Abstract
Presently polymer nanofibers have received much attention due to their unique properties such as large surface area, high porosity, small pore size, superior mechanical properties and ease of addition of surface functionalities compared with any other material. Nanofibers particularly polymeric nanofiber prepared by electrospinning process can be used as carriers for the controlled drug delivery of bioactive molecules such as cytokines, growth factors, anticancer drugs, enzymes and certain vitamins. This article presents an overview of nanofibers, various techniques involved in fabrication of nanofibers, their characterization, parameters affecting electrospinning process and their applications.
Introduction
Nanofibers are defined as fibers having at least one dimension of 100 nm or less. Nanofibers are a new class of material used for several value-added applications as medical, filtration, barrier, personal care, wipes, garments, composite, energy storage, and insulation (CitationRathinamoorthy 2012). The nanofibers possess unique properties that make them a suitable carrier for drug delivery. Owing to the smaller size possessed by nanofibers, drug can be delivered to the appropriate site in the body (CitationCharles and Owens 2003). Major advantages of nanofibers scaffold formulations are excellent stability, better targeting, minimum toxicity, high drug-loading capacity, exceptional mechanical properties, encapsulation of various ranges of drugs, and suitability for thermoliable drugs. Delivery of drugs to patients in a most physiologically acceptable manner has always been a matter of concern. A wide variety of polymeric materials either biodegradable or nonbiodegradable but compatible can be used as delivery matrices (). The biodegradable or nondegradable polymers can be used to control for drug release either via diffusion alone or via diffusion and scaffold degradation (CitationGarg et al. 2013, CitationParnami et al. 2013). The ultimate goal of drug delivery is to deliver a defined amount of drug in a precise, efficient, and controlled release manner. The nanofibers produced by electrospinning can be used as carriers for various types of drugs, genes, growth factors, proteins, antibiotics, and DNA (CitationGarg et al. 2012). As an essential and key element of the nanomaterials revolution, organic and inorganic nanofibers persist in an increasingly adaptable class of nanomaterials that assure to touch upon and improve different facets of human ailments, from improving human health to playing a key role in driving energy production.
Table I. Polymer and solvents used for fabrication of nanofiber.
Characteristics of nanofibers
The unique characteristics of nanofibers, such as biocompatibility, biodegradability, excellent mechanical property, sterility, and controlled release pattern, make it an ideal candidate for drug as well as cell delivery.
Nanofibers scaffold formulations exhibit excellent biocompatibility with incorporated substances as well as body tissues.
Nanofibers possess acceptable biodegradability profile and their degradation products are nontoxic and are eliminated easily from the implantation site of the body or are integrated with surrounding tissues.
Nanofibers formulations have open and interconnected pore structure, which allow for optimal interaction with bioactive molecules.
Nanofibers formulations have excellent ability to deliver their encapsulated substances to the target site and avoid their side effects.
Nanofibers have maximum entrapment as well as loading capacity so the drug is released continuously for longer duration upon insertion into the body.
Owing to biocompatiblility, nanofibers or its degradation products do not show toxicity in the body.
Nanofibers scaffold formulations have sufficient binding affinity to allow release of the encapsulated substance continuously for longer duration after insertion into the body or to allow retaining cells in their pore structures.
Production method of nanofibers
Various techniques have been successfully used for the fabrication of nanofibers:
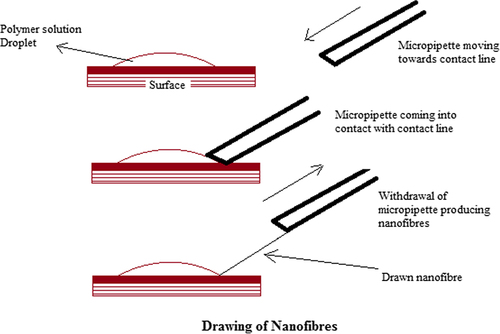
Drawing of nanofibers
In the drawing process, contact is made with the sharp tip of a micropipette or a glass rod with a previously deposited polymer solution droplet. The micropipette or glass rod is then withdrawn slowly, thus producing nanofibers (). While drawing the micropipette or glass rod, the solvent evaporates from the liquid fibers and ultimately solid nanofibers are formed. There is a specific time at which the fibers can be pulled. In the drawing process, a polymer solution should have proper viscoelastic properties. Owing to solvent evaporation from the deposited droplet, the viscosity of the droplet continuously increases, thus leading to shrinkage of the droplet. This affects the diameter of the fiber drawn and limits the continuous drawing of fibers (CitationBajáková et al. 2011, CitationRamakrishna et al. 2005).
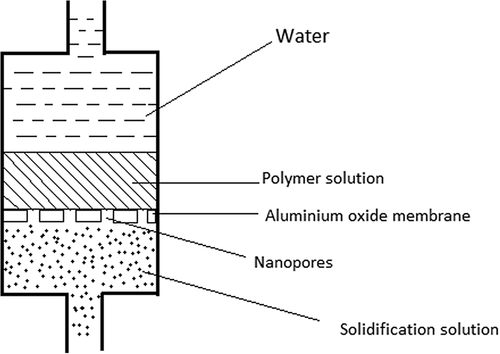
Template synthesis
Template synthesis involves the use of membranes or templates to obtain a desired structure. Nanoporous metal oxide membranes (e.g., aluminum oxide membrane) are commonly used, where a polymer solution is allowed to pass with a certain force through to a nonsolvent bath to produce nanofibers depending on the pore diameter (; CitationRamakrishna et al. 2005).
