?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Nanogels are robust nanoparticles that could be used to deliver active drug compounds in controlled drug delivery applications. Nanogels drug delivery system is more effective and safer for both hydrophilic and hydrophobic drugs due to their chemical composition and formulations that are inappropriate for other formulations. Nanogels have enabled enlargement of functionalized nanoparticles, which act as a drug carriers that can be loaded with drugs and other active material to be released in a controlled manner at specific site. This review aims at providing general introduction on nanogels, recent synthesis methodology and their novel application in different fields.
Introduction
Nanogels are nanoparticles composed of a hydrogel with cross-linked hydrophilic polymer with 100–200 nm particle size (CitationGarg et al. 2012b). Physically and chemically cross-linked synthetic polymers (CitationBencherif et al. 2009) or biopolymers (CitationKabanov and Vinogradov 2009) constitute nanogels. The pores of nanogels are filled with micromolecules or macromolecules (CitationLee et al. 2007). Nanogels have swelling and degradation properties with flexible size, large surface area and high water content (CitationHayashi et al. 2004). Nanogels are used to deliver all biologically active agents and are drugs in a controlled and sustained release manner. Nanogels occur in the form of three-dimensional structures in which drugs, polymers and dispersed phase of liquid can be entrapped (CitationAlvarez‐Lorenzo et al. 2011) (CitationVintiloiu and Leroux 2008). Availability of various polymer systems and ease of alteration of their characteristics have rendered nanogels formulations advantageous.
Advantages of nanogel
| A. | Nanogels occur withhave high biocompatibility and biodegradable formulation. | ||||
| B. | Nanogels can be controlled for sustained release of drug from the formulation by the addition of a polymeric network. Polymeric networks also control the particle size of the formulation (CitationSawada et al. 2011). | ||||
| C. | The free-flowing pearlescent solution of the nanogels is easily dispersed in aqueous media (CitationOh et al. 2008, CitationSamah et al. 2010, CitationSawada et al. 2011). | ||||
| D. | Nanogels can be easily administered in parenteral and mucosal administration (CitationGarg et al. 2013). | ||||
| E. | The biggest advantage of nanogels is reduced premature leakage of the drug from the solution (CitationGuerrero-Ramírez et al. 2008). | ||||
| F. | Both hydrophilic and hydrophobic drugs can be formulated in nanogels formulation (CitationGarg and Goyal 2014). | ||||
Limitations of nanogel
| A) | Nanogels have limited drug-loading efficiency and suboptimal regulation of drug release (CitationWang and von Recum 2011). | ||||
| B) | Sometimes a strong interaction between drug and polymer decreases the hydrophilicity of the nanogels and causes the structure to collapse, hence irreversibly entrapping the drug molecules and enhancing the hydrophilicity of the nanogel matrix (CitationKabanov and Alakhov 2002, CitationVinogradov et al. 2002). | ||||
| C) | Surfactant or monomers present in nano gel may cause adverse effects in the formulation (CitationGarg et al. 2014). | ||||
Classification of nanogels according to their structure
Nanogels are classified on the basis of their structures. Different types of nanogels are simple nanogels (artificial chaperons), hollow nanogels including pH- or temperature-sensitive nanogel, cross-linked core—shell nanogels also used to prepare stimuli-responsive nanogel, hairy cross-linked nanogels, multilayer nanogels and functionalized nanogels. Classification of nanogels according to their structure is given in .
Table I. Classification of nanogel according to their structure.
