy have been developed for controlled and targeted delivery. Microspheres, liposomes, lipid-based micro particle systems, and the conjugates that are acquired by modification of the active moiety with other chemical compounds, can be shown as examples for these types of systems (Gennaro 2000, CitationTomlinson et al. 1986, CitationChien 1992, CitationOppenheim 1981, CitationDelie 1998, CitationChen and Langer 1998).
The surfaces of nanoparticles can be modified with other molecules, which will provide better targeting of cells that cannot be identified by the immune system. They can be modified to cross the barriers such as the blood-brain barrier or the dermal tight junction. Nanoparticles can be modified as drug carriers which are prepared to deliver drugs to damaged tissues and achieve controlled drug release. The polymeric nanoparticles which are prepared and used as carrier systems for active matter have many advantages. The IONPs, which can be loaded with drugs, have properties of easy dispensability, small size, biocompatibility, superparamagnetism. Although chemotherapy is aimed at destroying cancer cells, it damages the normal cells as well (CitationHaley and Frenkel 2008).
The goal of this study is the development of a gemcitabine-loaded nano-scaled formulation for magnetically-targeted therapy of non-small cell lung cancer. In this study, gemcitabine was loaded onto magnetic gelatin nanoparticles by the adsorption technique. The adsorption isotherm and kinetic constants were calculated, and in vitro gemcitabine release from nanoparticles was determined by performing dialysis at 37°C.
Materials and methods
Materials
Gelatin type-B from bovine skin was purchased from Sigma, and glutaraldehyde (GA) was purchased from Merck. The IONPs were prepared according to the method described by CitationDung et al. (2009). Gemcitabine (“Gemzar”) was obtained from Lilly. All other chemicals were of analytical grade, and were used as received.
Synthesis of magnetic gelatin nanoparticles
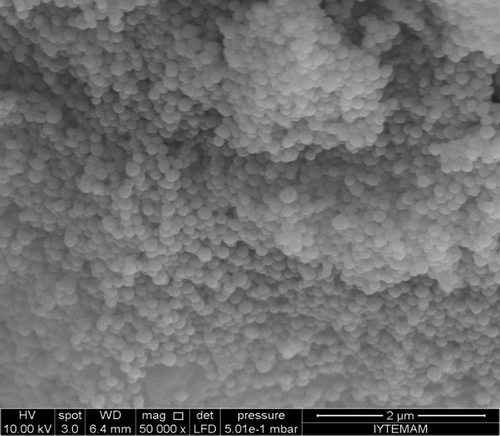
Magnetic gelatin nanoparticles (M-GNPs) were prepared using the two-step desolvation method reported by CitationYılmaz and Sanlıer (2013). Briefly, 50 mg of gelatin was dissolved in d-water at 37°C, and 2 mL of acetone was added to the gelatin solution. After the precipitation was complete, the supernatant was discarded and the pellet was dissolved in 1 mL of d-water at 37°C. The pH level of the solution was adjusted to the range of 12–13, and 0.5 mL of iron oxide suspension (10 mg/mL) was added to the solution under shaking at 480 rpm. Acetone was dropped into the solution using a tubing pump, at a rate of 1mL/min, and 12 μL of glutaraldehyde (4% v/v aqueous solution) was added to this mixture as a crosslinker. The mixture was left to shake for 24 h at room temperature. At the end of this time, M-GNPs were collected by centrifugation and washed with d-water. The drug adsorbed onto the M-GNPs was characterized using scanning electron microscopy (SEM) (Phillips XL-30S) in the Izmir Institute of Technology. The shape and dispersity of the nanoparticles were examined.
Adsorption of gemcitabine onto M-GNPs
Gemcitabine was loaded onto the M-GNPs by the adsorption technique. For this, 2 mg of M-GNPs were incubated with 1.5 mL of gemcitabine solution, and at the end of this process, the mixture was centrifuged. The supernatant was used for the analysis of the non-adsorbed drug. The concentrations of the initial and non-adsorbed gemcitabine were determined at 268 nm, based on the standard curve which was drawn for a concentration of gemcitabine ranging from 10 to 75 μg/mL, using the Perkin Elmer Lambda 35 UV/VIS spectrophotometer. Adsorbed drug amount and drug adsorption efficiency were calculated according to Eqs. (1) and (2) respectively.