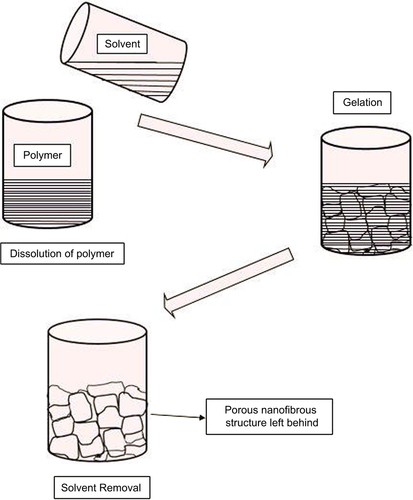
Phase separation
It is a method frequently used to prepare three-dimensional tissue engineering scaffolds. Phase separation of a polymer solution can be induced either by changing the temperature (thermally induced) or by adding a nonsolvent (nonsolvent induced) to the polymer solution to produce a polymer-rich phase and a solvent-rich phase (). The morphology of the polymer-rich phase can be fixed by quenching under low temperature (CitationRamakrishna et al. 2005). Solvent can be removed by either freeze-drying or extraction, thereby producing porous polymer scaffolds. Polymer scaffolds obtained by the phase separation method generally have a sponge-like porous morphology with micro-scale spherical pores (CitationHua et al. 2002, CitationNam and Park 1999). It is a simple technique that does not require much specialized equipment. It is easy to achieve batch-to-batch consistency and the mechanical properties of the scaffolds can be easily changed. However, this method has some drawbacks; that is, only a selected number of polymers can be used and is strictly a laboratory-scale technique.
Self-assembly
It is a promising technology for controlled build-up of defined nanostructured geometries from small units. It involves the organization of individual components spontaneously into an ordered and stable structure through noncovalent bond interaction (CitationZhang 2003, CitationHartgerink et al. 2002). Self-organization of molecules into a defined structure without any human intervention is common throughout technology and nature (CitationNumata and Sato 2013). Self- assembly of synthetic or natural macromolecules produces nanosized supramolecular structures, sometimes nanofibers (CitationChiti et al. 2003). Self-assembly can produce much thinner nanofibers—only several nanometers in diameter in comparison with electrospinning. Another problem is that mass production is not easy because of a complicated manufacturing process and low productivity.
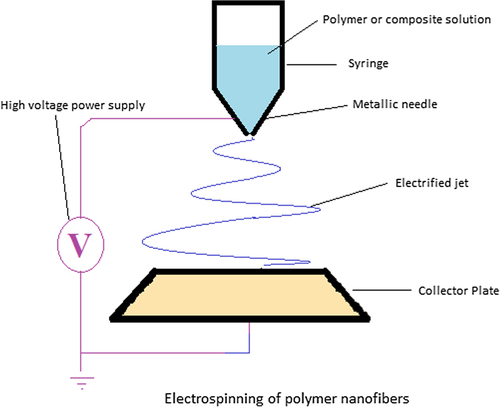
Electrospinning
Electrospinning is a versatile and simple process meant for the production of nanofibers by exposing a polymer solution/melt to a high voltage (30–50 KV) (CitationYu et al. 2009). Electrospun nanofibers show great promise for developing many types of novel drug delivery systems (DDSs) due to their special characteristics and the simple but useful fabricating process ().
An electrospinning unit consists of three essential components: a capillary tube with a pipette or needle of a small diameter, metal collecting screen, and a high-voltage source. In this process, one electrode is placed into the spinning solution/melt and the other attached to the collector. High voltage is applied to the end of a capillary tube containing polymer solution/melt held by its surface tension. This leads to the induction of charges on the surface of polymer solution/melt. When the applied electric field approaches a certain critical value, the repulsive electrical forces overcome the surface tension forces. As a result, a charged jet of the fluid is ejected from the tip of the Taylor cone. Finally, the solvent evaporates from the discharged polymer solution, leaving behind a charged polymer fiber. In the case of the melt, the discharged jet solidifies when it travels in the air stream (CitationDoshi and Reneker 1995, CitationHuang et al. 2003, CitationAgarwal et al. 2008). represents a comparison between the existing fabrication techniques of nanofibers.
Table II. Comparison between the existing fabrication techniques of nanofibers.
Various parameters affecting the electrospinning process (CitationSingh et al. 2014, CitationGagandeep et al. 2014, CitationKataria et al. 2014)
Solution properties
Molecular weight, viscosity, and surface tension
Adequate viscosity and molecular weight of the polymer is one of the prerequisites for electrospinning to occur in order to prevent breakage of fibers being formed. Polymer concentration affects the viscosity and surface tension of the solution. Therefore, the solution should neither be too dilute so that the fibers will break into droplets before reaching the collector electrode nor should not be too concentrated so that the fibers will not be formed.
Many experiments were conducted and it was found that when the polymer concentration was within optimal range, fiber diameter increased with increased polymer concentration.
Solution conductivity
Solution conductivity can influence the fiber size. Solutions with high charge-carrying capacity will have more charge on the surface and hence are easier to stretch. Conductivity of those polymers having less conductivity can be increased by adding salts or electrolytes that produce ions and hence voltage needed to produce smooth fibers decreases.
