Abstract
The transdermal route of drug delivery has gained immense interest for pharmaceutical researchers. The major hurdle for diffusion of drugs and bioactives through transdermal route is the stratum corneum, the outermost layer of the skin. Currently, various approaches such as physical approach, chemical approach, and delivery carriers have been used to augment the transdermal delivery of bioactives. This review provides a brief overview of mechanism of drug transport across skin, different lipid vesicular systems, with special emphasis on lipid vesicular systems including transfersomes, liposomes, niosomes, ethosomes, virosomes, and pharmacosomes and their application for the delivery of different bioactives.
Introduction
Bioactive are a group of physiologically active substances that exerts effects upon the physiology of organisms. The term bioactive covers a wide range of compounds which can be variously categorized as chemical types (small organic molecules, peptides/proteins, and oligonucleotides), therapeutic agents (drugs, biopharmaceuticals, and vaccines) and nontherapeutic (poisons and toxins) agents (CitationTucker 2010). Different types of bioactives compounds include recombinant proteins, antibiotics, genes, hormones, enzymes growth factors, vaccines, etc. Different delivery routes are available for the delivery of bioactives including oral, parenteral, intranasal, transdermal, etc.
Although numerous delivery routes are available for delivery of bioactives their delivery is still a challenge due to their several unfavorable properties including large molecular size, susceptibility to enzymatic degradation, short plasma half-life, ion permeability, immunogenicity, tendency to undergo aggregation, adsorption and denaturation, uptake by the reticuloendothelial system (RES), and accumulation in non-targeted organs and tissues. In addition, manufacturing processes and environmental factors may also damage bioactive-like proteins and also reduce their biological activity and render immunogenicity to the proteins leading to their precipitation (CitationSingh et al. 2013). Among numerous delivery routes, transdermal route have secured a unique position for the effective delivery of bioactives.
Transdermal drug delivery is an efficient technique for effective delivery of numerous drugs including bioactives. But the major problem faced in designing of transdermal drug delivery system is the natural transport barrier of the skin which must be resolved first. Highly organized crystalline lipid lamellae play a very important role in the barrier properties of the stratum corneum (CitationPrasanthi and Lakshmi 2012). Numerous techniques have been used to breakdown and weaken the highly organized structure of intercellular lipids with an effort to enhance drug transport across the intact skin or to increase the delivery force for the permeation of drugs across this skin barrier. Utilization of vesicle-based delivery system is one of the approaches which could reduce the natural transport barrier of the skin. Amongst the vesicle-based delivery system, lipid vesicles have confirmed their applicability in clinical use (CitationTiwary et al. 2007).
This review gives an overview about the different techniques to overcome the natural transport barrier of the skin including physical and chemical techniques with special emphasis on formulation techniques using novel vesicular carriers.
Transdermal drug delivery
Transdermal delivery offers numerous advantages over conventional methods for delivery of drug, which include enhanced efficacy, increased safety, greater convenience, and improved patient compliance. “Peak and valley” effect of oral injectable therapy could be bypassed by transdermal systems which delivers a steady flow of drugs into the bloodstream for an extended time period. Moreover it offers more controlled and effective treatment. This system avoids first-pass metabolism through the gastrointestinal tract and the liver, thus the therapeutic equivalent for the transdermal delivery of certain bioactive compounds could be significantly less than that of the corresponding oral delivery, thereby reducing related side effects (CitationPrausnitz et al. 2004).
Transportation of drugs across the skin
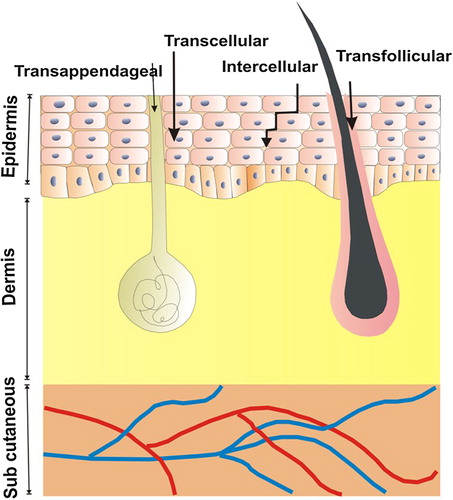
Human skin is the largest organ of the human body and is a potential candidate for delivery of drug because of its easy accessibility. In addition it provides the ability to visually monitor the genetically modified region and the possibility to surgically remove the aberrant tissue if unwanted side effects occur (CitationSubedi et al. 2010). Human skin consists of three layers, beginning from the surface; the epidermis, the dermis, and subcutaneous tissue or subcutis, each containing their own specific cells and respective functions (CitationKanitakis 2002). The transportation of drug across skin occurs through transappendageal, transfollicular, transcellular, and intercellular routes. Different routes of drug transport through skin are shown graphically in .
Natural barrier of skin
The major challenge in designing of transdermal drug delivery systems is natural transport barrier of the skin, which prevents transdermal delivery of therapeutic agents. So the different techniques have been utilized to overcome skin barrier which are as follows:
Physical technique: Iontophoresis, electroporation, sonophoresis, microniddle, magnetophoresis, thermophoresis, skin abrasion, etc.