In order to achieve maximum efficiency of gemcitabine adsorption onto the M-GNPs, the effect of incubation time and temperature, pH of the media, and the concentration of drug on adsorption, were studied. Firstly, 2 mg of M-GNP and 1.5 mL of gemcitabine dissolved in 10 mM sodium acetate buffer of pH 4 were incubated at 25°C for different periods of time (3–24h). At the end of this time, the mixture was centrifuged at 13,000 rpm, and the amount of gemcitabine in the supernatants was determined. The adsorption yield of each sample was calculated, and the optimum incubation time was determined. Secondly, the temperature of the adsorption process was investigated for optimum adsorption of the drug. M-GNPs and gemcitabine were mixed in a hot water bath at different temperatures ranging from 4 to 40°C, for 15 h, which was selected as the optimal incubation time. After 15 h, the supernatants were collected and the amount of gemcitabine was analyzed. Thus, optimum adsorption temperature was estimated. In the third step, effect of pH on drug adsorption was researched. Gemcitabine was dissolved in different buffer solutions in the pH range of 3.5–10, and M-GNPs were incubated with gemcitabine solution at 25°C (which was found to be the optimal incubation temperature). After centrifugation, the amount of adsorbed drug in each sample was calculated, and the optimal pH value of gemcitabine adsorption was determined. Finally, the effect of gemcitabine concentration on adsorption was investigated. Gemcitabine solutions at concentrations of 10–350 μg/mL were prepared in sodium acetate buffer of pH 4, the optimal buffer for adsorption. The nanoparticles and drug solutions were incubated for 15 h at 25°C. Maximum adsorption yield was estimated based on the amount of gemcitabine in the supernatants.
Adsorption kinetics, isotherms, and thermodynamic modeling
The experiments were performed to observe the effect of concentration at constant pH and temperature. Gemcitabine solutions were prepared at concentrations ranging from 50–350 μg/mL. Nanoparticles were dispersed equally in 10 mM Na-acetate-buffered gemcitabine solutions. The samples were incubated for 15 h in a hot water bath at 25°C and 30°C, with shaking at slow speed. After 15 h, the samples were centrifuged. The upper phases were taken and measured spectrophotometrically. The Langmuir isotherm was applied and constants were calculated. Thermodynamic constants were calculated using the constants that were obtained from the Langmuir isotherm.
A solution of 50 μg/mL of gemcitabine was prepared in a 10 mM Na-acetate buffer. M-GNPs were dispersed in the buffered drug solution. The samples were incubated in a hot water bath under constant shaking at 37°C and 25°C. The upper phases were removed at the predetermined time intervals and measured immediately. Kinetic values were calculated.
In vitro drug release
Gemcitabine-loaded M-GNPs were placed in a dialysis membrane after dissolving in PBS buffer (pH 6 and 7.4), and dialyzed against 15 mL of PBS buffer (pH 6 and 7.4) for 24 h at 37°C, under conditions of constant shaking. The data obtained was compared with the data of the release of free drug under the same conditions. The amount of gemcitabine released was spectrophotometrically determined from the receiver solution, and cumulative release was calculated with Formula 3. Likewise, free drug was dialyzed under the same conditions and the data obtained was calculated using Formula 3, and compared with drug release from nanoparticles.
