Abstract
Introduction
Adipose tissue-derived mesenchymal stem cells (AT-MSCs) are suitable choices in autologous stem cell treatment of liver-associated diseases due to their hepatic differentiation potential. Dimethyl sulfoxide (DMSO) is an amphipathic molecule with potential of delivering both lipophilic and hydrophilic agents into cells, also a common cryoprotectant for freezing of the cells. DMSO was used in some protocols for induction of AT-MSCs towards hepatocyte like cells. However, the effect of DMSO on hepatogenic differentiation of AT-MSCs were not surveyed, previously. In the present study, we aimed at evaluation of the effect of DMSO on differentiation of AT-MSCs into hepatic lineage.
Methods
We isolated mesenchymal stem cells (MSCs) from adipose tissue, and then verifies multi-potency and surface markers of AT-MSCs . Isolated AT-MSCs randomly dispensed in four groups including Group 1: HGF treated, 2: HGF+ DMSO treated, 3: HGF+ DMSO+ OSM treated, and group control for a period of 3 weeks in the expansion medium without serum; EGF and bFGF were also included in the first days of inductions. The morphologic changes during induction period was observed with microscopy. The secretion of albumin (ALB) of the differentiating MSCs was investigated using ELISA, and urea production was evaluated using colorimetric assay. The qRT-PCR was performed for quantitation of hepatocyte marker genes including AFP, ALB, CK18, HNF4a, and HNF6. The glycogen storage of differentiated cells was visualized by periodic-acid Schiff‘s staining.
Results
The results demonstrate that DMSO speeds up hepatic differentiation of AT-MSCs characterized by rapid changes in morphology; higher expression of hepatic marker gene (ALB) in both mRNA and protein level (P < 0.05); also increased transcriptional levels of other liver genes including CK18, HNF4a, and HNF6 (P < 0.01); and moreover, greater percentage of glycogen storage(p < 0.05) in DMSO-treated groups.
Conclusion
DMSO catalyzes hepatic differentiation; therefore, using DMSO for acceleration of the hepatogenic protocols of AT-MSCs appears advantageous.
Introduction
Hepatocytes constitute approximately 60% of all cells of the liver and are responsible for the majority of the biochemical functions of the organ (CitationKuo et al. 2011). Detoxification of xenobiotics and hazardous materials entering body is another main biologic duty of hepatocytes. These features make hepatocytes as not only an attractive tool for studying xenobiotic biotransformation, but also target cells for toxic reactions (CitationGomez-Lechon et al. 2007, CitationSturgill and Lambert 1997). Liver cell transplantation and bio-artificial liver may provide metabolic support for liver function temporarily and are prospective treatments for patients with liver failure (CitationWu and Tao 2012). The focus of both clinical and basic studies on stem cells is increasing due to their potentials in regenerative medicine and cell-based therapies (CitationBakhshandeh et al. 2012). Recent in vitro studies have demonstrated that mesenchymal stem cells (MSCs) from various sources, including human bone marrow, adipose tissue, and umbilical cord, can be differentiated into hepatocyte-like cells when appropriate conditions are used. In particular, interest exists for human adipose–derived stems cells as an attractive cell source for generating hepatocyte-like cells (CitationAl et al. 2011). In our last studies, the differentiation of adipose tissue-derived mesenchymal stem cell (AT-MSCs) into mesenchymal cell types including adipocytes, chondrocytes, and osteoblasts was well defined (CitationEslaminejad et al. 2006, Citation2007, Citation2013). In the present study, we focused on hepatocyte differentiation of AT-MSCs.
In order to induce MSC differentiation into mature hepatocytes invitro, it is essential to have adequate stimuli for the maintenance of cellular function, such as growth hormones, cytokines, extracellular matrix, or co-culture with other celltypes (CitationWu and Tao 2012). Accordingly, the development of efficient and quick methods for achievement of enough sources of hepatocytes applicable in different studies and therapeutic approaches seems necessary.
Chemical compounds such as dimethylsulfoxide (DMSO) were used with other reagents in differentiation protocols. DMSO is an amphipathic molecule with a highly polar domain and two apolar methyl groups, making it soluble in both aqueous and organic media (CitationSantos et al. 2003). DMSO is a safe and effective mechanism for facilitating the transdermal delivery of both hydrophilic and lipophilic medications to provide localized drug delivery (CitationMarren 2011), furthermore is routinely used for cryopreservation of the cells in freezing media.