Synthesis techniques
Polymerization of monomers in a homogeneous phase or in a microscale or nanoscale heterogeneous environment
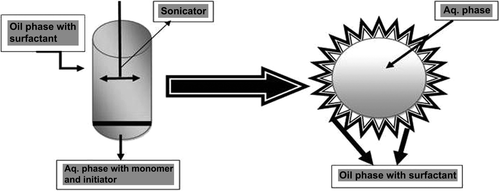
In this method, a colloidal suspension of the polymer is made by the homogeneous nucleation of water-soluble monomers that are used to synthesize stabilized nanogels (). This method is especially used to control the particle size. Nanogels of smaller particle size can be synthesized by adding an ionic surfactant, which also increases the colloidal stability of the formulations. As the surfactant concentration decreases, particle size increases in the nanogel formulation (CitationNayak and Lyon 2005). Donini and coworkers used the precipitation polymerization method to synthesize (PEG)-grafted poly (methacrylic acid) (PMA) nanosuspension in aqueous media. This method is only used for the hydrophobic and thermostable substances and cannot be used for biological molecules (CitationDonini et al. 2002). Some studies included monomer polymerization by inverse microemulsion (w/o) method with the addition of cross-linkers to produce a stabilized nano network (). First copolymerization of monomer solubilized in reverse micelles was carried out by Luisi and Straub (CitationLuisi and Straub 1984). Water-soluble nanoparticles are synthesized by several polymers such as polyacrylic acid (PAA) (CitationKriwet et al. 1998), poly (2-hydroxyethyl methacrylate (PHEMA) (CitationLandfester et al. 2000), and polyacrylamide (PAAm) (CitationLandfester et al. 2000). This method is also used to synthesize pH-sensitive diethylaminoethyl methacrylate nanogels used for controlled drug delivery system (CitationMarek et al. 2010). In inverse microemulsion method, atom transfer radical polymerization (ATRP) is used to synthesize stable cross-linked nanogels (CitationOh et al. 2006). Many disulfide cross-linkers were used to synthesize nanogels that are stable in extracellular environment and provide easy release of the drug. Biodegradable nanogels can synthesized by poly [oligo (ethylene oxide)-methyl methacrylate] polymer (CitationOh et al. 2006).
![Figure 1. Synthesis of nanogels by copolymerization in colloidal environments. Copolymerization of monomers [A] and bifunctional cross-linkers [B] in w/o microemulsions stabilized by surfactants [C] produces nanogels which can be then transferred into aqueous media after removal of surfactants and organic solvent.](/cms/asset/708d7109-d478-49b3-a498-4136fbbe0e13/ianb_a_930745_f0001_oc.jpg)
Physical self-assembly of interactive polymers
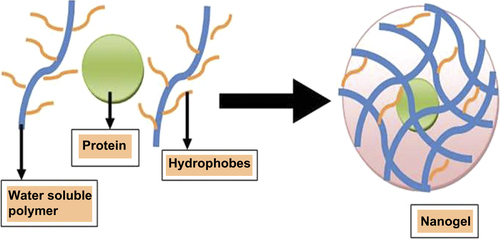
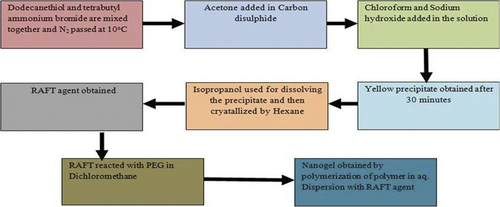
Nanogels are formulated using the physical self-assembled polymers method with amphiphilic polymers, where interaction between the drug and solvent occurs by the Van der Waals’ interaction, hydrogen bonding, etc. Micro- and macromolecules are entrapped within the nanogels’ structure during self-assembly (CitationBooth and Attwood 2000, CitationRiess 2003). Different protein-loaded nanogels are prepared by self-associating hydrophilic polymers. For example, Akiyoshi et al. prepared insulin hydrogels by the cholesterol-modified pullulan method, where they obtained 20– 30 nm particle size. In this method, the size of the nanogels are controlled by proper concentration of polymers and different environmental conditions, such as pH, ionic strength, and temperature (CitationAkiyoshi et al. 1998) (). For example, CitationYu et al. (2006) prepared oppositely charged protein (ovalbumin and lysozyme or ovotransferrin) nanogels by temperature-induced gelation method. Chitosan or ovalbumin can be used to prepare nanogel in the pH- and temperature-induced gelation method. Self-assembled nanogels (120–150 nm) are also prepared using different concentrations of two polymers that are more stable and also suitable for the long-term storage. Amphiphilic block copolymers (hydrophilic and hydrophobic polymers) are widely used to synthesize nanogels in the self-associating polymerization method. Amphiphilic polymers are synthesized by the living radical polymerization (LRP) techniques (e.g., reversible addition–fragmentation chain transfer [RAFT], and nitroxide-mediated polymerization [NMP] methods). Micellization of amphiphilic polymers structure can be modified by changing their nature, length, and composition (CitationBooth and Attwood 2000, CitationZamurovic et al. 2007). The RAFT process is a single-step synthesis of PEGylated poly (N, N′-dimethylaminomethyl methacrylate) nanogel using an amphiphilic macro-RAFT agent tri-thiocarbonate with hydrophobic do-decyl chain supporting polymerization (). The RAFT method is generally used for gene delivery system, because this method produces smaller particle size, which was appropriate for the gene delivery system (CitationYan and Tao 2010). Changes in temperature or addition of solvent or additives provide extra degree of freedom to tune the micellar behavior of the amphiphilic block copolymers (CitationCastro et al. 2008, CitationDenkova et al. 2008, CitationLin and Alexandridis 2002). Surface chemistry of nanogels can be modified for specific tissue and cell targeting. The functionalities of the core of nanogels can be tuned using specific chemical approaches to improve the drug-loading capacity.
