Abstract
Acne is a chronic inflammatory human skin disease, characterized by areas of skin with seborrhoea, comedones, papules, nodules, pimples, and possibly scarring with lesions occurring on face, neck, and back. Nanotechnological approaches such as particulate (solid lipid nanoparticles and microspheres), vesicular (liposomes and niosomes), colloidal drug delivery systems (micro-emulsion and nano-emulsion), and miscellaneous systems (aerosol foams and micro-sponges) have an important place in acne therapy. These approaches have an enormous opportunity for the designing of a novel, low-dose and effective treatment systems to control acne disease. In this review, we specially focus on the different nanotechnological approaches for an effective treatment of acne.
Introduction
Acne vulgaris (also known as simply acne) is a chronic inflammatory human skin disease, characterized by areas of skin with seborrhoea (scaly red skin), comedones (blackheads and whiteheads), papules (pinheads), nodules (large papules), pimples, and possibly scarring with lesions occurring on face, neck, and back. The swollen glands form pink papules, encircled by comedones from pustules or cysts initiated by anxiety, hereditary factors, hormones, and bacteria that is, Propionibacterium acnes (P. acnes; CitationKim and Mancini 2013). Acne arises due to the action of hormones on the sebaceous glands of skin leads to persevering of the pores with sebum and eruptions of the lesions called pimples or zits, and it follows as a result of extreme sebum production together with epidermal hyperproliferation. Acne affects approximately 650 million people or about 9.4% of the population globally. It affects almost 90% of people during their teenage years due to an increase in testosterone hormone level and sometimes persists into adulthood (CitationTanghetti 2013). It is slightly more common in females than males (9.8% vs. 9.0%, respectively). In those over 40 years old, 1% of males and 5% of females still have problems. Acne affects 40–50 million people in the United States (16%) and approximately 3–5 million in Australia (23%). Acne has been classified into four major categories such as comedonal, papular, pustular, and severe pustulocystic acne (CitationPatwardhan et al. 2013). shows characteristics of various grade of acne.
Table I. Characteristics of various grade of acne.
Causes of acne (CitationWell 2013)
Various factors such as environmental factors (humidity, heat, etc.), emotional factors, hormonal factors (Androgenic progestin), and micro-organisms are mainly responsible for causing acne.
High humidity, heat and other conditions cause recurrent and elongated sweating can aggravate acne.
Tight fitting clothes that confine air movement and avoid evaporation of skin moisture contribute to acne.
Exposure to grime, cooking oils/smoke or industrial chemicals such as petroleum derivatives can cause occupational acne.
Hair sprays can block the pilosebaceous gland cause acne.
Products that contain comedogenic oils cause acne due to occlusive and plugging the follicles.
Emotional factors will not cause acne but it subsidizes to acne together with severe or prolonged periods of stress or other emotional extremes.
Androgenic progestin and oral contraceptives are contributors to acne.
Some medications can exacerbate preexisting acne such as corticosteroids, androgens, azathioprine, bromides with a high progestin level, isoniazid, lithium, phenytoin, and thyroid preparations.
Acne vulgaris is mainly caused by gram-positive, non-spore forming, micro-aerophilic and rod-like bacterium P. acnes.
Pathogenesis of acne (CitationWilliams et al. 2012, CitationLolis et al. 2009, CitationPauli and Reunala 1987)
Acne is associated to hormones, sebum, follicle fallout, bacteria, and inflammation which initiate in the pilosebaceous units of the dermis. The major factors that cause acne include:
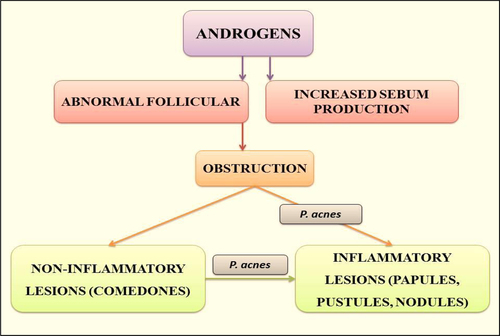
Increased sebum production: The one of the most important factors involved in the development of acne lesions is increased rate of sebum production. One imaginable role of sebum in the pathogenesis of acne is its primary or associative role in comedogenesis as well as providing the substrate for P. acnes growth. shows the basic mechanism involved in pathogenesis of acne.