Results
Synthesis of M-GNPs
In the present study, M-GNPs were synthesized by a two-step desolvation method. Since commercial gelatin shows a wide range of molecular weights, the presence of a low molecular weight fraction in the solution results in broad size distribution and aggregation of nanoparticles. The two-step desolvation method allows the removal of the lower molecular weight fraction in the first desolvation step. GA was added as a cross-linking agent to prevent redissolving of the gelatin nanoparticles. According to Gaihre and coworkers, when GA is added to the aqueous dispersion of the composite nanoparticles, the carboxylic acid residues and amine groups react with the cross-linking agent (CitationGaihre et al. 2008). A formulation of the temperature effect was explained with the high viscosity of gelatin at room temperature. Higher temperature was found to be preferable for the formation of GNPs, because the triple-helical structure of gelatin uncoils as the temperature rises (CitationNahar et al. 2008). Gelatine has been used in many studies as a drug, matrix, and nucleotide carrier (CitationOfokansi et al. 2010, CitationNahar et al. 2008, CitationLeo et al. 1997, CitationZwiorek et al. 2008). Due to the particle size, the nanoparticular system designed must be significiantly smaller than 5 μm, without forming aggregates, to ensure that the particles do not cause an embolism, since the smallest capillaries in the body are 5–6 μm in diameter (CitationSingh and Lillard 2009).
Gelatin is a natural polymer, with carboxyl, amine, and amide functional groups present in the polymer chain. So it is expected to be negatively charged in alkaline conditions, and to show higher efficiency in formation. The desolvating method is a modification of a salting-out technique. In salting-out, for protein precipitation, organic solvents may be used. As known, with the rapid addition of an organic solvent or a salting-out agent, hydrophobic sites of proteins emerge and interact with each other rapidly, so that proteins suddenly precipitate as aggregates. It means that with rapid addition of organic solvent, the particle grows so rapidly in size, that size control is not possible.
M-GNPs were synthesized as described in “Synthesis of magnetic gelatin nanoparticles”. According to the study by Gaihre B. and co-workers, for effective targeting, the percentage of IONPs in the composite nanoparticles plays an important role, and with increasing the percentage of IONPs in gelatin solution, there is no significiant change seen in the hydrodynamic size of the nanoparticles (CitationGaihre et al. 2009). It means that gelatin encapsulates the IONPs with apparent percentage, and there is no need to increase the percentage of IONPs. Increasing the IONP percentage also results in the increase in the viscosity of the gelatin solution, which leads to aggregation of gelatin as a gel, but not in the form of nanoparticles (CitationGaihre et al. 2009).
In the present study, M-GNPs were synthesized based on the method described in the study by CitationYilmaz. H and Hamarat Sanlier S. (2013). To show the shape of nanoparticles, SEM images were taken, and were as shown in . As seen in , the nanoparticles are homogenous and spherically shaped, with a diameter of ∼300 nm. This result is in good agreement with an earlier study (CitationYılmaz and Hamarat Sanlier 2013).
Adsorption of gemcitabine onto the M-GNPs
In the present work, the effects of temperature, pH, contact time, and drug concentration on adsorption were studied. Maximum adsorption occurred at 25°C, with a yield of 23.1% ± 5.8, at neutral pH. At 4°C, the adsorption yield was low, due to the lack of energy required for adsorption. Moreover, at high temperatures, the M-GNPs may expand due to the swelling property of gelatin, and it may result in decreased surface area for adsorption. The effect of temperature on the adsorption process can be seen in .
Table I. Effect of gemcitabine concentration, contact time, pH, and temperature on gemcitabine adsorption onto M-GNPs. M-GNP: Magnetic gelatin nanoparticle.
At higher temperatures such as 30°C and 40°C, the gelatin nanoparticles enlarge in size, and this enlargement causes a decrease in the area of the surface of the nanoparticle that comes into contact with drug molecules. Thus, the drug adsorption yield decreases. This result is similar to the results demonstrated in the study by in CitationYilmaz H. And Hamarat Sanlier S. (2013).
The contact times for adsorption were varied over a range of 3 to 24 h, at a temperature of 25°C. As seen in the , adsorption increased with contact time. With increasing contact time, gemcitabine molecules have a better chance to interact with the M-GNPs. Maximum adsorption occurred at 15 h, with 61.6 ± 0.4% yield. At 24 h, there was not much difference between the adsorption yield at 15 h and that at 24 h. However, in adsorption process, there is a risk of contamination during long adsorption times. A contact time of 15 h was chosen as the optimum, based on both adsorption efficiency and avoidance of the risk of contamination.