Recently several studies (CitationBanas et al. 2009, CitationOkura et al. 2010, CitationSeo et al. 2005) used DMSO in differentiation procedure of adipose-mesenchymal stem cells towards hepatocyte-like cells (HLCs). However, the effects of using DMSO agent on hepatogenic differentiation were not evaluated, previously.
In this study, we aimed at evaluation of using DMSO in differentiation of AT-MSCs into hepatic lineage. The isolation of AT-MSCs was performed, and then identity and multi-potency of them were verified. They were treated in four mediums including Group 1: HGF treated, Group 2: HGF+ DMSO treated, Group 3: HGF+ DMSO+ OSM treated, and Group control for a period of 3 weeks. The morphologic changes, secretion of albumin (ALB), urea production, and glycogen storage of the differentiated AT-MSCs were investigated. The qRT-PCR was performed for quantitation of hepatocyte marker genes including AFP, ALB, CK18, HNF4a, and HNF6. The results indicates that DMSO accelerates hepatic differentiation of AT-MSCs characterized by rapid morphologic specification from fibroblastic into polygonal shape; higher expression of hepatic marker gene (ALB) in both mRNA and protein level; also increased transcriptional levels of other hepatic genes including CK-18, HNF4a, and HNF6; and moreover, greater percentage of glycogen storage in DMSO-treated groups in comparison with non-treated and AT-MSCs. These results demonstrated that hepatic differentiation of AT-MSCs could be enhanced using DMSO.
Materials and methods
Ethical statement
This study was approved by University Ethics Committee, and written informed consent was signed by all donors before any experimental work.
AT-MSCs isolation and culturing
Adipose tissue was acquired from normal donors within the age range of 40–50 years. The isolation of AT-MSCs from adipose tissue was performed using previously published protocol (CitationBaglioni et al. 2009). Adipose tissue samples were immediately placed in DMEM/F12 containing 100 μg/ml streptomycin and 200 U/ml penicillin. Adipose tissue samples were washed in PBS, minced, and digested with 1% collagenase type I in 0.1% BSA for 1 h at 37°C in a shaking water bath. The pellet was collected by centrifugation and then treated with red blood cell lysis buffer (155 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA) for 10 min at room temperature. The pellet was carefully placed on Ficoll (histopaque) and centrifuged at 400 g for 30 min. The second layer, stromal vascular fraction (SVF), was picked up and centrifuged. The SVF pellet was re-suspended in flask with DMEM containing 10% fetal bovine serum (FBS), 1X ITS (Sigma), dexamethasone 1 × 10− 6 M, Penstrp (Gibco) and incubated at 37°C in humidified atmosphere with 5% CO2. After 24 h, non-adherent cells were removed by exchanging the medium. Media was changed twice weekly.
Immunophenotyping of AT-MSC
For immunophenotyping of surface antigens, passage 3 of AT-MSCs was cultivated at fibronectin-coated plates and trypsinized at 70% confluency. About 2 × 105 cells washed with PBS and then incubated with FITC or PE-conjugated antibodies including the following: CD44-FITC, CD31-FITC, CD90-PE, CD73-PE, CD105-PE, CD34-PE, and CD45-PE. Analysis of immuno-stained cell was performed using a flow cytometer (BD FACS Caliber, BD Biosciences, and San Jose, CA, USA).
Differentiation potential evaluation
AT-MSCs were seeded onto 6-well plates at a density of 2.5–3 × 104 cells/cm2. At a confluency of 70–80%, we induced differentiation into adipocytes or osteocytes. AT-MSCs were cultured in DMEM-containing Osteocyte Differentiation Medium (Bioidea, Iran) according to the manufacturer's instructions. After 21 days, cells were fixed and stained with Alizarin Red (Bioidea, Iran). To induce differentiation into adipocytes, AT-MSC were cultured in adipocyte differentiation medium (Bioidea, Iran). After induction period, cells were stained with Oil Red O (Sigma-Aldrich). The differentiation of AT-MSCs was observed using invert microscope (Hund wetzerland, Germany).