Solvent volatility
Solvent volatility affects the porosity of fibers. For sufficient solvent evaporation to occur, a volatile solvent must be used. During the emission of a fiber jet from the capillary tube toward the collector plate, phase separation occurs before the solid fibers are deposited onto the collector plate. This process of phase separation is greatly influenced by solvent volatility.
Processing parameters
Flow rate
Flow rate affects fiber size, fiber porosity, and fiber shape. Megelski et al. found that when the flow rate was increased, both the pore size and fiber diameter was reduced. Further increase in the flow rate leads to problems like bead defects in the fibers formed due to incomplete drying of the fibers.
Collector plate
The collector plate used in electrospinning process should be conductive in nature, grounded to maintain proper potential difference between the tip and collector. The charges collected at the collector plate will dissipate and the fibers will be well collected. Use of nonconducting plates instead of conducting plates leads to lower packing of fibers.
Diameter of needle
The smaller the diameter of the orifice, the lesser is the diameter of fibers being formed, because less volume of solution is collected at the tip of capillary tube.
Voltage
Application of a higher voltage (within the optimum range of electric field strengths) at the time of electrospinning reduces the fiber diameter and it also reduces the flight time between the collector and tip. For a certain polymer/solvent systems, there is an optimum range of electric field strengths, as either too weak or too strong a field leads to the formation of beaded fibers.
Humidity
High humidity will affect the fiber morphology because of water condensation on the fibers.
Distance between the tip and the collector
Distance between the collector plate and tip should be optimum because, if this distance is decreased then there will be lower flight time; in other words, there will be no time for the solvent to evaporate and it may lead to bead defects.
Characterization of nanofibers
Geometric characterization
Geometric properties of nanofibers include diameter distribution, fiber diameter, fiber morphology (e.g., cross-section shape and surface roughness), and fiber orientation. Techniques such as field emission scanning electron microscopy (FESEM), scanning electron microscopy (SEM), atomic force microscopy (AFM), and transmission electron microscopy (TEM) are used for the characterization of the geometric properties of nanofibers (CitationDemir et al. 2002, CitationLi et al. 2002, CitationMegelski et al. 2002, CitationSrinivasan and Reneker 1995). AFM is used to determine fiber diameter but the process of obtaining accurate measurements is difficult. TEM is used for obtaining diameters of extremely small fibers (< 300 nm). SEM is yet another technique used for detecting fiber diameters and morphologies but it suffers from a disadvantage that the resolution is less at extreme magnifications (CitationDoshi and Reneker 1995, CitationReneker and Chun 1996, CitationSrinivasan and Reneker 1995, CitationDemir et al. 2002, CitationFong et al. 2002). Still, SEM remains a quick method for observing the fibers produced and it requires a very small sample size for its operation. Many research scientists have been successful in determining the crystallinity of produced nanofibers by employing alternative methods such as X-ray diffraction and differential scanning calorimetry (DSC). Another geometric parameter, porosity and pore size, can be measured by mercury porosimetry (CitationKim et al. 2007, CitationNair et al. 2005).
Chemical characterization
Nanofibers molecular structure can be characterized by nuclear magnetic resonance (NMR) technique and Fourier transform infrared spectroscopy (FTIR) technique (CitationKwoun et al. 2000, CitationSchreuder-Gibson et al. 2002). With the application of these techniques, we can determine not only the molecular interactions of the polymers blended together for the fabrication of nanofibers but also their molecular structure (CitationGarg and Goyal 2014). The NMR spectrum of a blend of PEO and collagen revealed a new phase structure that was caused by hydrogen bond formation between the protons of the amino acid hydroxyl groups in collagen and ether oxygen of PEO (CitationHuang et al. 2001). The configuration of the macromolecules in a nanofiber can be characterized by wide-angle X-ray diffraction (WAXD), differential scanning calorimetry (DSC), and small-angle X-ray scattering (SAXC) (CitationBuchko et al. 1999, CitationChun et al. 1999, CitationChen et al. 2001, CitationZussman et al. 2002). FTIR-ATR analyses and water contact angle measurement are the techniques used for determining surface chemical properties (CitationDeitzel et al. 2002).
Mechanical characterization
Conventional techniques can be used for mechanical tests of nanofibrous nonwoven membranes (CitationLi et al. 2002, CitationHuang et al. 2001, CitationLee et al. 2002, CitationSchreuder-Gibson et al. 2002). CitationLi et al. (2002) obtained typical stress–strain curves of a PLLA nanofibrous mat for tissue engineering applications. It has been established that the tensile strength of a nanofibrous mat was analogous to that of natural skin. On the other hand, while the membranes were obtained from a rotating drum, it was found that the electrospun nonwoven mats had different properties in different directions (CitationLee et al. 2001, Citation2002). The orientation of fiber depended on other electrospinning parameters and on the linear velocity of the drum surface. To determine the mechanical behavior of conventional fibers, the established methods and standards are inadequate for testing of nanofibers (CitationWarner et al. 1998). Demczyk et al. directly measured the elastic modulus and tensile strength of multiwalled carbon nanotubes under TEM via a tensile testing device fabricated through a microfabrication technique. In future, it is anticipated that the analogous techniques can be applied to understand the mechanical properties of single nanofibers (CitationDemczyk et al. 2002).