Chemical technique: Use of penetration enhancers, prodrugs, salt formation, etc.
Formulation technique (using delivery vesicles): Liposome, transferosomes, niosomes, ethosomes, virosomes, phytosomes, cubosomes etc.
Physical technique
Iontophoresis. Iontophoresis also called as electromotive drug administration is a technique that uses a small electric charge to deliver a medicine or other chemical through the skin. It is basically an injection without the needle. Technically it is a non-invasive method of propelling high concentrations of a charged substance, normally a drug or bioactive agent, transdermally by repulsive electromotive force using a small electrical charge applied to an iontophoretic chamber containing a similarly charged active agent and its vehicle. One or two chambers are filled with a solution containing an active ingredient and its vehicle. The positively charged chamber (anode) repels a positively charged chemical, and the negatively charged chamber (cathode) repels a negatively charged chemical into the skin.
Iontophoresis is well classified for use in transdermal drug delivery. This method relies on the active transportation within an electric field unlike transdermal patches. In the presence of an electric field, the dominant forces in mass transport are electromigration and electroosmosis. Some common diseases treated using iontophoresis include plantar fasciitis, bursitis, and some types of hyperhidrosis (CitationDhote et al. 2012).
Phonophoresis. Phonophoresis is a technique that utilizes ultrasound to enhance the delivery of topically applied drugs. This has been used to enhance the absorption of topically applied analgesics and anti-inflammatory agents through the therapeutic application of ultrasound (CitationOgura et al. 2008). Phonophoresis has also been used to enhance the absorption of topically applied drugs and bioactives (CitationMerino et al. 2003).
Electroporation. In this technique short electric pulse (milliseconds or microseconds) is applied to the skin for the transitory structural perturbation of the lipid bilayer membranes. Electroporation possess potential to alter stratum corneum lipid domain. In addition electroporation have potentially enhanced the permeation of small compounds like fentanyl to moderately sized molecules like calcein and macromolecules like calcitonin. Enhanced transport has also been reported with lipophilic (e.g., timolol), hydrophilic (e.g. metoprolol), charged (e.g. heparin), and neutral molecules (e.g. mannitol) (CitationDenet et al. 2004).
Magnetophoresis. Magnetophoresis is a phenomenon of enhancing drug permeation across the biological barriers by the application of magnetic field. In vitro and in vivo studies reported that magnetophoresis leads to enhanced transdermal drug delivery. The predominant mechanism for drug permeation enhancement was found to be magnetokinesis and enhanced partitioning of drug into stratum corneum (CitationMurthy et al. 2010, CitationSammeta et al. 2011).
Skin abrasion. The abrasion technique involves the direct removal or disruption of the upper layers of skin to facilitate the permeation of topically applied medicaments. Some of the devices based on this particular techniques is widely employed by dermatologists for superficial skin resurfacing (e.g., microdermabrasion), which are used in the treatment of acne, scars, hyperpigmentaion, and other skin blemishes (CitationBenson 2005).
Microscissuining. It is a process which creates microchannels in the skin by eroding the impermeable outer layers using sharp microscopic metal granules. In addition, MedPharm Ltd. has recently developed a novel dermal abrasion device (D3S) for the delivery therapeutics which are difficult to formulate. This ranges from hydrophilic low molecular weight compounds to biopharmaceuticals. This device is noninvasive and histological studies on human skin have shown that the effects on the stratum corneum are reversible and nonirritating (CitationBenson 2005).
Temperature (Thermophoresis). This method involves the heat treatment of the skin to enhance the drug release. The increased permeation using heat treatment has been attributed to an increase in drug diffusivity in the vehicle and an increase in drug diffusivity in the skin due to increased lipid fluidity. In vivo delivery of drugs like nitroglycerin, testosterone, lidocaine, tetracaine, and fentanyl from transdermal patches with attached heating devices was shown to increase as a result of the elevated temperature at the site of delivery (CitationPatil et al. 2012).
Chemical techniques
As all the molecules for transdermal administration do not possess ideal physicochemical properties, modification of the drugs or addition of vehicles may become necessary to get therapeutic benefits. Various chemical approaches to reduce barrier property are discussed below.
Prodrug. Transdermal delivery of drugs which have unfavorable partition coefficient or solubility could be improved by prodrug (CitationSloan and Wasdo 2003). A promoiety is basically added to increase the transport of drug across the stratum corneum. Thereafter parent drug is released by hydrolysis in the viable epidermis (CitationBarry 2001).
Chemical enhancers. Chemical moieties which reduces the barrier function of stratum corneum are known as chemical enhancers. The enhancer can either disrupt lipid organization and enhance drug diffusion coefficient or interact with keratin in corneocytes, thereby opening the dense protein structure (CitationKumar and Philip 2007).
Formulation technique (using delivery vesicles)
This technique involves the use of vesicular delivery carriers for reducing the barrier properties of skin. Numerous vesicular carriers like liposomes, transferosomes, niosomes, virosomes, etc have proven themselves a potential tool for effective delivery of drugs and bioactives. These delivery vesicles have been discussed in detail in the subsequent part of the review.