Cell viability assessment
We utilized Trypan Blue dye for checking the percentage of the viable cells before and 48, 72 h, and one week after DMSO treatment (concentrations: 0.1, 0.5, 1, 1.5, and 2%). Cell suspensions were mixed with Trypan Blue, and the non-viable cells were counted under inverted microscope(Hund Wetzlar, Germany). All the experiments were performed in triplicates.
Hepatic differentiation
We classified AT-MSCs into four groups. The groups are as following: control group, and differentiation groups including HGF (Gibco, Invitrogen)-treated group (10 ng/ml), HGF + 0.1% DMSO (Sigma-Aldrich, USA) and HGF+ OSM (Sigma-Aldrich, USA) + DMSO (10 ng/ml, 10 ng/ml, and 0.1% DMSO) in serum-free expansion medium. All of differentiation groups were induced for 21 days and for all differentiation groups EGF (Gibco, Invitrogen), and bFGF (Sigma-Aldrich, USA) was administered both in the concentration of 10 ng/ml up to day 2 and in that from days 3–7, respectively.
Morphological observations of differentiation
The observation of the morphological changes in differentiating cells was performed using invert microscope (Hund wetzerland, Germany).
Periodic acid shiff's staining
For evaluation of polysaccharide storage potential among differentiation groups, we used PAS staining. After formalin fixation and Triton-X100 (Sigma-Aldrich, USA) permeabilization of HLCs in day 21, 1% periodic acid (Sigma-Aldrich, USA) was applied; finally, the cells exposed to Schiff reagent (Sigma-Aldrich, USA). The percent of PAS-positive cells was counted in four microscopic fields of each group after staining, and the means were calculated.
Albumin secretion
Culture supernatants harvested from each group of cells, after 21 days of induction and analyzed for albumin concentrations using enzyme-linked Immunosorbent assay (ELISA) kit (Pars Azmun, Iran). The results were compared with those of control group.
Urea production
The urea production is a detoxifying function of hepatocytes. On days 7, 14, and 21 of differentiation, the 6 mM NH4Cl was incubated (37°C, 5% CO2) for 1 day with each group and non-induced AT-MSCs as negative control. The urea concentrations was assayed in superior media using a colorimetric assay kit (Pars Azmun, Iran), according to the recommendations of the manufacturer.
Real-time quantitative PCR analysis
Total RNA was isolated from passage 3 of cultured AT-MSCs and day 21 of differentiation using Trizol reagent (Invitrogen, USA) according to the manufacturer's protocol. 1 μg of RNA was employed to synthesize cDNA by Revertide cDNA synthesis kit (Fermentase, Life Science, and USA) with Oligo dT18 primers. To detect the AFP, CK-18, HNF4a, and HNF6 mRNAs, we employed the SYBR Green Master mix (Takara, Japan) using the primers listed in . The samples were analyzed in triplicate for each group for verification of the results.
Table I. Primers used for qRT-PCR.
Statistical analysis
We used ANOVA and T-test for comparing different groups. P values of < 0.05 were considered significant. Each experiment was done in triplicate.
Results
Isolation, cultivation, and multi-lineage differentiation of AT-MSCs
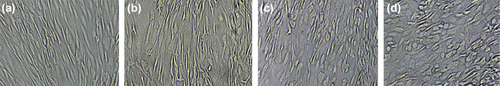
We successfully isolated AT-MSCs from adipose tissue of donors, by previously published protocol. On first day, isolated cells showed rounded morphology that start to attaching plastic after 48 h and then turned into spindle-shaped morphology () and a week later reached 60% confluency (). The potential of the AT-MSCs to differentiate into osteoblast and adipocytes was confirmed by osteogenic and adipogenic induction at passage 3; moreover, the accumulations of the calcium deposits and oil vacuoles after differentiation were visualized by alizarin red and oil red staining, respectively (, ).
Figure 1. The morphological appearance of isolated mesenchymal stem cells derived from adipose tissue. (a) Cells begin to adhere to the plastic surface in P0 after 48 h. (b) Cells after 7 days of culture in P0. Bars, 50 μm.
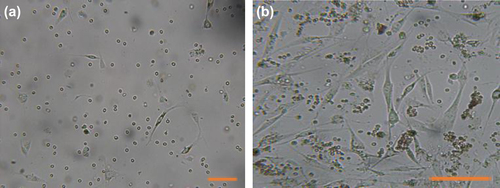
Figure 2. Differentiation potential of AT-MSC. Passage 3 of isolated AT-MSCs (a) Microscopic image views of the differentiation of AT-MSCs into adipogenic (b), osteogenic (c) lineages. Oil red staining shows lipid droplets stained red, deposits of calcium crystals stained red to brown by alizarin red staining (c). Bars, 50 μm.