Cross-linking of preformed polymers
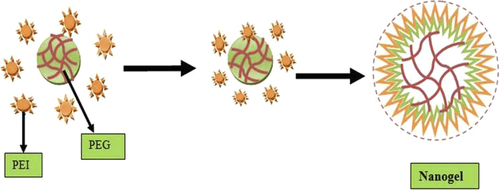
This method is especially used for preparing large-particle-size nanogels. Cross-linked cationic nanogel was used for polynucleotide delivery (CitationKohli et al. 2007). In cross-linking polymerization method, nanogel was prepared by oil-in-water emulsion, followed by solvent evaporation method where PEG was conjugated to a branched polyethyleneimine (CitationSun et al. 2005) in aqueous media (). Cationic PEI containing nanogels of 80–200 nm diameters were also obtained by the photo-Fenton reaction in aqueous media (CitationXu et al. 2007, CitationSun et al. 2006). Cationic nanogels were prepared by using PEI connected by disulfides linkers (CitationKohli et al. 2007), and hyaluronic acid (CitationHayashi et al. 2004) nanogels containing biodegradable disulfide linkages were prepared by the inverse water-in-oil emulsion method (CitationLee et al. 2007). DNA containing cross-linked nanogels was synthesized by mixing thiol-functionalized six-arm branched PEG and DMSO containing DNA by oxidation process (CitationMok and Park 2006). Both self-assembled method and cross-linking method provided opportunities to control distribution of polymer in nanogels. Various chemically cross-linked nanoparticles found morphologies with spheres, rods, and toroids (CitationMa et al. 2001). Covalent cross-linking of preformed polymer chains provides excellent opportunities for producing functional nanogels with large pore sizes for drug delivery (CitationMok and Park 2006). The cross-linking method is especially applied to control the particle size, shape, composition, and surface characteristics of the nanogels. It also provides complete entrapment of the drug in the formulation. Copolymerization method can be carried out in the presence of vinyl monomers, such as PEG triacrylate, PEG monomethyl ether monomethacrylate, and p-hydroxy styrene (CitationGratton et al. 2007)
Emulsion photopolymerization process
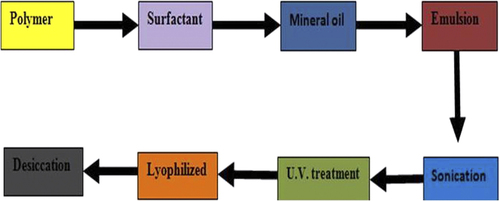
In the emulsion photopolymerization process, UV method is used to prepare dextran nanogel, where dextran hydroxyethyl methacrylate is emulsified with ABIL EM 90 as emulgent in mineral oil, the product is obtained in (1:1) acetone: hexane solution. General emulsion photopolymerization process is given in . Meso-tetraphenylporphinedisulfonate is used as a photosensitizer for the breakage of endosomal membranes to release of genes in the cytoplasm and nuclease (CitationRaemdonck et al. 2010).