Vesicular systems for transdermal delivery of bioactives
The rapid development of transdermal delivery formulations in the last few years is due to certain advantages offered by transdermal administration versus the conventional oral route:
Transdermal delivery circumvents the fluctuations which appear at gastro-intestinal absorption.
It increases the bioavailability of drugs because using the transdermal delivery the active principle enters directly into the circulatory system, bypassing the hepatic metabolism (CitationJain et al. 2003).
It can give a constant, controlled drug input decreasing the variations in drug plasma levels.
It increases the patient compliance by providing a simplified way of administration, minimum risk of trauma or any other injury of tissue (CitationJain et al. 2004).
In the last few years, vesicular systems have been promoted as a mean of sustained or controlled release of drugs and bioactives, because of their certain advantages, for example, lack of toxicity, biodegradation, capacity of encapsulating both hydrophilic and lipophilic molecules, capacity of prolonging the existence of the drug in the systemic circulation by encapsulation in vesicular structures, capacity of targeting the organs and tissues, capacity of reducing the drug toxicity, and increasing its bioavailability (CitationJain et al. 2005). The reason behind the use of vesicles in transdermal drug delivery is based on the fact that they act as drug carriers to deliver entrapped drug molecules across the skin, as well as acts as penetration enhancers because of their composition. Moreover, these vesicles serve as a depot for the sustained release of active compounds in the case of topical formulations, as well as rate-limiting membrane barrier for the modulation of systemic absorption in the case of transdermal formulations (CitationPirvu et al. 2010).
Vesicular carriers used in the transdermal delivery of bioactives can be classified on the basis of chief ingredient used such as lipid-based carrier system and polymer-based carrier system. Lipid-based carriers are further sub-classified into vesicular and non-vesicular lipid carriers. Vesicular lipid carriers include liposomes, niosomes, transferosomes, spingosomes, aquasomes, phtosomes etc, while non-vesicular lipid carriers include lipospheres, solid lipid nanoparticles, nano lipid constructs, micelles, dendrimers, etc. Polymer-based carriers include polymeric nanoparticles, microspheres, dendrimers polymeric micelles, nanocapsule, nanospheres etc. Among these delivery carriers lipid vesicular system have been proven a suitable carrier system for transcutaneous delivery of bioactives (CitationSingh et al. 2012, Citation2011). This review emphasizes on the lipid-based vesicular carriers used for the effective delivery of bioactives. Numerous lipid-based vesicular systems along with their advantage and disadvantage have been discussed in .
Table I. List of vesicular carriers used for transdermal drug delivery.
Lipid-based vesicular system
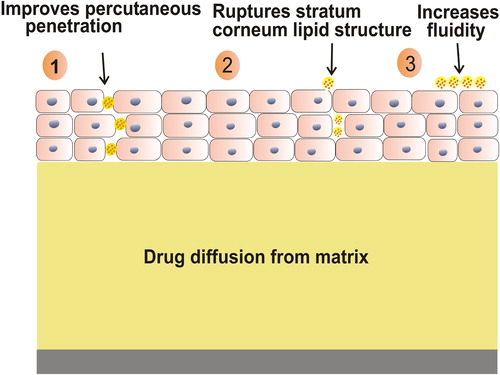
Lipid vesicular systems are a novel means of drug delivery that can improve the bioavailability of encapsulated drug and provide therapeutic activity in a controlled manner for a prolonged time period (CitationSingh et al. 2009). The mechanism of action of lipid vesicular carriers involves:
Percutaneous penetration of lipid vesicular carrier as they are made up of physiological lipids and their nano-size range allows the close contact of the carrier with skin thereby increasing the percutaneous penetration ().
Disruption of stratum corneum lipid structure by vesicular carrier leading to increase in fluidity and thus efficient transportation of carrier and drug across the skin.
Lipid vesicular system is a potential and efficient vehicle to effectively deliver bioactive substances like proteins, vitamins, enzymes, polypeptide, nucleic acid, antibodies as well as other synthetic drugs. The chief benefit of lipid vesicular system is their ability to target drugs to the tissue in the body (CitationRawat et al. 2008). In addition lipid-based carriers are nontoxic, biocompatible, and biodegradable (CitationSingh and Saraf 2008, CitationPradhan et al. 2013). Numerous lipid-based vesicular carriers for the effective delivery of bioactives have been discussed below:
Liposome
Liposomes are microscopical spherical vesicles mainly comprised of one or more lipidic bilayers, separated by aqueous compartments (CitationBarani and Montazer 2008). The methods of preparation includes physical dispersion involving handshaking and non-handshaking methods, solvent dispersion with ethanol and ether injection methods, double emulsion method, reverse phase evaporation method, and stable plurilamellar vesicle method.
Liposomes encapsulate a region on aqueous solution inside a hydrophobic membrane; dissolved hydrophilic solute cannot readily pass through lipid. Hydrophobic chemical can be dissolved into the membrane and in this way liposome can carry both hydrophilic and hydrophobic molecules. To deliver the molecule at the site of action, the lipid bilayer fuse with other bilayer such as cell membrane delivering the liposomal contents (CitationElsayed et al. 2006).