Applications of nanofibers
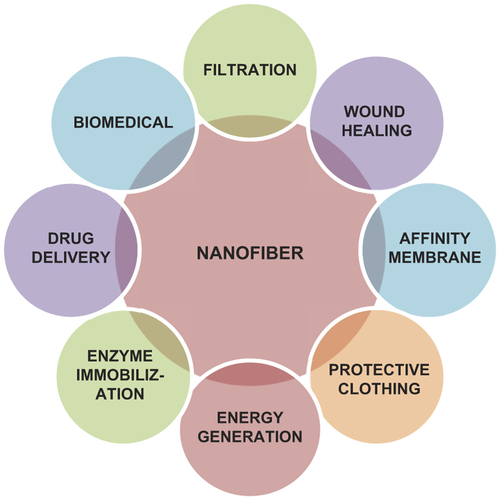
As the nanofibers possess excellent properties such as very high porosity, high surface area to volume ratio, and enhanced physico-mechanical properties, they are widely used in biomedical applications, such as tissue engineering scaffolds, in wound healing, filtration, as affinity membrane, drug delivery, health care, small-diameter vascular grafts, biotechnology, and immobilization of enzymes. shows the applications of nanofibers in different areas.
Nanofibers as wound-healing agent
Dressing meant for wound healing protects the wound, absorbs extra body exudates from the wound, and accelerates the wound healing process. To carry out these functions, wound dressing material provides physical barrier to a wound and at the same time is permeable to oxygen and moisture.
An ideal dressing meant for wound healing should have the following characteristics:
Absorption ability of excess exudates
Adequate gaseous exchange ability
Efficiency as bacterial barrier
Functional adhesion, that is, adherent to healthy tissue but nonadherent to wound tissue
Low cost
Ease of removal and painless
Electrospun nanofiber mat is a good wound dressing candidate because of its unique properties; the small pores and very high specific surface area not only inhibit exogenous microorganism invasions, but also assist in control of fluid drainage. In addition, the electrospinning process provides a simple way to add drugs into the nanofibers for any possible antibacterial purposes and medical treatment (CitationFang et al. 2011). A study conducted on polyurethane nanofibers formed by electrospinning technique revealed that the mat effectively exuded fluid from the wound without any fluid accumulation (CitationKhil et al. 2003). The mat also showed excellent oxygen permeability, besides inhibiting the invasion of exogenous microorganism. Dressing meant for wound healing protects the wound, exudes extra body fluids from the wound, and accelerates the wound healing process. To carry out these functions, the wound dressing material acts as a physical barrier to the wound and, at the same time, is permeable to oxygen and moisture.
Nanofibers as drug delivery system
To deliver the pharmaceuticals/drugs to the patient in a more physiologically acceptable manner has always been a matter of concern in medicine. The drug taken orally for various diseases is delivered to the damaged site, but the amount gets decreased against initial dosage. The release rate of a drug from electrospun nanofibers can be manipulated by altering the nanofiber composition, porosity, and its morphology so that effective drug concentration is maintained at the damaged site (CitationKaur et al. 2014). The electrospun nanofibers, being excellent carriers, protect the drug from decomposition in case of systemic application. The nanofibers can be used as carrier for drug delivery at the localized site of wound, thereby significantly reducing systemic absorption of drug and also, at the same time, reducing any side effects from the drugs. The delivery of drugs with polymer nanofibers is based on the fact that as the surface area of both the drug and the corresponding carrier is increased and dissolution rate of drug is also increased (CitationGoyal et al. 2013). Depending on the type of polymer carrier used for the formation of nanofibers, the release of pharmaceutical dosage form can be designed as delayed, immediate, rapid, or modified dissolution. Nanofibers have been effectively used as carriers for the delivery of some proteins (CitationCasper et al. 2005), polysaccharides (CitationZeng et al. 2005), growth factors (CitationMetwally et al. 2008), and some anticancer drugs (CitationLuong-Van et al. 2006). Researchers have electrospun a variety of solutions containing low-molecular-weight drugs including lipophilic drugs such as ibuprofen (CitationJiang et al. 2004), rifampin, Paclitaxel (CitationZeng et al. 2003), Cefazolin (CitationKatti et al. 2004) and hydrophilic drugs such as tetracycline hydrochloride (CitationKenawy et al. 2002) and mefoxin (CitationZong et al. 2002).
Cosmetics
Electrospun nanofibers have been used as a skin care mask for the treatment of skin cleansing, skin healing, and other medical and therapeutical properties (CitationLim and Ramakrishna 2005). The electrospun nanofibrous skin mask has high specific surface area that speeds up the transfer rate of additives to the skin. Owing to very high surface area to volume ratio and very small pore size, electrospun nanofibers can be used as face masks. To improve skin health, skin revitalizing factors can also be incorporated into nanofiber masks (CitationRamakrishna et al. 2006). The most interesting fact of the electrospun nanofibers mask is that it can be applied painlessly, gently, and directly to the skin to provide healing to the skin.
