Abstract
The aim of the present study is to design a mucoadhesive nano-carrier system which retains at the site of application and maximizes the therapeutic potential of anticancer drug as well as reduces their systemic side effects. In the present study PVA nanofibers of Docetaxel were prepared using electrospinning machine. The resulting nanofibers were characterized through various parameters such as surface morphology, drug loading, in-vitro drug release, tensile strength, mucoadhesiveness, drug permeability, degree of swelling and anticancer activities against selective cell lines to establish their therapeutics potential. On the basis of various evaluation results, we may conclude that the current approach comprising polymeric nanofibers can be successfully used for local delivery of anticancer drug.
Introduction
Buccal cancer is a subtype of head and neck cancer involving the tissue out growth in the cheek of the mouth (CitationWestra 2009). Several attempts have been made for local drug delivery via the mucous membranes lining of oral cavity to improve the therapeutics potential of the drugs and reduce their systemic side effects. Wu et al. investigated the therapeutic potential of Fenretinide mucoadhesive patch along with propylene glycol and menthol as permeation enhancer for oral cancer (CitationWu et al. 2012). Mallery et al. studied the anti-cancer activity of mucoadhesive gel of freeze dried black raspberries. Studies pertaining to mucoadhesive formulation for transmucosal drug delivery show promising results due to their affinity to be retained at the site of application and providing a continuous drug delivery at the local site (CitationChopra et al. 2006). Further transmucosal delivery of drug offer an alternative approach for non-invasive delivery of therapeutics with improved bioadhesion and patient compliance (CitationMathias and Hussain 2010). Mucoadhesive properties of the carrier system are the characteristic feature of its surface properties, more particularly relating to their contact surface area. Electrospun nanofibers provide large surface area thereby improving the mucoadhesive strength of the carrier system.
Electrospinning is a highly versatile and effective technique which uses a high electrical charge to draw ultrafine fibers from a melt or polymer solution (CitationGarg et al. 2012). Their unique properties which include microporous nature, high surface area, high drug loading capacity and mucoadhesive nature with sufficient mechanical strength make them a suitable candidate for controlled drug delivery at the site of application. Process parameters like applied voltage, flow rate, capillary collector distance and solution parameters like polymer concentration, viscosity, solution conductivity and solvent vapor pressure greatly influence the morphology and properties of the nanofibers (CitationCelebioglu and Uyar 2013, CitationChai and Wu 2013). In the present study polyvinyl alcohol (PVA) is chosen as a polymer due to its biodegradable nature, non-toxic, non-carcinogenic, electrospinnable and mucoadhesive nature which makes it a valuable candidate for buccal drug delivery.
Docetaxel is well known mitotic inhibitor for buccal cancer (CitationPircher et al. 2013). It is available only as I.V. formulation in the market which is facing problems of extravasations, vein inflammation and other side effects of chemotherapy. So to overcome these challenges, we are designing the delivery system for local delivery of anticancer drug. As the Docetaxel is lipophilic in nature, it binds to the local tissue and is retained in the tissue for longer period of time for their therapeutic action. The present study is oriented to maximize the therapeutic outcome of the anticancer drug and to reduce side effects.
Materials and methods
Materials
Docetaxel was supplied by United Biotech Pvt Ltd India as gift sample. PVA was purchased from Central Drug House (P) Ltd. All other chemicals used were of analytical grade.
Fabrications of PVA nanofibers
PVA solutions (6–10%) were prepared by dissolving required amount of PVA in 20 ml of distilled water with constant stirring (overnight) using magnetic stirrer to obtain the clear solution. The prepared solution was filled in a 5-ml syringe having needle with a tip diameter of 22 gauges (inner diameter 0.413 mm) and setup in the electrospinning apparatus (E-spin NANO, IIT Kanpur) (CitationZeng et al. 2005). A syringe pump was used to deliver the polymer solution at a rate from 0.25 to 0.35 ml/h by varying the voltage from 10 to 14 kV with a fixed needle to collector distance of 18 cm. The nanofibers were collected on Collector which was covered with an aluminum foil.