Ethosome
Ethosomes are novel carrier systems used for delivery of drugs possessing low penetration through the biological membrane, mainly skin. Ethosomes are lipid vesicles containing phospholipids, alcohol (ethanol and isopropyl alcohol), in relatively high concentration, and water, which are chiefly used for transdermal delivery of drugs. Although, the exact mechanism for better permeation of drugs into deeper layers of skin from ethosomes is still not clear. Vesicular formulations are composed of phospholipids and high concentration of ethanol. The synergistic effects of these two combinations are responsible for deeper distribution as well as penetration in the lipid bilayers of skin. Ethosome can be prepared by active loading, passive loading, hot method, and cold method (CitationSatyam et al. 2010).
“Ethanol effect” is one such effect which is thought to be a possible mechanism for interaction between skin lipids and ethosomes, whereby intercalation of the ethanol into intercellular lipids increases fluidity and decreases the density of multilayer of lipid. It is followed by the “ethosome effect”, which involves the opening of new pathways due to the malleability and fusion of ethosomes with skin lipids improving inter-lipid penetration and permeation thus resulting into drug release into deep layers of the skin (CitationAkiladevi and Basak 2010).
Niosomes
Niosome is a nonionic surfactant-based liposome. Niosomes may be unilamellar or multilamellar depending on their method of preparation. The niosome is made up of a surfactant bilayer whose hydrophilic ends expose toward outside and inside of the vesicle, whereas the hydrophobic chains face each other within the bilayer. Niosomes contain hydrophilic drugs within the enclosed space of vesicle, whereas hydrophobic drugs get embedded within the bilayer itself. Its method of preparation includes ether injection method, thin-film hydration technique, reverse-phase evaporation technique, and bubble method (CitationMujoriya and Bodla 2011).
Niosomes act as drug depots in the body offering the drug release in a controlled manner through its bilayer thus providing sustained release of the enclosed drug. Niosomes also provide targeted drug delivery as it delivers the drug directly to that particular part of body, where the therapeutic effect is needed. Hence it minimizes the dose required to get administered to achieve the desired therapeutic effect (CitationKarim et al. 2010).
Transferosome
The name is derived from the Latin word “transferre” which means “to carry across” and the Greek word “soma” means “body”. Conclusively it means “carrying body”. This is appropriate for controlled and potentially targeted drug delivery. Method of preparation of transferosome vesicle is nearly similar as liposomes, with a difference that separation of vesicle-associated and free drug is not needed. Two techniques which are generally used to design transferosomes include vortexing-sonication method and rotary evaporation sonication method.
Transferosome when interacts with skin, it searches and exploits hydrophilic pathway or “pores” between the cells in the skin. It opens pores between the cells wide enough to allow the entire vesicle to pass through together with its drugs. Generally transferosome exhibits following mechanism of action.
Transferosomes act as drug vectors, remaining intact after entering the skin.
Transferosomes act as penetration enhancers, disrupting the highly organized intercellular lipids from stratum corneum and therefore facilitating the drug molecules penetration in and across the stratum corneum (CitationHoneywell-Nguyen and Bouwstra 2005).
Sphingosome
It may be defined as concentric, bilayered vesicle where an aqueous volume is entirely enclosed by a membranous lipid bilayer mainly constituted of natural or synthetic sphingolipid. Sphingosomes possess several advantages over the other formulation. The sphingosomes are very stable to acid hydrolysis and have better drug retention characteristics. They are made up of sphingolipid (sphingomyelin) and cholesterol. There are various mechanisms by which small unilamellar sphingosomal vesicles (SUSV's) interact with cell such as stable adsorption, endocytosis, fusion, lipid transfer, etc. (CitationJain 2003).
Stable adsorption: Stable adsorption represents the association between intact vesicles and the cell surface. Such process is mediated by nonspecific electrostatic, hydrophobic or other forces or component present at the vesicles or cell surface.
Endocytosis: Endocytosis is the uptake of intact vesicles by endocytotic vesicles presumably following their delivery to the lysosomal apparatus.
Fusion: Fusion is the simple merging of vesicles bilayer with the plasma membrane bilayer, with release of vesicle content into the cytoplasmic space.
Lipid transfer: Transfer of individual lipid molecule between vesicles and the cell surface without the cell association of aqueous vesicle content.
Pharmacosomes
Pharmacosomes are the colloidal dispersions of drugs covalently bound to lipids and may exist as ultrafine vesicular, micellar or hexagonal aggregates, depending on the chemical structure of the drug–lipid complex. Since the system is formed by linking a drug (pharmakon) to a carrier (soma), they are called pharmacosomes. Pharmacosomes can efficiently pass through biomembranes and offer advantages over the use of other vesicular systems such as transferosome, liposomes, and niosomes. Any drug with a certain cut-off molecular weight can be formulated as pharmacosomes provided it has active functional groups to integrate with the vesicle-forming amphiphilic molecule (CitationBiju et al. 2006).
Pharmacosomes provide an efficient method for delivery of drugs directly to the site of action, resulting into reduction of drug toxicity and no adverse effects. It also provides economic therapy by increased bioavailability of drug, especially for poorly soluble drugs (CitationKavitha et al. 2010). Some of the advantages of pharmacosomes are discussed below:
Pharmacosomes may incorporate both hydrophilic and lipophilic drugs. The aqueous solution of these amphiphiles shows concentration dependent aggregation.