Filtration applications
Polymeric nanofibers have found wide applications in the field of filtration. For the purpose of filtration, the filter should have such properties so that the particles will get easily entrapped. Polymeric electrospun nanofibers have excellent properties of a filter because of their very high specific surface area to volume ratio, particles of the order of less than 0.5 mm can be easily trapped. The mechanism of trapping small particles, droplets is not only by physical entrapment but also by electrostatic attraction. Nanofiber membranes made of some special polymers can also be used for filtration and detection of some biological and chemical weapon agents (CitationGraham 2002). Nanofibers were used as a supporting scaffold in ultra-filtration (UF) for oil/water emulsion separation. The reported UF mat has a three-layered composite structure consisting of a cross-linked PVA electrospun nanofibrous mid-layer, a conventional nonwoven micro fibrous substrate, and a nonporous hydrophilic top layer (CitationYoon et al. 2006, CitationTang et al. 2009). The electrospun nanofibrous mat has large specific surface area and well-interconnected porous network. The UF filter provides excellent organic solute rejection capability. In military applications, they are used in isolating bags and uniform garments to decontaminate aerosol dusts, virus, and bacteria (CitationFang et al. 2011). To improve the filtration efficiency, nanofiber membranes can be electrostatically charged without increase in pressure drop by combining spinning and charging of polymer into nanofibers in one step (CitationTsai et al. 2002).
Protective clothing applications
Protective clothing find applications in warfare conditions as it maximizes the chances of sustainability, survivability of the solider against harsh environmental conditions, and biological chemical, and nuclear warfare agents. Electrospun nanofibers have been recognized as suitable candidates for protective clothing applications as they have all the properties of an ideal protective clothing such as light weight, high porosity, large surface area, resistant to penetration of harmful chemical agents, and good filtration efficiency (CitationSchreuder-Gibson et al. 2002, CitationGibson et al. 1999).
In immobilization of enzymes
Enzymes are used as catalysts in chemical reactions to increase the rate of reaction. Immobilization of enzymes improves the functionality and performances of enzymes for bioprocessing applications as they offer several advantages such as better control reaction and reusability (CitationJia et al. 2002, CitationBacheva et al. 2003, CitationXie and Hsieh 2003, CitationÖnal and Telefoncu 2003, CitationYang et al. 2004). Immobilization efficiency mainly depends upon matrix–enzyme interaction and porous structure of carrier system. Several approaches have been used for immobilizing enzymes on electrospun nanofibers, including physical adsorption, grafting enzyme on fiber surface, and incorporating enzyme into nanofiber by electrospinning, followed by cross-linking reaction. For grafting of enzyme on the nanofiber surface, the polymer should possess reactive groups for chemical interaction (CitationWang et al. 2004, Citation2006b, CitationStoilova et al. 2010). Enzymes can also be incorporated into nanofibers via electrospinning, and subsequent cross-linking the enzymes incorporated effectively prevented their leaching. Electrospun nanofiber may act as a catalyst carrier, because the large surface area could provide a large number of active sites, thus enhancing the catalytic capability. Nanofiber membranes provide a number of advantages as these can be synthesized into various structures such as well-aligned trays and are more durable than other carbon tubes or nanoparticles (CitationWang et al. 2006a). Several researchers have employed co-electrospinning method for immobilization of enzymes into nanofibers with high enzyme-loading activity. Jia et al. have produced polystyrene nanofiber by electrospinning technique for immobilization of α-chymotrypsin and found 65% hydrolytic activity of immobilized enzyme than that of free enzyme (CitationJia et al. 2002).
Energy harvest and storage applications
Nanofibers have been used as a storage media for energy sources such as natural gases and hydrogen gas. Nanofibrous materials have the ability to convert various forms of energies into electric power, thus providing solutions to energy crisis. Solar cells use solar energy for power generation. Presently, polycrystalline and single crystal–based solar cells are predominant in the solar cell market. Fuel cells are devices capable of converting hydrogen-rich fuels or hydrogen into electric current using a metal catalyst. Currently, different kinds of fuel cells, such as direct methanol fuel cells, proton exchange mat fuel cells, solid oxide fuel cells, and alkaline fuel cells, are available (CitationSundmacher 2010). Proton exchange mat fuel cells are the most important because of low operating temperature and high power density.
Affinity membrane
Affinity membranes find application in removing organic molecules from waste water. For example, a research was carried out in which β-cyclodextrin was introduced into a polymethyl methacrylate nanofiber membrane through physical mixing, and it was found that β-cyclodextrin formed inclusion complex with hydrophobic organic molecules present in waste water (CitationKaur et al. 2006). Several researches have been conducted wherein researchers have used electrospun nanofiber mesh as affinity membrane and functionalized surface with ligands. Gibson and Bamford have used electrospun nanofibers mesh as affinity membrane where the membrane surface was functionalized with ligands involving covalent bond interaction (CitationBamford et al. 1992, CitationGibson et al. 2001, CitationGopal et al. 2006). Ramakrishna and Ma have formulated and characterized protein A/G functionalized electrospun regenerated cellulose nanofiber mesh as affinity membrane for immunoglobulin G purification (CitationMa and Ramakrishna 2008).
Some challenges in nanofibers
Although electrospinning is the most widely used technique for the fabrication of nanofibers, there are some challenges associated with this process, particularly while fabricating electrospun nanofiber–based drug delivery system. The manufacturing process of nanofibers is quite expensive as compared to that of conventional fibers because of high cost of technology and low production rate. To keep control on properties and mass production of nanofibers, one needs to understand how electrospinning process transforms a polymers liquid system into solid nanofibers, which are ultrafine in diameter, through a millimeter-diameter capillary tube. The vapors emitted during the electrospinning process pose a health threat. Therefore, the vapors emitted need to be disposed of in an environmental-friendly manner.