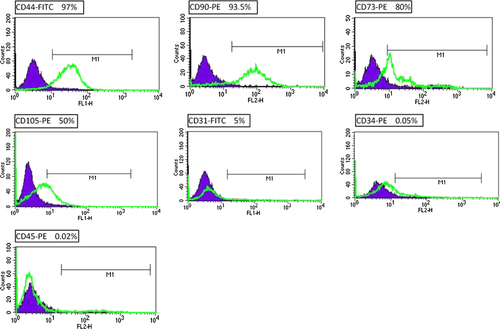
Immunophenotype of AT-MSCs
Flowcytometry analysis showed that AT-MSCs are positive for CD-44 (97%), CD-90(93.5%), CD73 (80%), and CD105 (50%), but negative for CD31 (5%), CD34 (0.05%), and CD45 (0.03%). These results confirmed the identity of AT-MSCs ().
Cell viability
We find out that higher concentrations are significantly (p < 0.05) cytotoxic in long-term culturing (for 1 week). Therefore, the concentration: 0.1% was used for induction.
Morphological observations
AT-MSCs were cultivated for 72 h at a density of 2.5–3 × 104 cells/cm2 on 6-well plates pre-coated with 5 μg/ml fibronectin (Sigma, USA). Subsequently, the culture medium was replaced with hepatic induction media containing: Group 1: HGF, Group 2: HGF+ DMSO, Group 3: HGF+ DMSO+ OSM, and Group control for a period of 21 days. After treatment with hepatocyte induction media, the morphological shift of AT-MSCs was not significant up to day 7 of induction period, but from day 8 in DMSO-treated groups, the fusiform morphology of AT-MSCs was changed and the cells displayed polygonal or near-polygonal shapes. Interestingly, the Groups 2 and 3 displayed an early conversion of the cellular shape on days 8 and 9, in comparison with Group1 (HGF treated group) day 12 ().
Urea production
Detoxification of ammonia into urea and its secretion are the main duties of hepatocytes. We investigated urea concentrations in the supernatant of hepatogenic media. The levels of urea between different groups in each time point were summarized in . The production of urea was increased in a time-dependent manner as compared with AT-MSCs, but the difference between three differentiation groups is not statistically significant (p > 0.05).
Figure 5. Urea and Albumin detection. Graph (a) the urea production after addition of NH4Cl in different days and different groups showed no significance among treatments. Graph (b) Concentrations of albumin secreted by AT-MSCs (control) HGF treated, HGF+ DMSO, and HGF+ DMSO+ OSM (at day 21); the albumin levels was significantly higher in DMSO-treated groups.
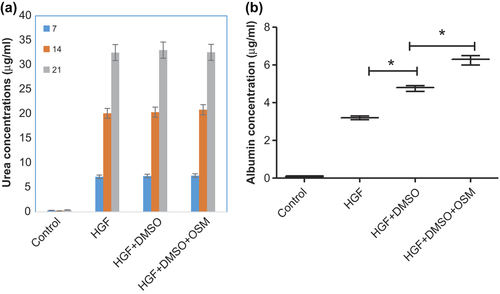
ALB secretion
Albumin protein concentrations in superior media was investigated on day 21 of induction and compared with that of non-induced AT-MSCs. While the ALB protein was undetectable in non-induced AT-MSCs, it was increased after 21 days of induction in cells. A significant (p < 0.01) increase was obvious in ALB levels of DMSO-treated groups (). The albumin levels of HGF+ DMSO+ OSM were higher than those of HGF+ DMSO group (p = 0.009).
Glycogen storage
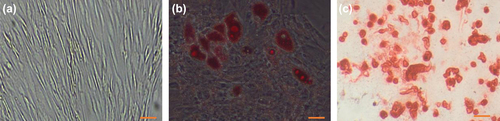
PAS staining was performed for comparison of glycogen storage in different groups (), and the percent of positive cells was counted and summarized in table of . The mean percentage of PAS-positive cells in HGF+ DMSO group (40.3%) was significantly greater than means of HGF-treated and control groups (26% and 2.3%) (p < 0.05), but lower than that of HGF+ DMSO+ OSM (55%) ().