Novel pullulan chemistry modification
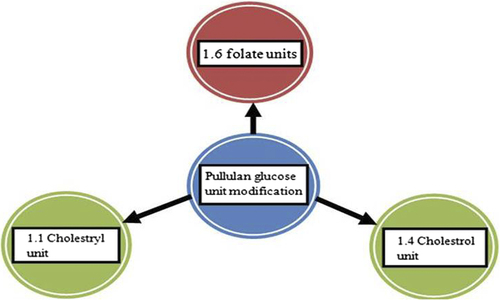
A mixture of cholesterol isocynate in dimethyl sulfoxide and pyridine was used to synthesize cholesterol-based pullulan (CHP) nanogel. Pullulan is substituted by 1.4 cholesterol moieties per 100 anhydrous glucoside units. All preparations synthesized by the CHP method should be freeze-dried. The CHP method has been especially known to act as good protein carrier nanogels formulation (CitationAlles et al. 2009). Michael addition reaction was used to modify CHP method where the acrylate group and the thiol group were substituted by polyethylene glycol (CitationHasegawa et al. 2009). A total of 1.1 units of cholesteryl group per 100 glucose units of parent pullulan were used to modify nanosystems of CHP (). This modification shows an interaction with Aβ oligomer and monomer for treatment of Alzheimer's disease and also increases microglia and cortical cell viability (CitationLee et al. 2009). Pullulan was substituted by 1.6 glucose units, which are particularly used for folate receptor targeting. Pullulan and photosensitizer (pheo-A) were coupled with carbodimide and they produced a conjugate, which was converted to nanogel by dialysis. This kind of nanogels is generally used for cancer therapy (CitationBae and Na 2010).
Novel photochemical approach
Photochemical approach is used to produce N-(2-aminoethyl)methylacrylamide and N,N′-methylene bis-acrylamide-coated ferric oxide nanoparticles nanogels, developed by magnetic resonance imaging (MRI), where nanogels are treated with UV radiation at 388 nm for 10 min (CitationGong et al. 2009). Similarly plasmid DNA-loaded diacrylated pluronic and glycidyl methyacrylated chito-oligo-saccharide nanogels were prepared at 365 nm UV light with the addition of a photo initiator (CitationLee et al. 2009).
Chemical modifications
Chemical modification of polymers supports release of drugs from the nanogels formulation. Acetylation of chondroitin sulfate will increase the release of doxorubicin for 3 weeks in the HeLa cells, which can be used in cancer treatment (CitationPark et al. 2010). Addition of polyion complexes of nanogels consisting of poly 2-(N, N-diethyl aminoethyl) methacrylate with quaternary group core increases the binding capacity of siRNA for cancer treatment and gene delivery (CitationTamura et al. 2010). When N-isopropylacrylamide and butylacrylate was saturated with sodium carbonate, it altered the drug absorption and release pattern of the Methotrexate (CitationSingka et al. 2010). Modified pH-sensitive hydroxypropylcellulose-polyacrylic acid nanogels are used for bio-imaging of cells by sensing the physicochemical environment. Hydroxyl group of hydroxypropylcellulose-polyacrylic acid was modified by removing cadmium (Hayashi et al.) ions quantum dots and polyacrylic acid to make the formulation pH sensitive and give a better response for cell bio-imaging (CitationWu et al. 2010). Pluronic nanogels were modified by RNase A with heparin conjugate for easy internalization of the enzyme (CitationChoi et al. 2010). Glycol–chitosan nanogels was grafted by 3-diethylaminopropyl that led to deaggregation of the product at lower pH and also altered the release of doxorubicin (CitationOh et al. 2010).
Drug release mechanisms of nanogel
Mechanisms of drug release have been investigated based on the characteristics of the polymer systems such as temperature, pH, and volume transition of nanogels as described further.
pH-responsive mechanism
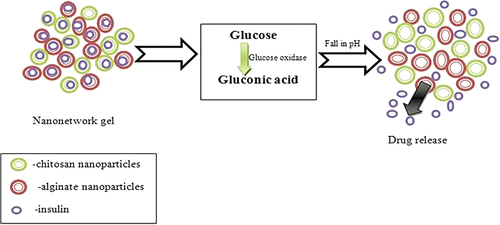
Dextran nanoparticles containing nanogel showed on and off catalytic activity for insulin delivery at specific pH. This system is known as glucose-mediated insulin delivery system composed of glucose oxidase, catalase, with chitosan and alginate polymer. The polymer used in the system is insoluble at neutral pH. As pH became acidic, the polymer swells and the drug starts to release from the system (). Glucose oxidase is responsible for converting glucose to gluconic acid, which reduces the biological pH (CitationGu et al. 2013). Owing to the swelling action of pH-sensitive polyacrylic acid chains, Temozolodine showed controlled release kinetics (CitationWu et al. 2010). The pH-sensitive glycol chitosan nanoparticles (grafted with diethylaminopropyl groups) significantly increases the release of Doxorubicin from the formulation (CitationOh et al. 2010).