Conclusion
Presently, nanofibers have gained wide importance, particularly in the controlled drug delivery systems, because of the numerous advantages such as high porosity and high surface area to volume ratio, which make them suitable for a wide range of applications. Electrospinning technique is the most widely used technique for the production of nanofibers. There are several problems that must be addressed for further applications such as the stability of active agents, the initial burst effect, the residual organic solvent, combined usage of new biocompatible polymers, and the drug loading. Improved large-scale nanofiber production techniques provide a large amount of high-quality nanofibers and reduce the application cost. According to the emerging trends, new discoveries and patents promote nanofibers in upcoming years and clinical studies soon to be expected.
Acknowledgement
Authors Goutam Rath and Amit K Goyal thank the Department of Biotechnology (DBT), New Delhi, India.
Declaration of interest
The author report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Agarwal S, Wendorff JH, Greiner A. 2008. Use of electrospinning technique for biomedical applications. Polymer. 49:5603–5621.
- Bacheva A, Baibak O, Belyaeva A, Oksenoit E, Velichko T, Lysogorskaya E, et al. 2003. Activity and stability of native and modified subtilisins in various media. Biochemistry (Moscow). 68:1261–1266.
- Bajáková J, Chaloupek J, Lukáš D, Lacarin M. 2011. “Drawing”–The Production of Individual Nanofibers by Experimental Method. 21–23 September 2011, Brno, Czech Republic, EU.
- Bamford C, Al-Lamee K, Purbrick M, Wear T. 1992. Studies of a novel membrane for affinity separations: I. Functionalisation and protein coupling. J Chromatogr A. 606:19–31.
- Buchko CJ, Chen LC, Shen Y, Martin DC. 1999. Processing and microstructural characterization of porous biocompatible protein polymer thin films. Polymer. 40:7397–7407.
- Casper CL, Yamaguchi N, Kiick KL, Rabolt JF. 2005. Functionalizing electrospun fibers with biologically relevant macromolecules. Biomacromolecules. 6:1998–2007.
- Charles PP Jr, Owens F. 2003. Introduction to Nanotechnology. Hoboken, NJ: A-Wiley Interscience Publication
- Chen Z, Foster MD, Zhou W, Fong H, Reneker DH, Resendes R, Manners I. 2001. Structure of poly (ferrocenyldimethylsilane) in electrospun nanofibers. Macromolecules. 34:6156–6158.
- Chiti F, Stefani M, Taddei N, Ramponi G, Dobson CM. 2003. Rationalization of the effects of mutations on peptide andprotein aggregation rates. Nature. 424:805–808.
- Chun I, Reneker DH, Fong H, Fang X, Deitzel J, Tan NB, Kearns K. 1999. Carbon nanofibers from polyacrylonitrile and mesophase pitch. J Adv Mater. 31:36–41.
- Deitzel J, Kosik W, Mcknight S, Beck Tan N, Desimone J, Crette S. 2002. Electrospinning of polymer nanofibers with specific surface chemistry. Polymer. 43:1025–1029.
- Demczyk B, Wang Y, Cumings J, Hetman M, Han W, Zettl A, Ritchie R. 2002. Direct mechanical measurement of the tensile strength and elastic modulus of multiwalled carbon nanotubes. Mater Sci Eng A. 334:173–178.
- Demir MM, Yilgor I, Yilgor EEA, Erman B. 2002. Electrospinning of polyurethane fibers. Polymer. 43:3303–3309.
- Doshi J, Reneker DH. 1995. Electrospinning process and applications of electrospun fibers. J Electrostat. 35:151–160.
- Fang J, Wang X, Lin T. 2011. Functional applications of electrospun nanofibers. Nanofibers-production, Properties and Functional Applications. Rijeka, Croatia: InTech - Open Access Publisher, pp. 287–326.
- Fong H, Liu W, Wang C-S, Vaia RA. 2002. Generation of electrospun fibers of nylon 6 and nylon 6-montmorillonite nanocomposite. Polymer. 43:775–780.
- Gagandeep Garg T., Malik B, Rath G, Goyal AK. 2014. Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur J Pharm Sci. 53:10–6.
- Garg T, Goyal AK. 2014. Biomaterial-based scaffolds – current status and future directions. Expert Opin Drug Deliv. 11:767–789.
- Garg T, Singh O, Arora S, Murthy R. 2012. Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst. 29:1–63.
- Garg T, Singh S, Goyal AK. 2013. Stimuli-sensitive hydrogels: an excellent carrier for drug and cell delivery. Crit Rev Ther Drug Carrier Syst. 30:369–409.
- Gibson P, Schreuder-Gibson H, Rivin D. 2001. Transport properties of porous membranes based on electrospun nanofibers. Colloids Surf A Physicochem Eng Asp. 187:469–481.
- Gibson P, Schreuder-Gibson H, Rivin D. 1999. Electrospun fiber mats: transport properties. AIChE J. 45:190–195.
- Gopal R, Kaur S, Ma Z, Chan C, Ramakrishna S, Matsuura T. 2006. Electrospun nanofibrous filtration membrane. J Memb Sci. 281: 581–586.
- Goyal G, Garg T, Malik B, Chauhan G, Rath G, Goyal AK. 2013. Development and characterization of niosomal gel for topical delivery of benzoyl peroxide. Drug Deliv.