In order to determine the effect of pH on adsorption yield, six pH levels were used, ranging from 3–8. Based on the obtained data, the best adsorption occurred at a pH of 4, with 74.1% efficiency. At a pH of 3, both M-GNPs and gemcitabine have a positive charge according to their isoelectric points. The isoelectric point of gemcitabine is 3.6, and it has a positive charge at a pH of 4. On the other hand, the isoelectric point of gelatin is nearly 5, and therefore, gelatin nanoparticles have a negative charge at pH 4. In the present study, it is hypothesized that at pH 4, M-GNPs and gemcitabine can interact due to their counter charges. Moreover, gemcitabine can be chemically adsorbed onto nanoparticles. Above this pH, gemcitabine loses its positive charge by giving its proton to the solvent, and gelatin becomes more negatively charged. According to this information, it is expected that the adsorption process will weaken and adsorption efficiency will decrease. The results can be seen in .
The effect of gemcitabine concentration on adsorption was studied at 25°C for 15 h. As seen in , the data revealed that maximum adsorption occurred at a concentration of 50 μg/mL, with ∼70% adsorption efficiency; above this concentration, desorption occurred and the adsorption reached an equilibrium. This data corresponds with data from an earlier study which was performed by CitationYilmaz H. and Hamarat Sanlier S (2013). In their study, with increasing concentration, adsorption efficiency was seen to increase until a concentration of 350 μg/mL was reached, and above this concentration, adsorption efficicency was seen to decrease, as in our study.
All of these experiments were repeated five times, and error bars were given. All of the drug adsorption yields are calculated from the standard curve of gemcitabine. The equation for this standard curve is y = 0.014x, and the R2 value is 0.999.
Adsorption kinetics, isotherms, and thermodynamic modeling
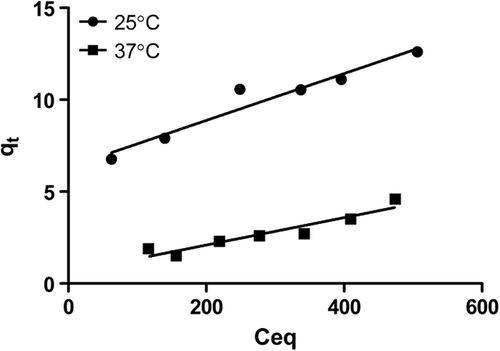
The Langmuir adsorption isotherm explains the monolayer coverage of the adsorbate on an adsorbent surface at a constant temperature. The assumption is that the forces exerted by chemically unsaturated surface atoms do not extend further than the diameter of one adsorbed molecule (CitationOfokansi et al. 2010). The linear equation of Langmuir's curve is:
Ceq is the concentration of gemcitabine solution (mol/L) at equilibrium, and Qeq is the amount of gemcitabine adsorbed per unit weight of M-GNP (mol/g). Q0 gives the adsorption capacity (mol/g) of the theoretical monolayer, and b is the Langmuir constant which is related with the energy of adsorption (L/mol). A plot of Ceq/Qeq versus Ceq yields a straight line with slope 1/Q0 and intercept b.
To identify the favorability of the adsorption, the dimensionless constant separation factor R is expressed as:
The value of RL indicates the state of adsorption favorability as follows: RL > 1, unfavorable; RL = 1, linear; 0 < RL < 1, favorable; RL = 0, irreversible (CitationMittal et al. 2008).
In the present study, the theoretical monolayer adsorption capacity was determined at two different temperatures, as 25°C and 37°C. The values are 83.8 mol/g at 25°C and 142.8 mol/g at 37°C, respectively. The Langmuir constant was calculated as 0.0019 at 25°C and 0.011 at 37°C. The constants can be seen in . The Langmuir isotherm curve can also be seen in .
Table II. Langmuir isotherm constants for adsorption of gemcitabine by M-GNPs. M-GNP: Magnetic gelatin nanoparticle.
At 25°C, the RL value was 0.768, while at 37°C, this value was 0.894, both of which are between 0 and 1, indicating that the adsorption of gemcitabine onto M-GNPs is favorable.These values indicate that the adsorption occurred spontaneously and the process was feasible.