Figure 6. Periodic PAS of control (a), HGF (b), HGF+ DMSO (c), HGF+ DMSO+ OSM (d). representing PAS-positive cells (%) in different groups.
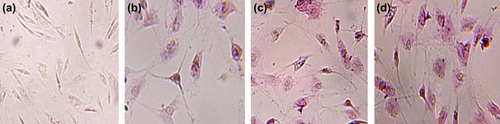
Table II. PAS-positive cells in each groups (%).
QRT-PCR results of other marker genes
The expression level of other marker genes was assessed using qRT-PCR. GAPDH used as normalization control. A total of five genes were analyzed in order to evaluate DMSO's effect on the hepatogenic differentiation of AT-MSCs genes.
The expression of AFP was quantitated at mRNA level on day 21 of differentiation () in three groups by qRT-PCR. The mRNA level of AFP was significantly higher in Group 3 than in Groups 1, 2, and control.
Figure 7. The expression of AFP (a), CK-18 (b), and ALB (c) was investigated by qRT-PCR in three differentiation groups on day 21 and control. GAPDH was used as internal standard. Hepatocyte marker genes, ALB and CK-18, are significantly up-regulated in DMSO-treated groups.
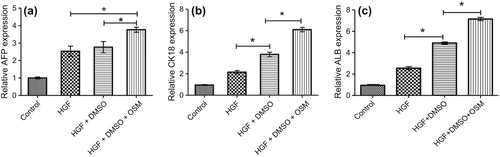
A significant increase (p < 0.05) in mRNA levels was observed between the HGF-treated and HGF + DMSO treated for the following genes: CK-18, ALB, HNF4a, and HNF6 ( and ). Moreover, the HGF+ DMSO+ OSM treatments showed significant increase in effects of DMSO in mRNA levels of ALB and CK-18. No significant increase was seen in the expression of AFP between HGF-treated and HGF+ DMSO-treated groups (P = 0.61), but the difference between HGF+ DMSO-treated and HGF+ DMSO+ OSM-treated groups was significant (P = 0.0046).
Figure 8. Expression of HNF6 (a) and HNF4a (b) treated with HGF, HGF+ DMSO and HGF+ DMSO+ OSM medias for a 21-day period. Genes were quantified and normalized against GAPDH. A significant increase was observed in the levels of both HNF6 and HNF4a in HGF+ DMSO and HGF+ DMSO+ OSM groups as compared with HGF-treated ones (p < 0.0001). Data are represented as the mean + SEM; p < 0.05 was considered significant.
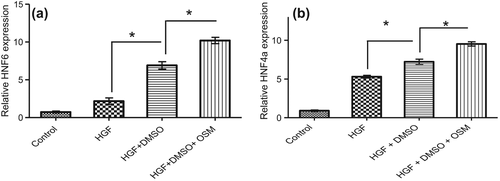
In the case of HNF4a and HNF6, the difference between HGF+ DMSO-treated and HGF+ DMSO+ OSM-treated groups was significant (p = 0.0037 and 0.0069, respectively; ).
Discussion
The International Society for Cellular Therapy (ISCT) has proposed a set of standards to define human Mesenchymal Stem Cells for laboratory investigations and preclinical studies: adherence to plastic in standard culture conditions; in vitro differentiation into osteoblasts, adipocytes, and chondroblasts; specific surface antigen expression in which ≥ 95% of the cells express the antigens recognized by CD105, CD73, and CD90, with the same cells lacking (≤ 2% positive) the antigens CD45, CD34, CD14 or CD11b, CD79a or CD19, and HLA-DR (CitationSousa et al. 2014). In the present study, we isolated AT-MSCs from adipose tissue and cultured in expansion medium. They gained typical morphology and adherence after several days. The multi-potency of them was surveyed by induction of differentiation towards mesodermal lineages including adipocytes and osteoblasts, and confirmed by two indicator stainings. The panel of surface markers was studied using flowcytometry, which were in accordance with the standards of ISCT.