- Graham S. 2002. Smart'silicon dust could help screen for chemical weapons. Scientific American, 3.
- Hartgerink JD, Beniash E, Stupp SI. 2002. Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proc Natl Acad Sci. 99:5133–5138.
- Hua FJ, Kim GE, Lee JD, Son YK, Lee DS. 2002. Macroporous poly (L-lactide) scaffold 1. Preparation of a macroporous scaffold by liquid–liquid phase separation of a PLLA–dioxane–water system. J Biomed Mater Res. 63:161–167.
- Huang L, Nagapudi K, Apkarian RP, Chaikof EL. 2001. Engineered collagen–PEO nanofibers and fabrics. J Biomater Sci Polym Ed. 12:979–993.
- Huang Z-M, Zhang Y-Z, Kotaki M, Ramakrishna S. 2003. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol. 63:2223–2253.
- Jia H, Zhu G, Vugrinovich B, Kataphinan W, Reneker DH, Wang P. 2002. Enzyme-carrying polymeric nanofibers prepared via electrospinning for use as unique biocatalysts. Biotechnol Prog. 18:1027–1032.
- Jiang H, Fang D, Hsiao B, Chu B, Chen W. 2004. Preparation and characterization of ibuprofen-loaded poly (lactide-co-glycolide)/poly (ethylene glycol)-g-chitosan electrospun membranes. J Biomater Sci Polym Ed. 15:279–296.
- Kataria K, Garg T, Goyal AK, Rath G. 2014. Novel technology to improve drug loading in polymeric nanofibers. Drug Deliv Lett. 4:79–86.
- Katti DS, Robinson KW, Ko FK, Laurencin CT. 2004. Bioresorbable nanofiber-based systems for wound healing and drug delivery: optimization of fabrication parameters. J Biomed Mater Res B Appl Biomater. 70:286–296.
- Kaur M, Garg T, Rath G, Goyal AK. 2014. Current nanotechnological strategies for effective delivery of bioactive drug molecules in the treatment of tuberculosis. Crit Rev Ther Drug Carrier Syst. 31:49–88.
- Kaur S, Kotaki M, Ma Z, Gopal R, Ramakrishna S, Ng S. 2006. Oligosaccharide functionalized nanofibrous membrane. Int J Nanosci. 5:1–11.
- Kenawy E-R, Bowlin GL, Mansfield K, Layman J, Simpson DG, Sanders EH, Wnek GE. 2002. Release of tetracycline hydrochloride from electrospun poly (ethylene-co-vinylacetate), poly (lactic acid), and a blend. J Control Release. 81:57–64.
- Khil MS, Cha DI, Kim HY, Kim IS, Bhattarai N. 2003. Electrospun nanofibrous polyurethane membrane as wound dressing. J Biomed Mater Res B Appl Biomater. 67:675–679.
- Kim GH, Han H, Park JH, Kim WD. 2007. An applicable electrospinning process for fabricating a mechanically improved nanofiber mat. Polym Eng Sci. 47:707–712.
- Kwoun SJ, Lec R, Han B, Ko F. 2000. A novel polymer nanofiber interface for chemical sensor applications. Frequency Control Symposium and Exhibition, Kansas City, MO, 07 Jun 2000–09. Proceedings of the 2000 IEEE/EIA International, 2000. IEEE, 52–57.
- Lee KH, Kim HY, La YM, Lee DR, Sung NH. 2002. Influence of a mixing solvent with tetrahydrofuran and N, N-dimethylformamide on electrospun poly (vinyl chloride) nonwoven mats. J Polym Sci Part B Polym Phys. 40:2259–2268.
- Lee S-H, Ku B-C, Wang X, Samuelson L, Kumar J. 2001. Design, synthesis and electrospinning of a novel fluorescent polymer for optical sensor applications. MRS Proceedings, 2001. Cambridge: Cambridge University Press.
- Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. 2002. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 60:613–621.
- Lim T-C, Ramakrishna S. 2005. Next-generation applications for polymeric nanofibres. In: Schulte J, Ed., Global Strategies, Industry Trends and Applications, Chapter 8. New York: John Wiley & Sons, pp. 137–147.
- Luong-Van E, Grøndahl L, Chua KN, Leong KW, Nurcombe V, Cool SM. 2006. Controlled release of heparin from poly (ϵ-caprolactone) electrospun fibers. Biomaterials. 27:2042–2050.
- Ma Z, Ramakrishna S. 2008. Electrospun regenerated cellulose nanofiber affinity membrane functionalized with protein A/G for IgG purification. J Memb Sci. 319:23–28.
- Megelski S, Stephens JS, Chase DB, Rabolt JF. 2002. Micro-and nanostructured surface morphology on electrospun polymer fibers. Macromolecules. 35:8456–8466.
- Metwally M, Cheong Y, Li TC. 2008. A review of techniques for adhesion prevention after gynaecological surgery. Curr Opin Obstet Gynecol. 20:345–352.
- Nair S, Natarajan S, Kim SH. 2005. Fabrication of electrically conducting polypyrrole-poly (ethylene oxide) composite nanofibers. Macromol Rapid Commun. 26:1599–1603.
- Nam YS, Park TG. 1999. Biodegradable polymeric microcellular foams by modified thermally induced phase separation method. Biomaterials. 20:1783–1790.