High entrapment efficiency is achieved, because drug itself forms vesicles in conjugation with lipids by covalent linkage.
Loss due to leakage of drug does not occur as the drug is covalently linked, however, loss may occur by hydrolysis.
Virosomes
These are spherical, unilamellar vesicles having a mean diameter of 150 nm. Essentially, they are empty influenza virus envelopes, containing the genetic material of the source virus but devoid of the nucleocapsid. Virosomes are not capable to replicate but are considered pure fusion- active vesicles. Unlike other lipid vesicles, they contain phospholipid bilayer membrane intercalated with functional viral envelope glycoproteins, influenza virus hemagglutinin (HA), and neuraminidase (NA). Characteristics of virosomes essentially depend on bilayer components chosen for preparation of virosomes. Modification of the content or type of lipids membrane could be used to optimize virosomes to achieve maximum incorporation of the drug or the best physiological effect. Numerous ligands like cytokines, peptides, and monoclonal antibodies can be adopted on the virosomal surface. Moreover, tumor-specific monoclonal antibody-fragments (Fab) could be linked to virosomes to achieve targeting for selected tumor cells.
All the above discussed vesicular carriers possess a great potential for transdermal delivery of bioactives. Meritorious role of these carriers for transdermal delivery of numerous bioactives have been discussed in .
Table II. List of vesicular carriers used for transdermal delivery of bioactives.
Applications of vesicular system for transdermal delivery of bioactives
Delivery of growth factors
Jeon and co-workers prepared nano-liposomes using high-pressure homogenization method. They reported that nano-liposomes encapsulated recombinant human epidermal growth factor system, when applied topically enhanced percutaneous delivery of recombinant human epidermal growth factor (CitationJeon et al. 2012). In another study, Foldvari and co-workers investigated the use of liposome for the delivery of interferon-α into human skin (CitationFoldvari et al. 2010). Jeschke and co-workers demonstrated that liposome-mediated integrin growth factor-I gene transfer enhanced the pathophysiology of a thermal injury by transdermal route (CitationJeschke et al. 1999). Brown and co-workers formulated liposomes entrapped growth factor for the treatment of incision wounds in mice by transdermal route. The liposomal formulation gave a transient increase in tensile strength, compared with blank liposomes. They reported that this formulation induced a sustained release of epidermal growth factor, which is very important for effective therapy (CitationBrown et al. 1988).
Delivery of antibiotics
Manosroi and co-workers prepared gallidermin-loaded anionic niosomes. They suggested that gallidermin-loaded niosomes is a superior topical antibacterial formulation because of the high accumulation in the skin with no risk of systemic effects. This formulation gave antibacterial activity against Propionibacterium acnes and Staphylococcus aureus (CitationManosroi et al. 2010). In another study by Grayson and co-workers developed gentamycin-loaded multivesicular liposomes as a prophylactic anti-infective treatment for surgical wounds. The liposomal formulation provides local sustained action. Evaluation of bioburden reduction 48 hr later showed that the liposomal formulation was significantly superior to empty liposomes for the treatment of wounds (CitationGrayson et al. 1993). Margalit and co-workers examined the response of infected wounds to treatment with cefazolin-encapsulating bioadhesive liposomes were studied in full thickness wounds in mice infected with Staphylococcus aureus. After 3 days treatment, the wound bacterial counts with cefazolin- encapsulating bioadhesive liposomes were down 100-fold from untreated controls, to the colonization infection boundary. Although all these studies can be taken as encouragement for exploring liposomes as drug delivery systems for topical treatment of wounds and burns (CitationMargalit et al. 1992).
Delivery of enzymes
Manosroi and co-workers developed papain containing niosomes and nanosphere and compared the penetration of papain with gel formulations. They reported that niosomes enhanced the transdermal absorption of papain in rat skin and improved scar reduction in rabbit ear model beneficial for the development of topical products for scar treatment (CitationManosroi et al. 2012). In another study, Simoesa and co-workers developed carriers for noninvasive administration of biologically important antioxidant enzymes Cu, Zn-superoxide dismutase, and catalase. They demonstrated the effect of cholate on solubilization and permeability of simple and protein-loaded phosphatidylcholine/sodium cholate mixed aggregates designed to provoke transdermal delivery of macromolecules (CitationSimoes et al. 2004).
Delivery of peptides
The transdermal delivery of peptides face enormous challenges, and at the same time has very significant potential for the treatment of both localized and systemic diseases. Cevc and co-workers formulated transferosomes containing insulin and they reported that this formulation increased the transdermal delivery of insulin (CitationCevc et al. 1995). Godin and Touitou designed erythromycin ethosomes and showed their antibacterial efficiency in vitro and in vivo. They reported that ethosomes are efficient carriers for drug delivery within the deep skin strata for eradication of staphylococcal infections (CitationGodin and Touitou 2005). In other study, Egbaria and co-workers formulated liposome encapsulated interferon by dehydration–rehydration method, which was twice as effective as was topical application of liposomes prepared by the reverse-phase evaporation method with respect to their ability to deposit interferon into the skin strata where the basal cell layers reside. They showed that the effects of liposomal composition and method of preparation on the ability of the formulation reduced the lesion scores in the cutaneous herpes simplex virus type 1 guinea pig model (CitationEgbaria et al. 1990). Malakar and co-workers developed transferosomal gel containing insulin using reverse-phase evaporation method for painless insulin delivery for use in the treatment of insulin-dependent diabetes mellitus. They reported that in vivo study of optimized transferosomal gel in alloxan-induced diabetic rat has demonstrated prolonged hypoglycemic effect in diabetic rats over 24 h after transdermal administration (CitationMalakar et al. 2012).