The changes in the standard Gibb's free energy were calculated using the Langmuir isotherm constant b. The equation is shown below. Changes in the thermodynamic parameters, that is free energy (G0), enthalpy (H0), and entropy (S0), were also calculated based on the adsorption isotherms, using Eqs. (6–8) (CitationMittal et al. 2008).
The negative standard Gibb's free energy values indicate the feasibility of the process and its spontaneous nature. The standard Gibb's free energy was determined as − 4.74 kJ/mol at 25°C and − 7.86 kJ/mol at 37°C. These values represent that the adsorption physically occurred. The ΔH0 value of this process was found to be 112.4 kJ/mol. This result indicates that the nature of the adsorption process is endothermic. The ΔS0 value was found to be 393.1 J/molK at 25°C and 387.9 J/molK at 37°C. The constants are represented in . The positive values of the entropy change show the increased randomness at the solid/solution interface, with some structural changes in the adsorbate and adsorbent, and an affinity of the M-GNP towards gemcitabine. All these values indicate that the adsorption occurred spontaneously and that the process was feasible.
Table III. Thermodynamic constants for adsorption of gemcitabine by M-GNPs. M-GNP: Magnetic gelatin nanoparticle.
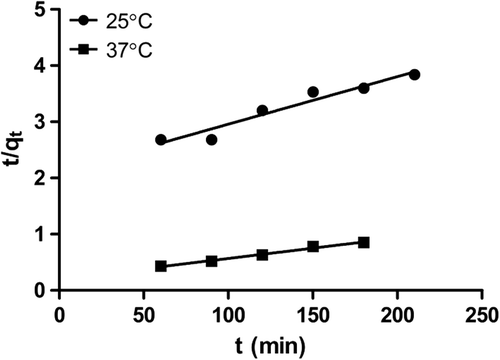
The kinetic model having an appropriate adsorption rate was chosen, using Eq. (9).
Where k2 (kg/g-min) is the pseudo-second-order rate constant. The linear plot of 1/q versus t gives the second-order rate constant and qe (g/kg). This model is used to predict behavior over the whole range of adsorption, and is in agreement with the chemisorption mechanism being the rate-controlling step. The pseudo-second-order kinetic curve is exhibited in .
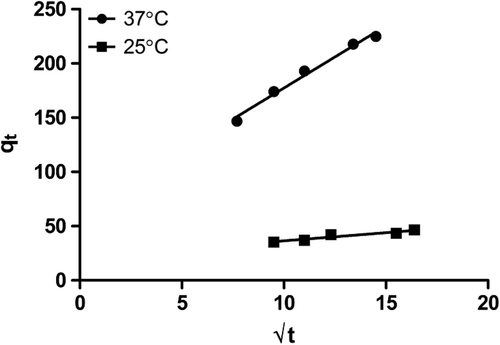
In order to identify the diffusion mechanism, the intra-particle diffusion model was applied and expressed as:
Where kp (mg/g-min½) is the intra-particle diffusion rate constant, and c (mg/g) is a constant that gives information about the thickness of the boundary layer. The plot of q versus t1/2 could yield a straight line passing through the origin, if the adsorption process obeys the sole intra-particle diffusion model. If the line does not pass through the origin, intra-particle diffusion is not the only rate-limiting step. It could be stated that this process is complex and involves more than one mechanism (CitationHamarat Sanlier et al. 2013).
When adsorption is preceded by diffusion through a barrier, the kinetics in most cases follow pseudo-first order kinetics. However, the pseudo-second order kinetics were not proved to be effective in representing the experimental kinetic data for the entire adsorption period. The diffusion mechanisms were considered independently, in accordance with the assumptions that the kinetics was controlled by pseudo-second order kinetics at the beginning of the experiment and then controlled by intra-particle diffusion. The intra-particle kinetic curve is shown in .