- Numata K, Sato K. 2013. Self-assembly of saponite nanoparticles influenced by interlayer H2O molecules. Int J Environ Sci Dev. 4:633.
- Önal S, Telefoncu A. 2003. Preparation and properties of α-galactosidase chemically attached to activated chitin. Artif Cells Blood Substit Biotechnol. 31:339–355.
- Parnami N, Garg T, Rath G, Goyal AK. 2013. Development and characterization of nanocarriers for topical treatment of psoriasis by using combination therapy. Artif Cells Nanomed Biotechnol.
- Ramakrishna S, Fujihara K, Teo W-E, Lim T-C, Ma Z. 2005. An Introduction to Electrospinning and Nanofibers. Singapore: World Scientific.
- Ramakrishna S, Fujihara K, Teo W-E, Yong T, Ma Z, Ramaseshan R. 2006. Electrospun nanofibers: solving global issues. Mater Today. 9:40–50.
- Rathinamoorthy R. 2012. Nanofiber for drug delivery system–principle and application. Pak Text J. 61:45–48.
- Reneker DH, Chun I. 1996. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology. 7:216.
- Schreuder-Gibson H, Gibson P, Senecal K, Sennett M, Walker J, Yeomans W, et al. 2002. Protective textile materials based on electrospun nanofibers. J Adv Mater. 34:44–55.
- Singh H, Sharma R, Joshi M, Garg T, Goyal AK, Rath G. 2014. Transmucosal delivery of Docetaxel by mucoadhesive polymeric nanofibers. Artif Cells Nanomed Biotechnol.
- Srinivasan G, Reneker DH. 1995. Structure and morphology of small diameter electrospun aramid fibers. Polym Int. 36:195–201.
- Stoilova O, Manolova N, Gabrovska K, Marinov I, Godjevargova T, Mita DG, Rashkov I. 2010. Electrospun polyacrylonitrile nanofibrous membranes tailored for acetylcholinesterase immobilization. J Bioact Compat Polym. 25:40–57.
- Sundmacher K. 2010. Fuel cell engineering: toward the design of efficient electrochemical power plants. Ind Eng Chem Res. 49: 10159–10182.
- Tang Z, Qiu C, Mccutcheon JR, Yoon K, Ma H, Fang D, et al. 2009. Design and fabrication of electrospun polyethersulfone nanofibrous scaffold for high-flux nanofiltration membranes. J Polym Sci Part B Polym Phys. 47:2288–2300.
- Tsai PP, Schreuder-Gibson H, Gibson P. 2002. Different electrostatic methods for making electret filters. J Electrostat. 54:333–341.
- Wang H, Shao H, Hu X. 2006a. Structure of silk fibroin fibers made by an electrospinning process from a silk fibroin aqueous solution. J Appl Polym Sci. 101:961–968.
- Wang X, Kim Y-G, Drew C, Ku B-C, Kumar J, Samuelson LA. 2004. Electrostatic assembly of conjugated polymer thin layers on electrospun nanofibrous membranes for biosensors. Nano Lett. 4:331–334.
- Wang ZG, Xu ZK, Wan LS, Wu J, Innocent C, Seta P. 2006b. Nanofibrous membranes containing carbon nanotubes: electrospun for redox enzyme immobilization. Macromol Rapid Commun. 27:516–521.
- Warner SB, Buer A, Ugbolue S, Rutledge G, Shin M. 1998. A fundamental investigation of the formation and properties of electrospun fibers. National textile center annual report, 83–90.
- Xie J, Hsieh Y-L. 2003. Ultra-high surface fibrous membranes from electrospinning of natural proteins: casein and lipase enzyme. J Mater Sci. 38:2125–2133.
- Yang F, Murugan R, Ramakrishna S, Wang X, Ma Y-X, Wang S. 2004. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials. 25:1891–1900.
- Yoon K, Kim K, Wang X, Fang D, Hsiao BS, Chu B. 2006. High flux ultrafiltration membranes based on electrospun nanofibrous PAN scaffolds and chitosan coating. Polymer. 47:2434–2441.
- Yu D-G, Zhu L-M, White K, Branford-White C. 2009. Electrospun nanofiber-based drug delivery systems. Health. 1:67–75.
- Zeng J, Aigner A, Czubayko F, Kissel T, Wendorff JH, Greiner A. 2005. Poly (vinyl alcohol) nanofibers by electrospinning as a protein delivery system and the retardation of enzyme release by additional polymer coatings. Biomacromolecules. 6:1484–1488.
- Zeng J, Xu X, Chen X, Liang Q, Bian X, Yang L, Jing X. 2003. Biodegradable electrospun fibers for drug delivery. J ControlRelease. 92: 227–231.
- Zhang S. 2003. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 21:1171–1178.
- Zong X, Fang D, Kim K, Ran S, Hsiao B, Chu B, et al. 2002. Nonwoven nanofiber membranes of poly (lactide) and poly (glycolide-co-lactide) via electrospinning and applications for anti-adhesions. Abstracts of papers of the American Chemical Society, 2002. Amer Chemical Soc 1155 16TH ST, NW, Washington, DC 20036 USA, U466–U466.
- Zussman E, Yarin A, Weihs D. 2002. A micro-aerodynamic decelerator based on permeable surfaces of nanofiber mats. Exp Fluids. 33:315–320.