Conclusion
Transdermal delivery of drugs can be the promising area provided the problems associated with it are properly resolved. Lipid vesicular system can provide the novel solution for the problems related to transport of drugs and bioactives. These vesicles possess a great prospective in the efficient noninvasive delivery of wide range of drugs and bioactives, which includes large molecules like peptides, hormones and antibiotics, other bioactives with poor penetration due to unfavorable physicochemical characters, drugs for immediate and targeted action, etc. Enhanced delivery of bioactive molecules through the skin by means of vesicular carrier opens new challenges and opportunities for the development of novel improved therapies.
Acknowledgment
The authors are thankful to Director, University Institute of Pharmacy, Pt. Ravishankar Shukla University, Raipur (C.G.) India for this study and UGC-MRP-41-748-2012, UGC-RA 70-371/2012, DST-FIST for financial assistance relating to this work.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Akiladevi D, Basak S. 2010. Ethosomes: A noninvasive approach for transdermal drug delivery. Int J Curr Pharm Res. 2:1–4.
- Barani H, Montazer M. 2008. A review on applications of liposomes in textile processing. J Liposome Res. 18:249–262.
- Barry BW. 2001. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci. 14:101–114.
- Benson HAE. 2005. Transdermal drug delivery: penetration enhancement techniques. Curr Drug Deliv. 2:23–33.
- Biju SS, Talegaonkar S, Mishra PR, Khar RK. 2006. Vesicular systems: an overview. Indian J Pharm Sci. 68:141–153.
- Brown GL, Curtsinger LJ, White M, Mitchell RO, Pietsch J, Notelquist R, et al. 1988. Acceleration of tensile strength of incisions treated with EGF and TGF-beta. Ann Surg. 208:788–794.
- Cevc G, Blume G, Schatzlein A. 1995. Transdermal drug carriers: basic properties, optimization and transfer efficiency in the case of epicutaneously applied peptides. J Control Release. 36:3–16.
- Das MK, Palei NN. 2011. Sorbitan ester niosomes for topical delivery of rofecoxib. Indian J Exp Biol. 49:438–445.
- Denet AR, Vanbever R, Preat V. 2004. Skin electroporation for transdermal and topical delivery. Adv Drug Deliv Rev. 56:659–674.
- Dhote V, Bhatnagar P, Mishra PK, Mahajan SC, Mishra DK. 2012. Iontophoresis: a potential emergence of a transdermal drug delivery system. Sci Pharm. 80:1–28.
- Egbaria K, Ramachandran C, Kittayanond D, Weiner N. 1990. Topical delivery of liposomally encapsulated interferon evaluated by in vitro diffusion studies. Antimicrob Agents Chemother. 34:107–110.
- El-Nabarawi MA, Bendas ER, El Rehem RTA, Abary MYS. 2013. Transdermal drug delivery of paroxetine through lipid-vesicular formulation to augment its bioavailability. Int J Pharm. 443: 307–317.
- Elsayed MMA, Abdullah OY, Naggar VF, Khalafallah NM. 2006. Deformable liposome and ethosome: Mechanism of enhanced skin delivery. Int J Pharm. 322:60–66.
- Foldvari M, Baca-Estrada ME, Zhihong H, Hu J, Poku SA, King M. 2010. Dermal and transdermal delivery of protein pharmaceuticals: lipid-based delivery systems for interferon α. Biotechnol Appl Biochem. 3:154–156.
- Gill B, Singh J, Sharma V, Kumar SLH. 2012. Emulsomes: a emerging vesicular drug delivery system. Asian J Pharm. 6:87–94.
- Godin B, Touitou E. 2003. Ethosomes: new prospects in transdermal delivery. Ther Drug Carriers Syst. 20:63–102.
- Godin B, Touitou E. 2005. Erythromycin ethosomal systems: physicochemical characterization and enhanced antibacterial activity. Curr Drug Deliv. 2:269–275.
- Gonzalez-Rodriguez ML, Mouram I, Cozar-Bernal MJ, Villasmil S, Rabasco AM. 2012. Applying the Taguchi method to optimize sumatriptan succinate niosomes as drug carriers for skin delivery. J Pharm Sci. 101:3845–3863.
- Grayson LS, Hansbrough JF, Zapata-sirvent RL, Kim T, Kim S. 1993. Pharmacokinetics of DepoFoam gentamicin delivery system and effect on soft tissue infection. J Surg Res. 55:559–564.