Fabrications of drug loaded nanofibers
Drug loading was carried out by passive loading technique (CitationMaretschek et al. 2008). Docetaxel was dissolved in 1 ml ethanol and added to the polymeric solution prior to electrospinning. The mixture of drug and PVA solution (containing 5% w/v sucrose as a sweetening agent) was stirred for 24 h at room temperature to obtain a clear homogenous solution. The solution was then electrospun using the optimized spinning parameters (discussed above). The drug loaded nanofibers were collected and dried overnight under vacuum at room temperature.
Nanofiber characterization
Morphology analysis
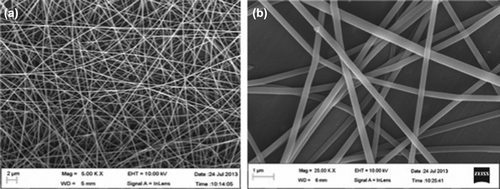
Surface morphology of the nanofibers and their diameter was examined by scanning electron microscopy (EVO M-10, Carl Zeiss, Thornwood, NY, United States), which provides a photomicrograph image of sample by scanning it with a beam of electrons in a raster scan pattern (CitationMisra et al. 2007). This mode provides high-resolution imaging of fine surface morphology of the sample. In this technique, 5–10 different positions on the fiber mat were tested to measure the average diameter of the electrospun nanofibers. Small portion of the fiber mat was placed on the SEM sample holder and sputter coated with platinum. Accelerating voltage of 10 kV was employed to take the SEM images.
FT-IR analysis
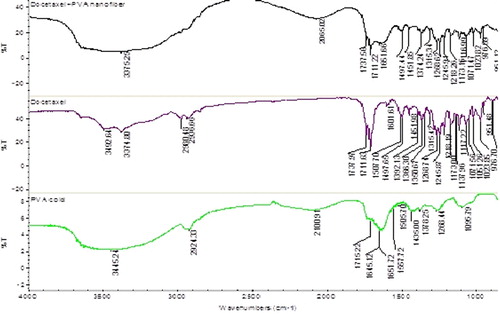
The infrared spectrum of the PVA, drug and drug loaded nanofibers were observed in a range between 4000 and 800 cm− 1 using IR spectrometer (Perkin-Elmer spectrum RX1, Canton, Massachusetts, USA). Small pieces of drug loaded electrospun nanofibers was cut and mixed with KBr to make sample pellets. Measurements were taken with a resolution of 2 cm− 1.
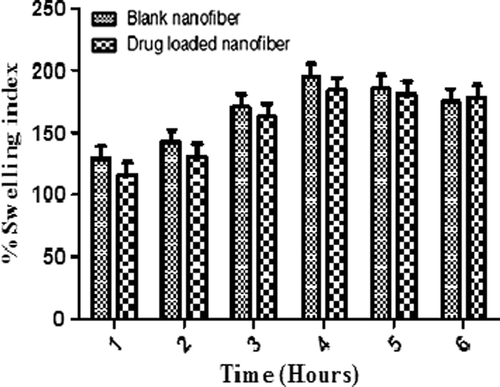
Degree of swelling
An Eq. (1) is used to determine the % degree of swelling of the optimized nanofibers. The test was carried out in PBS 6.8, at 37°C for 1–6 h. Swelling index of unloaded and drug loaded nanofibers was compared.
Where, M is the weight of swollen nanofiber sample which is wiped dry with filter paper, Md is the dried mass of immersed sample in buffer medium, measured by drying the swollen nanofibers mats in an oven at 40°C until constant weight was reached.
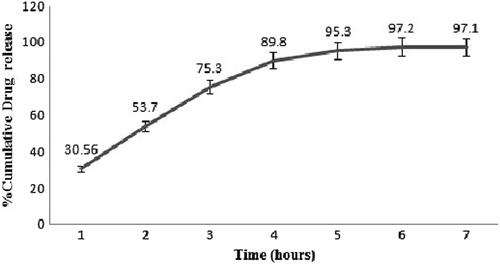
In-vitro Docetaxel release studies
The in-vitro release study of the optimized nanofibers was carried out by placing nanofibers (1 × 1 cm2) in 100 ml PBS solution (pH 6.8) with 0.5% Tween 20, at 37°C at 100 rpm in a thermostatically shaking incubator. Samples were taken at fixed intervals of time from the solution after 1, 2, 3, 4, 5, 6 and 7 h. The amount of drug present in the samples was determined by using UV-visible spectrophotometer at a λmax of 231 nm followed by the addition of fresh media to maintain sink conditions.