In autologous stem cell therapy, the patient‘s own stem cells are expanded and induced to differentiate into favorite cells, subsequently, transplanted back into the same patient. Multipotential mesenchymal stem cells, present in many organs and tissues, represent an attractive tool for the establishment of a successful stem cell-based therapy in the field of regeneration medicine (CitationBanas 2012). These autologous cells are immuno-compatible and exhibit controlled differentiation and multi-functional abilities, and do not undergo post-transplantation rejection or unwanted differentiation such as formation of teratomas (CitationIshikawa et al. 2010).
A number of recent studies show that extra-hepatic mesenchymal stem cells from different tissues can be differentiated into hepatocyte-like cells (HLCs) (CitationZhang et al. 2012). Therefore, AT-MSC-based therapies may provide a novel approach for hepatic regeneration and hepatocyte differentiation, thereby supporting hepatic function in diseased individuals (CitationIshikawa et al. 2010). Additionally, HLCs derived from AT-MSCs can be used for in vitro studies of cytotoxicity, side effects of drugs, and development of new therapeutic materials. Thus, there is an urgent need for providing hepatocytes applicable in laboratory and regenerative studies.
Several hepatic differentiation protocols of MSC have been published in recent years, based on cellular stimulation with exogenous cytokines/growth factors, co-culture with fetal or adult hepatocytes, 2- or 3-dimensional (2D, 3D) matrices to favor differentiation (CitationZhang et al. 2012) and other methods. In addition, small molecules able to direct hepatic differentiation of MSCs could be the useful chemical tools to clarify the molecular mechanisms that control hepatic fate specification, and to improve the trans-differentiation efficiency of MSCs into hepatocyte-like cells (CitationOuyang et al. 2012).
Simple chemical reagents such as SJA710–6 (CitationOuyang et al. 2012), Trichostatin A (CitationSnykers et al. 2007), 5-azacytidine (CitationAurich et al. 2009), and DMSO (CitationBanas et al. 2009, CitationOkura et al. 2010, CitationSeo et al. 2005) were used in few studies, either separately or with growth factors and cytokines in protocols of hepatic differentiation. Chetty et al. developed a protocol that improves directed differentiation of pluripotent stem cells into multiple lineages by DMSO treatment (CitationChetty et al. 2013).
DMSO has been used for years as a cryoprotectant agent; it acts by penetrating the cell and binding water molecules, and it has been described as harmless for the individual who receives it in limited amounts (CitationRuiz-Delgado et al. 2009). Some reports applied DMSO for differentiation protocols of AT-MSCs towards HLCs (CitationBanas et al. 2009, CitationOkura et al. 2010, CitationSeo et al. 2005); However, the effect of DMSO on hepatic conversion of AT-MSCs was not studied previously. In this work, we assessed the effect of utilizing DMSO at trans-differentiation of AT-MSCs into HLCs. The morphological changes, ureogenesis, ALB secretion, glycogen storage, and the expression of hepatocyte marker gene mRNAs including: AFP, CK-18, HNF4a, and HNF6 were examined.
Previous studies used 0.1% of DMSO for hepatogenic induction of MSCs (CitationBanas et al. 2009, CitationOkura et al. 2010, CitationSeo et al. 2005), and we also performed our study by 0.1% DMSO, because we find out that higher concentrations are cytotoxic in long-term culturing (data not shown); in addition, Wang and Scott reported that DMSO inhibits multiple steps in the process of adipocyte differentiation of MSCs; the maximum inhibitory effects occur at 2% DMSO (CitationWang and Scott 1993).
Liu et al., used DMSO for improvement of differentiation fetal liver stem/progenitor cells(FLSPCs) (CitationLiu et al. 2012); they found that for inducing FLSPCs differentiation, treatment with HGF+ DMSO was most effective, which was strongly supported by the typical morphological change and the significant decrease of OV-6 positive cells. In addition, the percentage of glycogen synthetic cells, and the expressions of ALB, G-6-P, CK-8, CK-18 and CYP450–3A1 in HGF+ DMSO-treated group were higher than in any other group (CitationLiu et al. 2012). Although, we studied AT-MSCs in hepatic fate specification, our PAS, ALB and CK-18 results in HGF+ DMSO-treated group are higher than in HGF-treated and control groups that is in accordance with the study of FLSPCs by Liu and colleagues. We also studied two liver-enriched transcription factors including HNF4a and HNF6. The qRT-PCR results implies that DMSO treatment caused significant increase of both HNF4a and HNF6 in comparison with non-treated cells.