Follicular hyper keratinization: Hindrance of the pilosebaceous canal heads the expansion of acne lacerations. The hindrance is produced by the growth of supporter keratinized cells within the channel that form an impaction blocking the flow of sebum. It may also be due to an irregularity in the sebaceous lipids resulting in a relative hyper proliferation of corneocytes. Comedones formation may be due to a localized deficiency of linoleic acid in pilosebaceous duct.
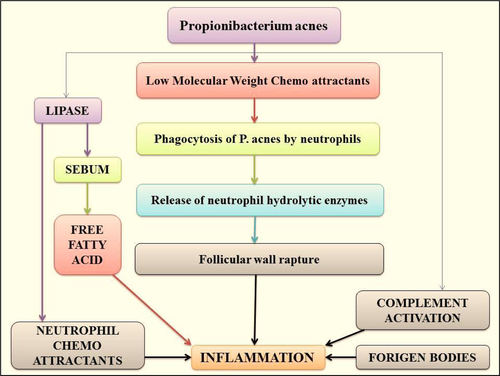
Abnormal bacterial function: Skin surface in acne disposed to areas are colonized with Staphylococcus epidermidis and P. acnes. P. acnes subsidize to inflammation through stimulation of various chemotactic factors and separation the comedones. shows the sequence of events leading to acne inflammation primarily induced by P. acnes.
Various approaches for acne treatment
Topical approach (CitationFu and Vender 2011)
Topical therapy is active as first-line treatment in mild acne. Topical therapy along with systemic therapy control moderate and severe acne. Drugs employed for topical application are shown in .
Table II. Topical drug for acne treatment.
Systemic approach (CitationBettoli et al. 2010)
Systemic therapy is active as first-line treatment in moderate and severe acne along with topical therapy. Drugs employed for systemic application are shown in .
Table III. Systemic drug for acne treatment.
Novel approach
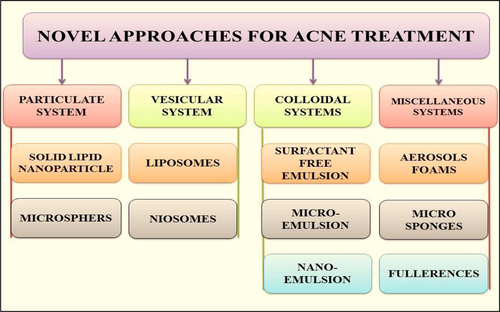
Novel drug delivery strategies play an essential role in refining the topical delivery of anti-acne agents by enhancing their dermal localization with a concomitant reduction in their side effects. Pointing is the capability to direct the drug-loaded system to the site of attention. Controlled drug release and following biodegradation are important for developing successful formulations (CitationCastro and Ferreira 2008). Potential releases mechanisms involve desorption of surface-bound/adsorbed drugs, diffusion through the carrier matrix, carrier matrix erosion, or combined erosion/ diffusion process. Novel carriers are gaining wide acceptance, which include utilization of carriers such as niosomes, liposome, emulsomes, transferosomes, micro emulsion, nano emulsion, and nano lipid carriers that are efficient to transfer the drug across the skin (CitationGarg et al. 2013; ).
The main advantages of nanocarriers are:
excellent entrapment efficiency;
maintain the physiochemical properties of entrapped drug;
act as penetration enhancers;
act as local depot for sustained release of drugs;
deliver the drug molecule to target site without effecting the normal organ or tissue;
minimize the toxic effects;
accommodate drug molecules with a wide range of solubility;
accommodate both lipophilic and hydrophilic drugs;
provide protection from hydrolysis and oxidation;
provide rapid and efficient penetration of the drug moiety;
increase the rate and extent of absorption of the drug;
increase the residence time of drugs; and
improve the horny layer properties.