Differential scanning calorimetry
Differential scanning calorimetry (DSC) is a thermo analytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. DSC of plain unloaded nanofibers and drug loaded nanofibers mats was performed to confirm the stability of polymers in the formulation. DSC measurement was done with a sample weight of 2–3 mg under a nitrogen atmosphere (60 ml/min) with a scanning speed of 10°C/min. The samples were heated from 0 to 350°C at a rate of 10°C/min.
Drug entrapment efficiency
To calculate the entrapment efficiency of (1 cm2) area of drug loaded nanofibers, the defined size of polymeric nanofibers was dried at 40°C and dissolved in the solvent in which drug and polymer is soluble. The amount of drug in the solutions was calculated by UV spectrophotometer (CitationGarg et al. 2013). The entrapment efficiency was calculated by using the following equation:
Mucoadhesive test
The mucoadhesive detachment force of the nanofibers from goat mucosa is measured using Brookfield texture analyzer (CT3, Middleboro, MA, United States). Firstly, goat mucosa was placed on one of the attachment of the device and nanofibers with 1.2 cm2 diameter were stuck to the other attachment of the analyzer with the help of double sided tape. Both of these were made in contact by applying a weight of 1 kg for 15 s after base location (CitationParnami et al. 2013). The force needed for the fibers to detach from the mucosa was calculated.
MTT assay
The cytotoxic effects of plain Docetaxel and Docetaxel loaded nanofibers were evaluated by MTT assay using T47D Breast carcinoma cell lines. The cultured cells were plated in 24-well culture dishes and incubated for 24 h at 37°C to promote their adhesion to the plates. Formulation with different concentration was added to the plate with one vehicle control. After 4 h of incubation MTT assay was added and reading was taken at 595 nm using iMark Micro plate Absorbance Reader (Bio-Rad, Hercules, California, US). Cell viability (%) was calculated by taking the ratio of absorbance of treated cells to that of control cells.
Tensile strength of nanofibers
Nanofibers (8 cm length and 4 cm width) were placed in vertical position along the axis of the Brookfield texture analyzer with the help of clamps. The instrument is then allowed to run with a minimum sensitivity force of 3 gm/cm2 after base location. The force required to split the mat in to two pieces was determined (CitationSharma et al. 2011).
Permeability studies
Nanofibers (1 cm2) were applied on the goat mucosal membrane and mounted on the Franz diffusion cell, and the top cell was clamped and covered with a parafilm to prevent the evaporation of the receptor medium. The receptor medium was filled with phosphate buffer (pH 6.8) which was maintained at constant temperature by a circulating water bath. The temperature was maintained at 37°C in all diffusion studies. At predetermined time intervals, the samples were withdrawn from the receptor compartment and replaced by an equal volume of fresh buffer solution. The samples were analyzed by a UV spectrophotometer.
Results and discussion
PVA nanofibers
The process and formulation parameters were carefully optimized to achieve the desired characteristic in electrospun nanofibers necessary for local and controlled drug delivery. Parameters which were selected in this process including concentration of polymer, applied voltage, flow rate and tip to collection distance (TCD) were optimized in the process of electrospinning. In this study the polymer solution (PVA) was electro spun under the applied electrical potential of 10–14 kv over a tip to collector distance of 18 cm and a flow rate of 0.25–0.35 ml/h. At 8% polymer concentration under the applied electric potential of 12 kV over a TCD of 18 cm optimum fibers with an average diameter of 180–200 nm were produced (CitationWon et al. 2012). Below 8% concentration of PVA, fiber density was very less along with the formation of beads at regular intervals because of low concentration of PVA and higher conductivity of polymeric solution (CitationSong et al. 2005) but as the concentration of PVA was increased there was a gradual increase in the fiber density with no droplet formation till a concentration of 8%, beyond this constant droplet formation was observed at 10%. With an increase in the PVA concentration there is an increase in the electrical force exerting on the jet, which in turn increases the mass throughput but results in the formation of drops. The parameters were optimized on the basis of physical observation of fibers under motic microscope and the morphology of blank optimized fibers was observed using scanning electron microscope.