- Gupta A, Aggarwal G, Singla S, Arora R. 2012. Transfersomes: a novel vesicular carrier for enhanced transdermal delivery of sertraline: development, characterization and performance evaluation. Sci Pharm. 80:1061–1080.
- Gupta M, Vaidya B, Mishra N, Vyas SP. 2011. Effect of surfactants on the characteristics of fluconazole niosomes for enhanced cutaneous delivery. Artif Cells Nanomed Biotechnol. 39:376–384.
- Honeywell-Nguyen PL, Bouwstra JA. 2005. Vesicles as a tool for transdermal and dermal delivery. Drug Disco Today Technol. 2:67–74.
- Jain NK. 2003. Advances in Controlled and Novel Drug Delivery. New Delhi, India: CBS Publishers,. 326:273–278.
- Jain S, Jain P, Umamaheshwari RB, Jain NK. 2003. Transfersomes: a novel vesicular carrier for enhanced transdermal delivery: development, characterization and performance evaluation. Drug Dev Ind Pharm. 29:1013–1026.
- Jain S, Sapre R, Tiwary AK, Jain NK. 2005. Proultraflexible lipid vesicles for effective transdermal delivery of levonorgestrel: Development, characterization and performance evaluation. AAPS Pharm SciTech. 6:513–522.
- Jain S, Umamaheshwari RB, Bhadra D, Jain NK. 2004. Ethosomes: A novel vesicular carrier for enhanced transdermal delivery of an antiHIV agent. Indian J Pharm Sci. 66:72–81.
- Jeon SO, Hwang HJ, Oh DH, Seo JE, Chun KH, Hong SM, et al. 2012. Enhanced percutaneous delivery of recombinant human epidermal growth factor employing nano-liposome system. J Microencapsul. 29:234–241.
- Jeschke MG, Barrow RE, Hawkins HK, Yang K, Hayes RL, Lichtenbelt BJ, et al. 1999. IGF-1 gene transfer in thermally injured rats. Gene Ther. 6:1015–1020.
- Jones DS, Moss GP. 2010. Themed issue: recent advances in transdermal drug delivery. J Pharm Pharmacol. 62:669–670.
- Kanitakis J. 2002. Anatomy, histology and immunohistochemistry of normal human skin. Eur J Dermatol. 12:390–401.
- Karim KM, Mandal AS, Biswas N, Guha A, Chatterjee S, Behera M, Kuotsu K. 2010. Niosome: A future of targeted drug delivery systems. J Adv Pharm Technol Res. 1:374–380.
- Kavitha D, Sowjanya N, Panaganti S. 2010. Pharmacosomes: an emerging vesicular system. Int J Pharm Sci Rev Res. 5:168–171.
- Khan A, Sharma PK, Visht S, Malviya R. 2011. Niosomes as colloidal drug delivery system: a review. J Chronotherapy Drug Deliv. 2:15–21.
- Khositsuntiwong N, Manosroi A, Gotz F, Werner RG, Manosroi W, Manosroi J. 2012. Enhancement of gene expression and melanin production of human tyrosinase gene loaded in elastic cationic niosomes. J Pharm Pharmacol. 64:1376–1385.
- Kulkarni PR, Yadav JD, Vaidya KA, Gandhi PP. 2011. Transferosomes: an emerging tool for transdermal drug delivery. Int J Pharm Sci Res. 2:735–741.
- Kumar R, Philip A. 2007. Modified transdermal technologies: breaking the barriers of drug permeation via the skin. Trop J Pharm Res. 6:633–644.
- Kumar R, Kumar S, Jha SS, Jha AK. 2011. Vesicular system-carrier for drug delivery. Der Pharmacia Sinica. 2:192–202.
- Malakar J, Sen SO, Nayak AK, Sen KK. 2012. Formulation, optimization and evaluation of transferosomal gel for transdermal insulin delivery. Saudi Pharm J. 20:355–363.
- Manosroi A, Chankhampan C, Manosroi W, Manosroi J. 2013. Transdermal absorption enhancement of papain loaded in elastic niosomes incorporated in gel for scar treatment. Eur J Pharm Sci. 48:474–483.
- Manosroi A, Chankhampan C, Manosroi W, Manosroi J. 2012. Transdermal absorption enhancement of papain loaded in elastic niosomes incorporated in gel for scar treatment. Eur J Pharm Sci. 48:474–483.
- Manosroi A, Khanrina P, Lohcharoenkala W, Wernerc RG, Gotzd F, Manosroie W, Manosroia J. 2010. Transdermal absorption enhancement through rat skin of gallidermin loaded in niosomes Pharmaceutical Nanotechnology. Inter J Pharmaceutics. 392:304–310.
- Merino G, Kalia YN, Delgado-Charro MB, Potts RO, Guy RH. 2003. Frequency and thermal effects on the enhancement of transdermal transport by sonophoresis. J Control Release. 88:85–94.
- Mujoriya RZ, Bodla RB. 2011. Niosomes challenge in preparation for pharmaceutical scientist. Int J Appl Pharm. 3:11–15.
- Murthy SN, Sammeta SM, Bowers C. 2010. Magnetophoresis for enhancing transdermal drug delivery: mechanistic studies and patch design. J Control Release. 148:197–203.