Novel drug delivery systems used for acne treatment include:
Particulate systems
Solid lipid nanoparticles. Solid lipid nanoparticle (SLN) fabrication by the purpose of topical usage should be made up of lipids that remain in solid state at skin (32°C) and room temperature. Its particle surface is protected by a surfactant which stabilizes the dispersion. The major advantages are providing protection of labile compounds against chemical degradation as well as producing controlled release of the active ingredients. SLN with a drug-enriched shell shows burst release characteristics whereas SLN with a drug-enriched core leads to sustained release. SLN has occlusive assets, that is, they can be used in direction to increase the water content of the skin (CitationFireman et al. 2011). CitationRaza et al. (2013) systematically optimized biocompatible isotretinoin (ITR)-loaded SLNs for topical treatment of all types of acne, including recalcitrant, severe and nodulocystic. The developed system was characterized and evaluated for skin compliance, skin transport characteristics and anti-acne potential against testosterone-induced acne in male Laca mice. The results showed that SLNs were able to transport the drug to various skin layers effectively while formed drug micro-reservoirs. The optimized SLNs exhibited drug entrapment of 89.49 ± 4.1% and showed marked anti-acne potential as well as tolerability on the mouse skin as compare the marketed product. The results assure massive promise of the optimized SLNs of ITR in reducing dermal irritation and increasing the therapeutic performance, thus resulting in an efficacious and patient-compliant formulation (CitationRaza et al. 2013).
Microspheres. Microsphere technology improves the treatment tolerability, encourage adherence, and contribute to better long-term therapeutic outcomes. Microsphere technology removes the quick delivery of high concentrations of active drug to the application site and instead facilitates controlled release of potentially irritating drugs. It is associated with improved treatment outcomes and minimal irritation. Microsphere formulations of topical Tretinoin and benzoyl peroxide (BPO) currently on the market have demonstrated good efficacy and tolerability and are expected to encourage adherence and long-term therapeutic benefit (CitationKircik 2011). CitationEichenfield et al. (2012) studied the safety and efficacy of Tretinoin microsphere gel (TMG) 0.04% pump in children aged 9–11 years with acne vulgaris. The results showed that greater improvement in the least-squares mean change in non-inflammatory lesions with TMG 0.04% than with vehicle (− 19.9 vs. − 9.7, p = 0.04) and a significant difference in Investigator Global Assessment of improvement at Week 12 between the children treated with TMG 0.04% pump and those treated with vehicle (p = 0.02), but there were no discernible differences in static acne severity scales. This study confirmed statistically significant differences in the reduction of non-inflammatory lesions between TMG 0.04% pump and vehicle in patients aged 9–11 years with acne vulgaris (CitationEichenfield et al. 2012).
Vesicular systems
Nowadays, vesicular systems, both liposomes and niosomes, play an increasingly important role in drug delivery as they can reduce drug toxicity and modify drug pharmacokinetics and bioavailability.
Liposomes. Liposomes are recurrently used as vehicles in pharmaceuticals and cosmetics for a controlled and optimized delivery to particular skin layers. Liposomes are spherical vesicles whose membrane consists of amphiphillic lipids (i.e., lipids that are hydrophilic on one side and lipophilic on the other side) that enclose an aqueous core, similar to the bilayer membranes of living cells. Because of the amphiphillic nature of liposomes, they may encapsulate both hydrophilic as well as lipophilic drugs. This unique dual release capability enables the delivery of two types of substances once they are applied on the skin; each differs in its effects on skin permeability, which may enhance the desired therapeutic benefit (CitationParnami et al. 2013). CitationPornpattananangkul et al. (2013) developed and evaluated of liposomal lauric acids (LipoLA) as an effective and safe therapeutic agent for the treatment of acne infection. It was observed that the resulting LipoLA readily fuse with bacterial membranes, causing effective killing of P. acnes by disrupting bacterial membrane structures. Topically applying LipoLA in a gel form onto the infectious sites leads to eradication of P. acnes bacteria in vivo. Further skin toxicity studies showed that LipoLA does not induce acute toxicity to normal mouse skin within 24 h. These results suggested that LipoLA hold a high therapeutic potential for the treatment of acne infection and other P. acnes-related diseases (CitationPornpattananangkul et al. 2013).
Niosomes. Niosomes appear as an interesting drug delivery system in the treatment of dermatological disorders especially for acne. In fact, topically applied niosomes can increase the residence time of drugs in the stratum corneum and epidermis, while reducing the systemic absorption of the drug. They are assumed to increase the horny layer properties, both by reducing trans-epidermal water loss and by increasing smoothness via replenishing lost skin lipids (CitationGarg et al. 2012). CitationLiu and Huang (2013) developed lipid vehicles to deliver curcumin and inhibit P. acnes in the skin. The inhibitory activities of the curcumin-containing vehicles against P. acnes were studied using the bioluminescence assay. Curcumin (0.43 μg/mL) in the vehicles significantly inhibited the growth of P. acnes. The formation of curcumin reservoir in the skin through the curcumin-loaded vehicles is confirmed using confocal laser scanning microscopy. Curcumin-loaded vehicles could efficiently accumulate in the skin and inhibit P. acnes in vitro. Our results highlight the potential of using vehicles containing lauric acid and curcumin as an alternative treatment for acne vulgaris (CitationLiu and Huang 2013).