Woodbury and coworkers showed that neuronal differentiation could be rapidly induced in human MSCs by DMSO as a chemical inducer (CitationWoodbury et al. 2000). The microscopic observations during differentiation period represented a faster appearance of hepatocyte-like morphology in DMSO-treated groups, emphasizing the inducer capacity of DMSO.
Stephens et al. reported that numerous osteoblast-expressed genes were elevated in response to DMSO treatment in MSCs and correlated with enhanced mineralization (CitationStephens et al. 2011).
Likewise, our results showed elevation of hepatoblast-expressed genes after treatment with DMSO and enhanced ALB levels and polysaccharide storage in AT-MSCs.
Shi et al., optimized the differentiation medium for differentiation of mouse bone marrow mesenchymal stem cells into hepatocytes. They also find out that the percentage of ALB-expressed cells correlated with OSM, and the percentage of CK18-expressed cells correlated with both FGF-4 and OSM (CitationShi et al. 2008). These results are near to over findings about the significant increase in expression levels of both CK-18 and ALB at HGF+ DMSO+ OSM as compared with HGF+ DMSO group, highlighting the key role of OSM in maturation of hepatocytes.
Here, we described the catalyzing effect of DMSO for trans-differentiation of AT-MSCs towards HLCs, but the mechanism responsible for these effects is unknown. CitationSnykers et al. (2007) attributed it to histone hyper acetylation induction effects, like effects of Trichostatin A, we further offer the delivering potential of both lipophilic and hydrophilic agents by DMSO through cell membranes. In a study directed by Wang and colleagues, DMSO treatment markedly increased internalization of TAT protein into carcinoma cells (CitationWang et al. 2010). This potential might makes DMSO as an active vehicle for enhancement of hepatogenic factors entrance into AT-MSCs.
In conclusion, using DMSO in the hepatic differentiation of AT-MSCs can improve efficiency of differentiation. The findings of this study may help development of an efficient and advantageous protocol for hepatocyte differentiation of AT-MSCs.
Authors’ contributions
EA conceived the study and participated in its design and coordination. NZ participated in the sequence alignment and drafted the manuscript. AA, AB, MBE, and SAM helped in drafting the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors thank the Department of Medical Biotechnology, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, and the Umbilical Cord Stem Cell Research Center (UCSRC), Tabriz University of Medical Sciences.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Al BF, De KJ, Vanhaecke T, Rogiers V. 2011. Current status of human adipose-derived stem cells: differentiation into hepatocyte-like cells. ScientificWorldJournal. 11:1568–1581.
- Aurich H, Sgodda M, Kaltwasser P, Vetter M, Weise A, Liehr T, et al. 2009. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut. 58:570–581.
- Baglioni S, Francalanci M, Squecco R, Lombardi A, Cantini G, Angeli R, et al. 2009. Characterization of human adult stem-cell populations isolated from visceral and subcutaneous adipose tissue. FASEB J. 23:3494–3505.
- Bakhshandeh B, Soleimani M, Hafizi M, Ghaemi N. 2012. A comparative study on nonviral genetic modifications in cord blood and bone marrow mesenchymal stem cells. Cytotechnology. 64:523–540.
- Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Osaki M, et al. 2009. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol. 24:70–77.
- Banas A. 2012. Purification of adipose tissue mesenchymal stem cells and differentiation toward hepatic-like cells. Methods Mol Biol. 826:61–72.
- Chetty S, Pagliuca FW, Honore C, Melton DA. 2013. A protocol to improve pluripotent stem cell differentiation. Protocol Exchange.
- Eslaminejad M, Karimi N, Shahhoseini M. 2013. Chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells treated by GSK-3 inhibitors. Histochem Cell Biol. 140:623–633.
- Eslaminejad MB, Mirzadeh H, Mohamadi Y, Nickmahzar A. 2007. .Bone differentiation of marrow-derived mesenchymal stem cells using beta-tricalcium phosphate-alginate-gelatin hybrid scaffolds. J Tissue Eng Regen Med. 1:417–424.
- Eslaminejad MB, Nikmahzar A, Taghiyar L, Nadri S, Massumi M. 2006. Murine mesenchymal stem cells isolated by low density primary culture system. Dev Growth Differ. 48:361–370.
- Gomez-Lechon MJ, Castell JV, Donato MT. 2007. Hepatocytes–the choice to investigate drug metabolism and toxicity in man: in vitro variability as a reflection of in vivo. Chem Biol Interact.168:30–50.