Pseudo-second order kinetics showed that with increasing temperature, the drug adsorption capacity of M-GNPs was reduced. On the contrary, as can be seen in intra-particle diffusion kinetics, drug diffusion into the M-GNPs at 37°C is higher when compared to that at 25°C. Based on the data, we presume that at the beginning of the adsorption process, the rise in temperature increases the drug adsorption by M-GNPs, but afterwards, adsorption follows intra-particle diffusion and lower temperatures are likely to be better for this stage of adsorption. In light of the results obtained, it can be stated that the process of adsorption may be implemented in two stages for better adsorption, which means that it can be started with high temperature and followed with low temperature. The data obtained about kinetic constants can be seen in .
Table IV. Pseudo-second-order and intra-particle diffusion kinetic constants for adsorption of gemcitabine by M-GNPs. M-GNP: Magnetic gelatin nanoparticle.
In vitro drug release studies
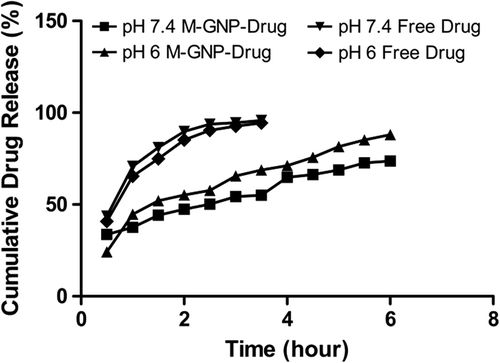
In order to determine the drug release behavior of M-GNPs, release studies were conducted with the aid of a dialysis membrane using PBS buffer at pH levels of 6 and 7.4, at 37°C. shows the release profile of gemcitabine from M-GNPs and the release profile of free gemcitabine. The data was compared with the release of the free drug under the same circumstances. The physiologic pH value of the blood is 7.4. On the other hand, based on data in the literature, the pH level in the microenvironment of the tumor decreases to a range of 5–6.5 (CitationSingh and Lillard 2009). For this reason, drug release from the nanoparticles was observed both at the physiologic pH and at pH 6, and compared with the release of free drug.
As seen in the , the cumulative release of free gemcitabine reached 95.8% at pH 7.4, and 95% at pH 6, in 3.5 h, which means that the release of free gemcitabine is not pH-dependent. On the other hand, the cumulative release of gemcitabine from M-GNPs was slower and pH-dependent. At pH 6, only 69% of gemcitabine was released from M-GNPs in 3.5 h, and at the end of 24 h, 91.1% was released. At pH 7.4, 55% of gemcitabine was released from M-GNPs in 3.5 h, and 68.8% gemcitabine was released after 24 h. The results showed that there is 25% more release at pH 6 than at pH 7.4. However, because of the magnetic property of nanoparticles, it is expected that the targeting of M-GNPs will take 30 min (CitationLubbe et al. 2001). Hence, it is very important to compare the release profiles of gemcitabine in the first hour, at pH 7.4. The data showed that in the first hour, 37% of gemcitabine was released from M-GNPs at a pH of 7.4, but the release of free gemcitabine was 71%. The fast release of gemcitabine from nanoparticles at the beginning of the drug release process can be explained by the burst effect. However, based on the data obtained, it still has a better release profile compared with that of free gemcitabine. The faster release of gemcitabine from M-GNPs in an acidic medium can be explained by the surface charge of the M-GNPs. At acidic medium, the surface charge of M-GNPs is expected to be positive, which leads to the weakening of the interaction between gemcitabine and M-GNPs (CitationGaihre et al. 2009).
Moreover, the release of free drug was not changed at any pH level, and the release amount was completed in 3.5 h. Our nanoparticle drug carrier system achieved more controlled and sustained release of gemcitabine compared to the free drug. Based on our data, the prepared nanoparticle system is thought to have the potential to decrease unwanted side effects.
According to the report by B. Gaihre and his coworkers, who studied doxorubicin-loaded M-GNPs, drug release at the physiologic pH of 7.4 was 32%. A fast drug release profile was seen at acidic pH. The release value was 61%. The drug release in our study is higher when compared to the drug release in this study (CitationGaihre et al. 2009).