Colloidal dispersions
Surfactant-free emulsions. Emulsifier-free formulations are a developing area for dermatologic and cosmetic products. Most skin care products are emulsions, that is, a mixture of two or more materials that are not miscible with each other. As a result, they require the addition of surfactants (emulsifiers) that stabilize the formulation and increase its shelf life. Furthermore, once these surfactant agents are applied on the skin, they tend to emulsify and remove the natural lipids of the epidermis. Accordingly, the pharmaceutical industry has been developing surfactant-free emulsions as alternatives to conventional formulations using stabilizers, such as polymeric emulsifiers or solid particles. These stabilizers produce sufficiently stable products with a cosmetically pleasant appearance (CitationDominguez-Delgado et al. 2011). CitationDominguez-Delgado et al. (2011) prepared and characterized surfactant-free emulsions of Triclosan intended to be used for the treatment of acne. Differential scanning calorimetry, transmission electron microscopy, and scanning electron microscopy studies suggested that Triclosan-loaded emulsions show good encapsulation efficiency (95.9%). Emulsions, being free of surfactants or other potentially irritant agents, can be a good option for the delivery of Triclosan to the skin, representing a good alternative for the treatment of acne (CitationDominguez-Delgado et al. 2011).
Micro-emulsion. These are translucent mixtures of oil, surfactant, co-surfactant, and water, in which either the oil globules are dispersed in water (o/w) or water globule are dispersed in oils (w/o). Various studies have discovered the significance of micro-emulsion for dermal and transdermal delivery both in-vitro and in-vivo. Due to the high solubilization capacity, a large quantity of drug can be incorporated in this formulation. The components of microemulsion can interact with the lipid layers of stratum corneum and changes its structural integrity leading to enhance transdermal permeation of drug (CitationKumar et al. 2011). CitationLiu and Huang (2012) studied the antimicrobial activity of curcumin-loaded myristic acid micro-emulsions against Staphylococcus epidermidis. Curcumin distribution in neonate pig skin was visualized using confocal laser scanning microscopy. Curcumin (0.86 μg/mL) in the myristic acid micro-emulsion could inhibit 50% of the bacterial growth, which was 12 times more effective than curcumin dissolved in dimethyl sulfoxide (DMSO). The cocktail combination of myristic acid and curcumin in the micro-emulsion carrier synergistically inhibited the growth of S. epidermidis (CitationLiu and Huang 2012).
Nano-emulsion. Nano-emulsions (NEs) can also be defined as “ultrafine emulsions” because of the formation of droplets in the submicron range. A nano-emulsion seems to be transparent and translucent with a bluish color. The small-size droplets give them characteristic stability against creaming, sedimentation, flocculation, and coalescence. It allows the effective transport of active ingredients to the skin (CitationBorges et al. 2013). CitationLin et al. (2013) developed lipid nanocarriers, NEs, and nanostructured lipid carriers (NLCs) that combine Tretinoin and tetracycline for the efficient topical delivery to treat acne vulgaris. The antibacterial activities of the nanosystems against Staphylococcus aureus, Pseudomonas aeruginosa, and P. acnes were evaluated using an agar diffusion assay. NEs and NLCs exhibited high entrapment of Tretinoin which ranged 60–100%. This is the first report examining skin permeation and antibacterial activities of dual-drug nanocarriers for acne treatment (CitationLin et al. 2013).