Nanofibers of Docetaxel
When the optimized parameters of unloaded PVA nanofibers were used for the fabrication of drug loaded nanofibers, the formation of beads happens due to increase in the viscosity and poor conductivity of the resultant drug polymer solution. Therefore, the spinning parameters are further optimized for fabrication of drug loaded nanofibers parameters, like TCD and applied voltage, to get bead free, continuous, thin and high density nanofibers. Non polar nature of the drug decreases the conductivity of polymer solution and, moreover, the presence of ethanol facilitates the penetration of water into the polymeric structure which leads to decrease in aqueous volume in the continuous phase. In addition, the presence of 5% w/v sucrose also influences physicochemical properties of the polymer solution which in turn decreases the overall polarity and conductivity of the resultant solution. So the solution requires a higher potential to form an electrical jet under the specified spinning conditions. Smooth and uniform drug loaded nanofibers were obtained at higher applied voltage, i.e., 16 KV against 12 KV of unloaded polymeric nanofibers. The optimized spinning conditions for drug loaded nanofibers were found to be 16 KV applied voltage and 14 cm TCD. From these results, we may infer that a higher applied voltage and lower TCD would increase the voltage gradient across the electrode to address the poor conductivity issues of resultant drug polymer solution and would help to develop jet under the above conditions. shows optimization parameters and microscopic observations of different trials of nanofibers.
Table I. Optimization parameters and microscopic observations of different trials.
Morphology of optimized formulation
The SEM images of the unloaded and drug loaded nanofibers shows that the nanofibers has smooth surface, uniformity, non-beaded having average diameter of 180–200 nm in unloaded nanofibers and 190–220 nm in drug loaded nanofibers (). The larger diameter of drug loaded nanofibers attributed to partial congealing of polymer induced by ethanol (CitationHosseini et al. 2012) during the preparation of drug polymer solution. Moreover the partial congealing of the polymer increases polymer density per unit area of the polymer solution and also subsidized the water evaporation from the electrospun nanofibers under the defined spinning condition. Further the higher potential at fixed TCD of 14 cm increases the mass throughput of the polymer solution leading to larger diameter of drug loaded nanofibers.
Drug-polymer compatibility studies
IR spectra of PVA and drug as well as its corresponding electrospun nanofibers show that there is no shift in the characteristic peaks of drug and polymer. There is broadening of the 3375−1 peak in the IR spectrum of drug loaded nanofibers than the Docetaxel alone. This is due to the presence of more tertiary-OH groups in PVA. shows that there was no change in wave number of functional groups indicating the stability of nanofibers with the polymer and hence it concludes that there are no drug–polymer compatibility issues.
Degree of swelling
Release of the drug from the nanofiber mat depends upon the degree of swelling as it determines the release pattern of the drug from the Nanofibrous mat. shows the graphical representation in degree of swelling of blank and drug loaded nanofibers at different time intervals. The swelling of unloaded PVA nanofibers is found to be higher than drug loaded nanofibers. This could be attributed to the higher surface area of unloaded nanofibers. Moreover the partial congealing of the polymer in the drug loaded nanofibers restricts the water permeability. This allows controlled release of drug present from the core of polymeric nanofibers. However, the swelling index of both polymeric nanofibers and drug loaded nanofibers was decreased after 4 h indicating the erosion of the polymer. Drug loaded polymeric nanofibers show slow erosion rate, which could be attributed to a higher polymer density per unit mass of the drug loaded nanofibers.
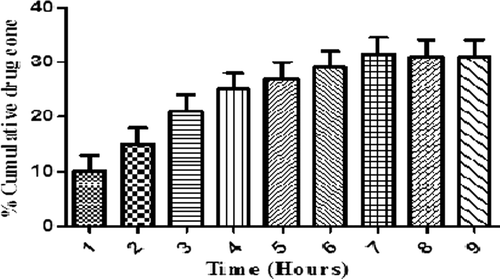
In-vitro drug release studies
depicts the cumulative drug release study of the optimized formulation showing initial burst release of the drug of about 30.6% in first hours. This could be attributed to the drug release from the surface of nanofibers associated to the high surface to volume ratio of polymeric nanofibers. Initially burst release is due to slow drug release could be due to the partial congealing of polymer which restricts the diffusion of water into the polymeric structure, thereby reducing the drug release. Further, the % cumulative release of 97.1% observed after 6 h was attributed to the drug release governed by both erosion and diffusion process.