Conclusion
In this paper, M-GNPs were synthesized by a simple method, and characterized using SEM. The SEM image of M-GNPs shows that M-GNPs are spherical nanoparticles (). Gemcitabine, which is an anti-cancer agent, was loaded onto the M-GNPs by a process of adsorption. It is important to discover the adsorption mechanism which can be explained using thermodynamic isotherm models. Due to the importance of the nature of adsorption, the Langmuir isotherm is drawn with the results obtained. According to Langmuir isotherm, the Gibb's free energy of the adsorption process at 25°C was − 4.74 kJ/mol, and at 37°C it was -.86 kJ/mol, which indicates that the process spontaneously occurred at 25°C and 37°C. Thus, it was estimated that the adsorption was physical. The kinetic values showed that the adsorption process cannot be explained either by pseudo-second-order kinetics or by the intra-particle diffusion kinetic model. Kinetic studies showed that adsorption process may be completed in two stages; at the beginning of adsorption, high temperature is required, and afterwards, the temperature may be decreased, to have a better adsorption profile.
Although the adsorption was physical, drug release occurred in a controlled manner. Free drug release was completed in 3.5 h at both pH values, that is pH 7.4 and pH 6. On the other hand, the drug release from nanoparticles was pH-dependent and showed more controlled behavior. Also, the drug release from nanoparticles was completed in 24 h instead of 3.5 h. Moreover, the results showed that there is a 25% more release at pH 6 than at pH 7.4.
The further studies should include studies on hyperthermic drug release due to the magnetic property of nanoparticles, cell culture studies, and in vivo animal studies, to determine the biodistribution profile of nanoparticles.
Acknowledgement
We would like to thank the Scientific and Technological Research Council of Turkey (TUBITAK) for financial support received.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Arya G, Vandana M, Acharya S, Sahoo SK. 2011. Enhanced antiproliferative activity of Herceptin (HER2)-conjugated gemcitabine-loaded chitosan nanoparticle in pancreatic cancer therapy. Nanomedicine. 7:859–870.
- Barbara S, Arpicco S, Rocco F, Marsaud V, Renoir JM. 2007. Encapsulation of gemcitabine into lipophilic derivatives polycyanoacrylate nanospheres and nanocapsules. Int J Pharm. 344:71–77.
- Chen H, Langer R. 1998. Oral particulate delivery: status and future trends. Adv Drug Deliv Rev. 34:339–350.
- Chien YW. 1992. Oral drug delivery and delivery systems. In: Y. W. Chien, Eds. Novel Drug Delivery Systems New York: Marcel Dekker Inc, pp. 139–196.
- Delie F. 1998. Evaluation of nano- and microparticle uptake by the gastrointestinal tract. Adv Drug Deliv Rev. 34:221–233.
- Ding D, Zhu Z, Qin L , Jing W , Yong H , Xiqun J, Baorui L. 2011. Cisplatin-loaded gelatin-poly(acrylic acid) nanoparticles: Synthesis, antitumor efficiency in vivo and penetration in tumors Eur J Pharm Biopharm. 79:142–149.
- Dung DKT, Hai TH, Phuc LH, Long DB, LKVinh, Truc PN. 2009. Preparation and characterization of magnetic nanoparticles with chitosan coating. J Phys Conf Ser. 187:1–5.
- Gabr Y, Assem N, Michael A, Fahmy L. 1996. Evaluation studies on oxypolygelatin and degraded gelatin as plasma volume expander Arzneimittelforschung. 46:763–766.
- Gaihre B, Aryal S, Barakat NAM, Kim HY. 2008. Gelatin stabilized iron oxide nanoparticles as a three dimensional template for the hydroxyapatite crystal nucleation and growth. Mater Sci Eng C. 28 :1297–1303.
- Gaihre B, Aryal S, Khil MS, Kim HY. 2008. Encapsulation of Fe3O4 in gelatin nanoparticles: Effect of different parameters on size and stability of the colloidal dispersion. J. Microencapsul. 25:21–30.
- Gaihre B, Khil MS, Lee DR, Kim HY. 2009. Gelatin-coated magnetic iron oxide nanoparticles as carrier system: drug loading and in vitro drug release study. Int. J. Pharm. 365:180–189.