Miscellaneous systems
Aerosols foams. Aerosol foams have converted an increasingly standard type of topical formulation for a variety of skin conditions including acne vulgaris. The vehicle base of the foam can have a liquid or semi-solid consistency that shares the same physicochemical characteristics of conventional vehicles like creams, lotions, and gels, but it maintains desirable properties such as moisturizing, fast-drying effects, or higher drug bioavailability. The aerosol base is dispensed through a gas pressurized can that discharges the foam. The product characteristics (i.e., texture, bubble size and thickness, viscosity, stability, and spreadibility) are determined by the type of formulation and the dispensing container that are selected to suit the specific treatment needs (CitationSimonart 2012). CitationDel Rosso (2009) successfully prepared sodium sulfacetamide 10%-sulfur 5% emollient foam in the treatment of acne vulgaris. Recently, an emollient foam sodium sulfacetamide 10%-sulfur 5% formulation indicated for topical therapy of acne vulgaris, rosacea, and seborrhea dermatitis has become available (CitationDel Rosso 2009).
Microsponges. Microsponges are biologically inert particles prepared of synthetic polymers with the capacity to store a volume of an active agent up to their own weight. Additionally, the particles assist to defend the entrapped active compound from physical and environmental degradation. The microsponge technology can be utilized in a variety of formulations, but is more frequently manufactured as gels. Once applied on the skin, microsponges slowly release the active agents (CitationJelvehgari et al. 2006). CitationJelvehgari et al. (2006) developed microsponge delivery system of BPO for the treatment of acne and athletes foot. The micrograph of microsponges showed that they were spherical in shape and contained pores. One of the research studies showed that an increase in the ratio of drug: polymer resulted in a reduction in the release rate of BPO from microsponges which were attributed to a decreased internal porosity of the microsponges (CitationJelvehgari et al. 2006).
Fullerenes. Fullerenes (resemble a hollow sphere) are molecules composed entirely of carbon. Once fullerenes come into contact with the skin, they migrate through the skin intracellularly, as opposite to affecting over cells. So, a fullerene could be used to “trap” active compounds and then release them into the epidermis on the skin after the application. Moreover, fullerenes, themselves, are thought to be potentially potent antioxidants (CitationInui et al. 2011). CitationInui et al. (2012) developed polyhydroxylated fullerene (capable of potent radical-scavenging activity) and investigated its inhibitory effects in vitro on sebum production in hamster sebocytes and in P. acnes lipase activity. These results suggested that fullerene could be a beneficial skin care reagent for controlling acne vulgaris by suppressing sebum in the inflammatory response and by reducing P. acnes lipase activity (CitationInui et al. 2012).
shows the various applications of novel nano-carrier systems in the effective treatment of acne.
Table IV. Applications of nano-carrier systems for treatment of acne.
Conclusion
In this review, we have discussed about the whole global consequence of the disease by familiarizing drug delivery systems or by modifying the current ones as chemotherapy regimen for acne. Particulate, vesicular, and colloidal drug delivery approaches have their important place in acne therapy. From the above study, we can conclude that the above nanotechnology approaches has an enormous opportunity for the designing of a novel, low-dose and effective treatment systems to control acne disease. From the future perception, development of vaccines using combined strategic approach like nanocarriers can play a major role in the treatment of acne.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- An JS, Kim JE, Lee DH, Kim BY, Cho S, Kwon IH, et al. 2011. 0.5% Liposome-encapsulated 5-aminolevulinic acid (ALA) photodynamic therapy for acne treatment. J Cosmet Laser Ther. 13:28–32.
- Bettoli V, Sarno O, Zauli S, Borghi A, Minghetti S, Ricci M, et al. 2010. [What's new in acne? New therapeutic approaches]. Ann Dermatol Venereol. 137 Suppl 2:S81–S85.
- Borges VR, Simon A, Sena AR, Cabral LM, de Sousa VP. 2013. Nanoemulsion containing dapsone for topical administration: a study of in vitro release and epidermal permeation. Int J Nanomedicine. 8:535–544.
- Brown EJ. 1977. A povidone-iodine skin cleanser foam in the management of acne vulgaris. Br J Clin Pract. 31:218–219.
- Castro GA, Ferreira LA. 2008. Novel vesicular and particulate drug delivery systems for topical treatment of acne. Expert Opin Drug Deliv. 5:665–679.
- Castro GA, Oliveira CA, Mahecha GA, Ferreira LA. 2011. Comedolytic effect and reduced skin irritation of a new formulation of all-trans retinoic acid-loaded solid lipid nanoparticles for topical treatment of acne. Arch Dermatol Res. 303:513–520.
- Castro GA, Orefice RL, Vilela JM, Andrade MS, Ferreira LA. 2007. Development of a new solid lipid nanoparticle formulation containing retinoic acid for topical treatment of acne. J Microencapsul. 24:395–407.