Drug entrapment efficiency
The drug entrapment efficiency of the optimized formulation was found to be 95%. During the collection of the fiber over the collector, all nanofibers were not collected at the collector site; some of the fiber got collected on the other conducting surfaces of the equipment. This happens due to the decreasing potential drainage capacity of the collector with the fiber deposition on the surface of collector leading to distraction of fibers from the collecting surface.
Mucoadhesive test
Mucoadhesive detachment force for drug loaded nanofibers were found to be 239 gm/cm2 and for unloaded nanofibers 299 gm/cm2. This could be attributed to the diameter of nanofibers where the plain nanofibers possess smaller fiber diameter than drug loaded nanofibers. As a result of that the fiber densities per unit mass is more in case of unloaded nanofibers, in turn increasing the overall surface area of the polymeric nanofibers than that of drug loaded nanofibers which serve as a rate limiting factor in determining the mucoadhesive strength.
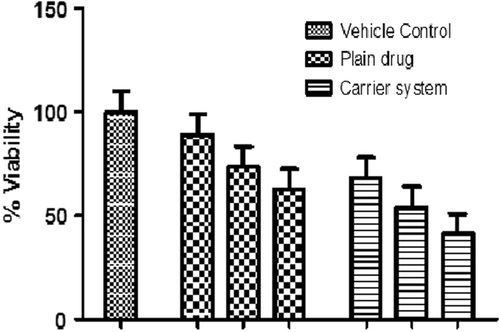
MTT assay
shows the comparative cytotoxic activity of the developed formulation. Results show that the carrier system exhibits higher cytotoxicity in comparisons to plain drug at same concentration. This could be attributed to the higher solublization and permeation of the drug in the developed carrier system due to PVA. Further, the DSC results interpret that the drug is present in the amorphous state in the developed carrier system, which can improve drug solubility, and therefore, the combined effect of PVA and physical state of the drug improve drug permeability and in turn produce higher cytotoxicity. Higher cytotoxicity of the developed carrier system could give an impetus that a lesser drug concentration would require to achieve desired therapeutic outcome with minimum side effects.
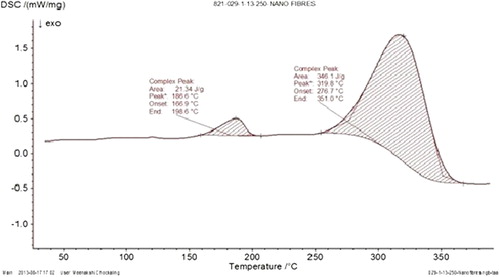
DSC of drug loaded nanofibers
DSC of drug loaded nanofibers represents the peak at 165.9°C and maximum peak value at 186.6°C. indicated that the thermal stability of drug as the native drug shows phase transition temperature at 166.5°C. This could be attributed to the amorphous state of the drug in the developed carrier system.
Tensile strength of nanofibers
Result of mechanical strength indicates that the drug loaded fibers (65 gm/cm2) exhibit better mechanical strength than plain polymeric nanofibers (58 gm/cm2). This happens due to partial congealing of PVA caused by ethanol during the preparation of drug polymer solution. Ethanol due to its low surface tension, approaches quickly to the surface of polymer which leads to displacement of water from the polymer surface. It results in the formation of extra hydrogen bond and additional hydrophobic interaction could be attributed to higher mechanical strength of drug loaded polymeric nanofibers.
Permeability studies
Results of this study shown in indicated that the Docetaxel is retained in the tissue for its therapeutics action due to the lipophilic nature of the drug, only 32% of the drug was released in the medium after 9 h. Permeability studies indicated that the maximum drug is available at the site of application thereby reducing the systemic toxicity of the drug.
Conclusions
Electrospinning is a simple, versatile and cost-effective technology which generates non-woven polymeric nanofibers. Solution and processing parameters such as viscosity, molecular weight, concentration of the polymer, applied voltage, tip to collector distance, conductivity, etc. significantly affect the fiber morphology and by manipulation of these parameters one can get desired properties for specific application. The results show that the combination of nanotechnology with electrospinning process can open up new doors for development of carrier system for local drug delivery.