- Gennaro AR. 2006. Remington: The Science and Practice of Pharmacy. Part 5: Pharmaceutical Manufacturing/Extended-Release and Targeted Drug Delivery Systems, The New Drug Approval Process and Clinical Trial Design. In: Philadelphia, PA: Lippincott Williams Wilkins, pp. 939–965.
- Gomez-Guillen MC, Gimenez B, Lopez-Cabellero ME, Montero MP. 2011. Functional and bioactive properties of collagen and gelatin from alternative sources: a review. Food Hydrocolloids. 25(8):1813–1827.
- Haley B, Frenkel E. 2008. Nanoparticles for drug delivery in cancer treatment. Urol Oncol. 26:57–64.
- Hamarat Sanlier S, Ak G, Yilmaz H, Ozbakir G, Cagliyan O 2013. Removal of textile dye, direct red 23, with glutaraldehyde cross-linked magnetic chitosan beads. Prep Biochem Biotechnol. 43:163–176.
- Hodge LS, Taub ME, Tracy TS. 2011. Effect of its deaminated metabolite, 2′,2′-difluorodeoxyuridine, on the transport and toxicity of gemcitabine in HeLa cells. Biochem. Pharmacol. 81:950–956.
- Jongjareonrak A, Rawdkuen S, Chaijan M, Benjakul S, Osako K, M, Tanaka. 2010. Chemical compositions and characterisation of skin gelatin from farmed giant catfish (Pangasianodon gigas). LWT - Food Science and Technology. 43:161–165.
- Kalevi K, Paola E, Kim B, Ernest KJP. 2008. Nanoparticles in cancer. Curr Radiopharm. 1:30–36.
- Leo E, Vandelli MA, Cameroni R, Forni F. 1997. Doxorubicin-loaded gelatin nanoparticles stabilized by glutaraldehyde: Involvement of the drug in the cross-linking process. Int J Pharm. 155:75–82.
- Lippincort R. 1997. American Joint Commitee on Cancer (AJCC), In: Cancer Staging Manual, 5th edn. , Lippincott Williams & Wilkins: Philadelphia, pp. 127–37.
- Lubbe AS, Alexiou C, Bergemann C 2001. Clinical applications of magnetic drug targeting J Surg Res. 95:200–206.
- Martín-Banderas L, Sáez-Fernández E, Holgado A, Durán-Lobato M, Prados JC, Melguizo C, Arias JL. 2013. Biocompatible gemcitabine-based nanomedicine engineered by flow focusing for efficient antitumor activity. Int J Pharm. 443:103–109.
- Mittal A, Gajbe V, Mittal J. 2008. Removal and recovery of hazardous triphenylmethane dye, Methyl Violet through adsorption over granulated waste materials. J. Hazard. Mater. 150:364–375.
- Nahar M, Mishra D, Dubey V, Jain NK. 2008. Development, characterization, and toxicity evaluation of amphotericin B-loaded gelatin nanoparticles. Nanomedicine. 4:252–261.
- Ofokansi K, Winter G, Fricker G, Coester C. 2010. Matrix-loaded biodegradable gelatin nanoparticles as new approach to improve drug loading and delivery. Eur. J. Pharm. Biopharm. 76:1–9.
- Oppenheim RC. 1981. Solid colloidal drug delivery systems: nanoparticles. Int J Pharm. 8:217–234.
- Singh R, Lillard JW. 2009. Nanoparticle-based targeted drug delivery Exp. Mol. Pathol. 86:215–223.
- Tomlinson E, Davis S. 1986. Site Spesific Drug Delivery. Chichester: Wiley, New York: Plenum press, p. 93.
- Yılmaz H, Hamarat Sanlıer S. 2013. Preparation of magnetic gelatin nanoparticles and investigating the possible use as chemotherapeutic agent Artif. Cells Nanomed. Biotechnol. 41:69–77.
- Zwiorek K, Bourquin C, Battiany J, Winter G, Endres S, Hartmann G, Coester C. 2008. Delivery by cationic gelatin nanoparticles strongly increases the immunostimulatory effects of CpG oligonucleotides. Pharm Res. 25:551–562.