- Del Rosso JQ. 2009. The use of sodium sulfacetamide 10%-sulfur 5% emollient foam in the treatment of acne vulgaris. J Clin Aesthet Dermatol. 2:26–29.
- Dominguez-Delgado CL, Rodriguez-Cruz IM, Escobar-Chavez JJ, Calderon-Lojero IO, Quintanar-Guerrero D, Ganem A. 2011. Preparation and characterization of triclosan nanoparticles intended to be used for the treatment of acne. Eur J Pharm Biopharm. 79:102–107.
- Eichenfield LF, Hebert AA, Schachner L, Paller AS, Rossi AB, Lucky AW. 2012. Tretinoin microsphere gel 0.04% pump for treating acne vulgaris in preadolescents: a randomized, controlled study. Pediatr Dermatol. 29:598–604.
- Fireman S, Toledano O, Neimann K, Loboda N, Dayan N. 2011. A look at emerging delivery systems for topical drug products. Dermatol Ther. 24:477–488.
- Fluhr JW, Barsom O, Gehring W, Gloor M. 1999. Antibacterial efficacy of benzoyl peroxide in phospholipid liposomes. A vehicle-controlled, comparative study in patients with papulopustular acne. Dermatology. 198:273–277.
- Fu LW, Vender RB. 2011. Newer approaches in topical combination therapy for acne. Skin Therapy Lett. 16:3–6.
- Garg T, Singh O, Arora S, Murthy R. 2012. Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst. 29:1–63.
- Garg T, Singh S, Goyal AK. 2013. Stimuli-sensitive hydrogels: an excellent carrier for drug and cell delivery. Crit Rev Ther Drug Carrier Syst. 30:369–409.
- Honzak L, Sentjurc M. 2000. Development of liposome encapsulated clindamycin for treatment of acne vulgaris. Pflugers Arch. 440: R44–R45.
- Inui S, Aoshima H, Ito M, Kobuko K, Itami S. 2012. Inhibition of sebum production and Propionibacterium acnes lipase activity by fullerenol, a novel polyhydroxylated fullerene: potential as a therapeutic reagent for acne. J Cosmet Sci. 63:259–265.
- Inui S, Aoshima H, Nishiyama A, Itami S. 2011. Improvement of acne vulgaris by topical fullerene application: unique impact on skin care. Nanomedicine. 7:238–241.
- Jelvehgari M, Siahi-Shadbad MR, Azarmi S, Martin GP, Nokhodchi A. 2006. The microsponge delivery system of benzoyl peroxide: preparation, characterization and release studies. Int J Pharm. 308: 124–132.
- Kaur N, Puri R, Jain SK. 2010. Drug-cyclodextrin-vesicles dual carrier approach for skin targeting of anti-acne agent. AAPS PharmSciTech. 11:528–537.
- Kim W, Mancini AJ. 2013. Acne in childhood: an update. Pediatr Ann. 42:418–427.
- Kircik LH. 2011. Microsphere technology: hype or help?J Clin Aesthet Dermatol. 4:27–31.
- Kumar A, Agarwal SP, Ahuja A, Ali J, Choudhry R, Baboota S. 2011. Preparation, characterization, and in vitro antimicrobial assessment of nanocarrier based formulation of nadifloxacin for acne treatment. Pharmazie. 66:111–114.
- Leyden JJ, Tanghetti EA, Miller B, Ung M, Berson D, Lee J. 2002. Once-daily tazarotene 0.1% gel versus once-daily tretinoin 0.1% microsponge gel for the treatment of facial acne vulgaris: a double-blind randomized trial. Cutis. 69:12–19.
- Leyden J, Wortzman M, Baldwin EK. 2009. Tolerability of clindamycin/tretinoin gel vs. tretinoin microsphere gel and adapalene gel. J Drugs Dermatol. 8:383–388.
- Lin CH, Fang YP, Al-Suwayeh SA, Yang SY, Fang JY. 2013. Percutaneous absorption and antibacterial activities of lipid nanocarriers loaded with dual drugs for acne treatment. Biol Pharm Bull. 36:276–286.
- Liu CH, Huang HY. 2012. Antimicrobial activity of curcumin-loaded myristic acid microemulsions against Staphylococcus epidermidis. Chem Pharm Bull (Tokyo). 60:1118–1124.