Acknowledgement
Authors would like to thank the United Biotech Pvt Ltd, Baddi for providing gift sample of Docetaxel for the research work and ISF College of pharmacy, Moga for providing research facilities.
Declaration of Interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Celebioglu A, Uyar T. 2013. Electrospinning of nanofibers from non-polymeric systems: electrospun nanofibers from native cyclodextrins. J Colloid Interface Sci. 404:1–7.
- Chai JH, Wu QS. 2013. Electrospinning preparation and electrical and biological properties of ferrocene/poly(vinylpyrrolidone) composite nanofibers. Beilstein J Nanotechnol. 4:189–197.
- Chopra S, Mahdi S, Kaur J, Iqbal Z, Talegaonkar S, Ahmad FJ. 2006. Advances and potential applications of chitosan derivatives as mucoadhesive biomaterials in modern drug delivery. J Pharm Pharmacol. 58:1021–1032.
- Garg T, Singh O, Arora S, Murthy R. 2012. Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst. 29:1–63.
- Garg T, Singh S, Goyal AK. 2013. Stimuli-sensitive hydrogels: an excellent carrier for drug and cell delivery. Crit Rev Ther Drug Carrier Syst. 30:369–409.
- Hosseini S, Niaei A, Salari D. 2012. Preparation and characterization of nano- and non-nanoscale Co(3)O(4) spinels obtained from different methods and study of their performance in combustion of aromatics from polluted air-A comparison with Pt/gamma-Al(2)O(3) performance. J Environ Sci Health A Tox Hazard Subst Environ Eng. 47:1728–1732.
- Maretschek S, Greiner A, Kissel T. 2008. Electrospun biodegradable nanofiber nonwovens for controlled release of proteins. J Control Release. 127:180–187.
- Mathias NR, Hussain MA. 2010. Non-invasive systemic drug delivery: developability considerations for alternate routes of administration. J Pharm Sci. 99:1–20.
- Misra SK, Nazhat SN, Valappil SP, Moshrefi-Torbati M, Wood RJ, Roy I, Boccaccini AR. 2007. Fabrication and characterization of biodegradable poly(3-hydroxybutyrate) composite containing bioglass. Biomacromolecules. 8:2112–2119.
- Parnami N, Garg T, Rath G, Goyal AK. 2013. Development and characterization of nanocarriers for topical treatment of psoriasis by using combination therapy. Artif Cells Nanomed Biotechnol.
- Pircher A, Wasle IK, Mian M, Gamerith G, Ulsperger E, Studnicka M, et al. 2013. Docetaxel in the treatment of non-small cell lung cancer (NSCLC) – an observational study focusing on symptom improvement. Anticancer Res. 33:3831–3836.
- Sharma S, Mohanty S, Gupta D, Jassal M, Agrawal AK, Tandon R. 2011. Cellular response of limbal epithelial cells on electrospun poly-epsilon-caprolactone nanofibrous scaffolds for ocular surface bioengineering: a preliminary in vitro study. Mol Vis. 17: 2898–2910.
- Song M, Li N, Sun S, De Villiers MM. 2005. Effect of processing variables on the release and particulate properties of sustained release amoxicillin microcapsules prepared by emulsion solvent evaporation. Pharmazie. 60:278–282.
- Westra WH. 2009. The changing face of head and neck cancer in the 21st century: the impact of HPV on the epidemiology and pathology of oral cancer. Head Neck Pathol. 3;78–81.
- Won JJ, Nirmala R, Navamathavan R, Kim HY. 2012. Electrospun core-shell nanofibers from homogeneous solution of poly(vinyl alcohol)/bovine serum albumin. Int J Biol Macromol. 50: 1292–1298.
- Wu X, Desai KG, Mallery SR, Holpuch AS, Phelps MP, Schwendeman SP. 2012. Mucoadhesive fenretinide patches for site-specific chemoprevention of oral cancer: enhancement of oral mucosal permeation of fenretinide by coincorporation of propylene glycol and menthol. Mol Pharm. 9:937–945.
- Zeng J, Aigner A, Czubayko F, Kissel T, Wendorff JH, Greiner A. 2005. Poly(vinyl alcohol) nanofibers by electrospinning as a protein delivery system and the retardation of enzyme release by additional polymer coatings. Biomacromolecules. 6:1484–1488.