- Ishikawa T, Banas A, Hagiwara K, Iwaguro H, Ochiya T. 2010. .Stem cells for hepatic regeneration: the role of adipose tissue derived mesenchymal stem cells. Curr Stem Cell Res Ther. 5:182–189.
- Kuo TK, Ping Y-H, Lee OK. 2011. Mesenchymal Stem Cells for Liver Regeneration.Circ Res. 108:1340–1347.
- Liu WH, Liu ZC, You N, Zhang N, Wang T, Gong ZB, et al. 2012. Several important in vitro improvements in the amplification, differentiation and tracing of fetal liver stem/progenitor cells. PLoS One. 7:e47346.
- Marren K. 2011. Dimethyl sulfoxide: an effective penetration enhancer for topical administration of NSAIDs. Phys Sportsmed. 39:75–82.
- Okura H, Komoda H, Saga A, Kakuta-Yamamoto A, Hamada Y, Fumimoto Y, et al. 2010. Properties of hepatocyte-like cell clusters from human adipose tissue-derived mesenchymal stem cells. Tissue Eng Part C Methods. 16:761–770.
- Ouyang J, Shao J, Zou H, Lou Y, Yu Y. 2012. Hepatic differentiation of rat mesenchymal stem cells by a small molecule. ChemMedChem. 7:1447–1452.
- Ruiz-Delgado GJ, Mancias-Guerra C, Tamez-Gomez EL, Rodriguez-Romo LN, Lopez-Otero A, Hernandez-Arizpe A, et al. 2009. Dimethyl sulfoxide-induced toxicity in cord blood stem cell transplantation: report of three cases and review of the literature. Acta Haematol. 122:1–5.
- Santos NC, Figueira-Coelho J, Martins-Silva J, Saldanha C. 2003. Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects. Biochem Pharmacol. 65: 1035–1041.
- Seo MJ, Suh SY, Bae YC, Jung JS. 2005. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 328:258–264.
- Shi XL, Mao L, Xu B-Y, Xie T, Zhu Z-H, Chen J-H, et al. 2008. Optimization of an effective directed differentiation medium for differentiating mouse bone marrow mesenchymal stem cells into hepatocytes in vitro. Cell Biol Int. 32:959–965.
- Snykers S, Vanhaecke T, De Becker A, Papeleu P, Vinken M, Van Riet I, Rogiers V. 2007. Chromatin remodeling agent trichostatin A: a key-factor in the hepatic differentiation of human mesenchymal stem cells derived of adult bone marrow. BMC Dev Biol. 7:24.
- Sousa BR, Fonseca EA, Amaya MJ, Tonelli FMP, Lacerda SMSN, Lalwani P, et al. 2014. Human adult stem cells from diverse origins: an overview from multiparametric immunophenotyping to clinical applications. Cytometry A. 85:43–77.
- Stephens AS, Stephens SR, Hobbs C, Hutmacher DW, Bacic-Welsh D, Woodruff MA, Morrison NA. 2011. Myocyte enhancer factor 2c, an osteoblast transcription factor identified by dimethyl sulfoxide (DMSO)-enhanced mineralization. J Biol Chem. 286:30071–30086.
- Sturgill MG, Lambert GH. 1997. Xenobiotic-induced hepatotoxicity: mechanisms of liver injury and methods of monitoring hepatic function. Clin Chem.43:1512–1526.
- Wang H, Scott RE. 1993. Inhibition of distinct steps in the adipocyte differentiation pathway in 3T3 T mesenchymal stem cells by dimethyl sulphoxide (DMSO). Cell Prolif. 26:55–66.
- Wang H, Zhong CY, Wu J-F, Huang Y-B, Liu C-B. 2010. Enhancement of TAT cell membrane penetration efficiency by dimethyl sulphoxide. J Control Release. 143:64–70.
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. 2000. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 61:364–370.
- Wu XB, Tao R. 2012. Hepatocyte differentiation of mesenchymal stem cells. Hepatobiliary Pancreat Dis Int. 11:360–371.
- Zhang L, Ye JS, Decot V, Stoltz J-F, de Isla N. 2012. Research on stem cells as candidates to be differentiated into hepatocytes. Biomed Mater Eng. 22:105–111.