- Liu CH, Huang HY. 2013. In vitro anti-propionibacterium activity by curcumin containing vesicle system. Chem Pharm Bull (Tokyo). 61:419–425.
- Lolis MS, Bowe WP, Shalita AR. 2009. Acne and systemic disease. Med Clin North Am. 93:1161–1181.
- Lucky AW, Sugarman J. 2011. Comparison of micronized tretinoin gel 0.05% and tretinoin gel microsphere 0.1% in young adolescents with acne: a post hoc analysis of efficacy and tolerability data. Cutis. 87:305–310.
- Parnami N, Garg T, Rath G, Goyal AK. 2013. Development and characterization of nanocarriers for topical treatment of psoriasis by using combination therapy. Artif Cells Nanomed Biotechnol. (Epub ahead of print).
- Patwardhan SV, Kaczvinsky JR, Joa JF, Canfield D. 2013. Auto- classification of acne lesions using multimodal imaging. J Drugs Dermatol. 12:746–756.
- Pauli SL, Reunala T. 1987. [Acne fulminans—a systemic disease refractory to treatment]. Duodecim. 103:835–839.
- Pornpattananangkul D, Fu V, Thamphiwatana S, Zhang L, Chen M, Vecchio J, et al. 2013. In vivo treatment of Propionibacterium acnes infection with liposomal lauric acids. Adv Healthc Mater. 2:1322–1328.
- Prasad S, Mukhopadhyay A, Kubavat A, Kelkar A, Modi A, Swarnkar B, et al. 2012. Efficacy and safety of a nano-emulsion gel formulation of adapalene 0.1% and clindamycin 1% combination in acne vulgaris: a randomized, open label, active-controlled, multicentric, phase IV clinical trial. Indian J Dermatol Venereol Leprol. 78:459–467.
- Raza K, Singh B, Singal P, Wadhwa S, Katare OP. 2013. Systematically optimized biocompatible isotretinoin-loaded solid lipid nanoparticles (SLNs) for topical treatment of acne. Colloids Surf B Biointerfaces. 105:67–74.
- Ridolfi DM, Marcato PD, Justo GZ, Cordi L, Machado D, Duran N. 2012. Chitosan-solid lipid nanoparticles as carriers for topical delivery of tretinoin. Colloids Surf B Biointerfaces. 93:36–40.
- Rolland A, Wagner N, Chatelus A, Shroot B, Schaefer H. 1993. Site-specific drug delivery to pilosebaceous structures using polymeric microspheres. Pharm Res. 10:1738–1744.
- Schafer-Korting M, Korting HC, Ponce-Poschl E. 1994. Liposomal tretinoin for uncomplicated acne vulgaris. Clin Investig. 72: 1086–1091.
- Shah KA, Joshi MD, Patravale VB. 2009. Biocompatible microemulsions for fabrication of glyceryl monostearate solid lipid nanoparticles (SLN) of tretinoin. J Biomed Nanotechnol. 5: 396–400.
- Simonart T. 2012. Newer approaches to the treatment of acne vulgaris. Am J Clin Dermatol. 13:357–364.
- Tanghetti EA. 2013. The role of inflammation in the pathology of acne. J Clin Aesthet Dermatol. 6:27–35.
- Vender R. 2012. Double-blinded, vehicle-controlled proof of concept study to investigate the recurrence of inflammatory and noninflammatory acne lesions using tretinoin gel (microsphere) 0.04% in male patients after oral isotretinoin use. Dermatol Res Pract. 2012:736532.
- Viyoch J, Pisutthanan N, Faikreua A, Nupangta K, Wangtorpol K, Ngokkuen J. 2006. Evaluation of in vitro antimicrobial activity of Thai basil oils and their micro-emulsion formulas against Propionibacterium acnes. Int J Cosmet Sci. 28:125–133.
- Well D. 2013. Acne vulgaris: a review of causes and treatment options. Nurse Pract. 38:22–31.
- Williams HC, Dellavalle RP, Garner S. 2012. Acne vulgaris. Lancet. 379:361–372.
- Yeung CK, Shek SY, Yu CS, Kono T, Chan HH. 2011. Liposome- encapsulated 0.5% 5-aminolevulinic acid with intense pulsed light for the treatment of inflammatory facial acne: a pilot study. Dermatol Surg. 37:450–459.