- Ogura M, Paliwal S, Mitragotri S. 2008. Low-frequency sonophoresis: current status and future prospects. Adv Drug Deliv Rev. 60:1218–1223.
- Patel SS, Patel MJ, Patel NM. 2010. Need, development and application of virosomal system in medicine. Int J Pharm Sci Nanotechnol. 3:1065–1074.
- Patil PM, Chaudhri PD, Patel JK, Kedar KA, Katolkar PP. 2012. Recent trends in challenges and opportunities of transdermal drug delivery system. Int J Drug Dev Res. 4:39–50.
- Pirvu CD, Hlevca C, Ortan A, Prisada R. 2010. Elastic vesicles as drugs carriers through the skin. Farmacia. 58:128–135.
- Prasanthi D, Lakshmi PK. 2012. Vesicles-mechanism of transdermal permeation: a review. Asian J Pharm Clin Res. 5:18–25.
- Prausnitz MR, Mitragotri S, Langer R. 2004. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 3:115–124.
- Margalit R, Okon M, Verushalmi N, Avidor E. 1992. Bioadhesive liposomes for topical drug. Delivery: molecular and cellular studies. J Control Release. 19:275.
- Pradhan M, Singh D, Singh MR. 2013. Novel colloidal carriers for psoriasis: current issues, mechanistic insight and novel delivery approaches. J Control Release. 170:380–395.
- Rajan R, Vasudevan DT. 2012. Effect of permeation enhancers on the penetration mechanism of transfersomal gel of ketoconazole. J Adv Pharm Technol Res. 3:112–116.
- Rawat M, Singh D, Saraf S, Saraf S. 2008. Lipid carriers: a versatile delivery vehicle for proteins and peptides. Yakugaku Zasshi. 128:269–280.
- Sammeta SM, Repka MA, Murthy SN. 2011. Magnetophoresis in combination with chemical enhancers for transdermal drug delivery. Drug Dev Ind Pharm. 37:1076–1082.
- Saraf S, Paliwal S, Saraf S. 2011. Sphingosomes a novel appoach to vesicular drug delivery. Int J Cur Sci Res. 1:63–68.
- Satyam G, Shivani S, Garima G. 2010. Ethosomes: a novel tool for drug delivery through the skin. J Pharm Res. 3:688–691.
- Semalty A, Semalty M, Rawat BS, Singh D, Rawat MSM. 2009. Pharmacosomes: The lipid based novel drug delivery system. Expert Opin Drug Deliv. 6:599–612.
- Simoes SI, Marques CM, Cruz ME, Cevc G, Martins MB. 2004. The effect of cholate on solubilisation and permeability of simple and protein-loaded phosphatidylcholine/sodium cholate mixed aggregates designed to mediate transdermal delivery of macromolecules. Eur J Pharm Biopharm. 58:509–519.
- Singh D, Dubey P, Pradhan M, Singh MR. 2013. Ceramic nanocarriers: versatile nanosystem for protein and peptide delivery. Expert Opin Drug Deliv. 10:241–259.
- Singh D, Singh MR, Saraf S, Saraf S. 2011. Development of delivery cargoes for debriding enzymes effective in wound healing. Trends Appl Sci Res. 6:863–876.
- Singh MR, Pradhan K, Singh D. 2012. Lipid matrix systems with emphasis on lipid microspheres: Potent carriers for transcutaneous delivery of bioactives. Curr Drug Deliv. 9:243–254.
- Singh MR, Saraf S. 2008. Lipospheres: emerging carriers for proteins and peptides. Int J Pharm Sci Nanotech. 13:207–213.
- Singh M.R, Singh D, Swarnlata S. 2009. Development and in vitro evaluation of polar lipid based lipospheres for oral delivery of peptide drugs. Int J Drug. 1:15–26.
- Singodia D, Verma A, Khare P, Dube A, Mitra K, Mishra PR. 2012. Investigations on development of amphotericin B liposomes for industrial applications. J Liposome Res. 22:8–17.
- Sloan KB, Wasdo S. 2003. Designing for topical delivery: prodrugs can make the difference. Med Res Rev. 23:763–793.
- Subedi RK, Oh SY, Chun MK, Choi HK. 2010. Recent advances in transdermal drug delivery. Arch Pharm Res. 33:339–351.
- Tadwee IK, Gore S, Giradkar P. 2012. Advances in topical drug delivery system: a review. Int J Pharm Res Allied Sci. 1:14–23.
- Tiwary AK, Sapra B, Jain S. 2007. Innovations in transdermal drug delivery: formulations and techniques. Recent Patents Drug Deliv Formul. 1:23–36.
- Tucker IG. 2010. The 12th conference on the formulation and delivery of bioactives. Ther Deliv. 1:25–27.
- Uchino T, Lefeber F, Gooris G, Bouwstra J. 2013. Characterization and skin permeation of ketoprofen-loaded vesicular systems. Eur J Pharm Biopharm. [Epub ahead of print].
- Verma P, Pathak K. 2012. Nanosized ethanolic vesicles loaded with econazole nitrate for the treatment of deep fungal infections through topical gel formulation. Nanomed Nanotechnol Biol Med. 8:489–496.