Thermosensitive and volume transition mechanism
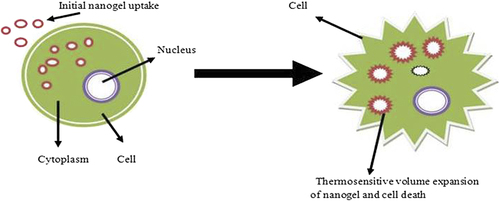
Thermosensitive nanogel was developed by poly (N- isopropylacrylamide), which releases indomethacin due to maintenance of the temperature above lower critical solution temperature (LCST), which leads to sudden shrinkage in the volume of gel () (CitationShin et al. 2001). Thermo-responsive nanogel was synthesized by modification of polyethyleneimine with pluronic group, which gives decreased particle size, and was successfully used for gene delivery systems (CitationLee and Yoo 2008). Thermo-responsive expanded volume poly alkylene oxides nanogel starts to release drug due the destruction of cellular network (CitationLee et al. 2009). Poly (N-isopropylacrylamide) thermosensitive nanogels of doxorubicin are prepared by chitosan-modified chemically reduced graphene oxide (CRGO) process, which is based on near infrared (NIR) method. Graphene used as photo thermal material to respond to NIR, and chitosan is used to prevent aggregation in the formulation of protein. Doxorubicin starts to release drug at 42°C produced by NIR after the shrinkage in poly (N-isopropylacrylamide) polymer (CitationWang et al. 2013).
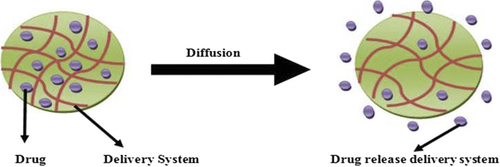
Diffusion of the drug from nanogel
Diffusion occurs when a drug or other active agent passes through a polymer that forms a controlled released device. The diffusion can occur on a macroscopic scale as through pores in the polymer matrix or on a molecular level by passing between polymer chains. A normal process of diffusion is given in . A polymer and an active agent have been mixed to form a homogeneous system, referred to as a matrix system. In this kind of a system, the combinations of polymer matrices and bioactive agents chosen allow for the drug to diffuse through the pores or macromolecules’ structure of the polymer upon introduction of the delivery system into biological environment without including any changes in the polymer itself. The timing of drug release from the delivery system by diffusion can be controlled by two factors: (1)the strength of binding of the drug in the micelle core (e.g., hydrophobic binding in hydrophobic cores), which is characterized by the partitioning of drug between the micelle and external environment, and (2) the polymer chain binding to each other in micelles structure and characterized by cmc (CitationKabanov et al. 1995). The aforementioned factors show “thermodynamic” and “kinetic stability” of the formulation, which helps to link both drug and micelles and control the drug release (CitationKabanov and Alakhov 2002). Diffusion of the drug from the smaller size micelle core is usually not rate limiting (for low-molecular-mass drugs). Simple diffusive process involves polymeric release of doxorubicin for a long period and the initial release is controlled by addition of cationic and anionic polyelectrolyte, which increases the size of the nanogel, and doxorubicin starts to release layer by layer without sudden initial outburst (CitationTan et al. 2008).
Techniques used to characterize nanogels
Nanogel formation
Nanogel formation was determined by techniques such as dark field microscopy, NMR studies, RAMAN spectra, and Fourier transform infrared (FTIR).
Dark field microscopy
This technique shows the direct image of nanogels within one second after mixing the two polymer solutions. Eclipse E60 Nikon microscope was used to observe the image of β-cyclodextrin polymers (pβ-CD) and dextran (with hydrophobic lauryl side chains) self-assembled nanogels. Both solutions (dextran and pβ-CD) penetrated the capillary and in the little space came in contact with each other and finally reflected as a white spot to give complete formation of nanogel (CitationGref et al. 2006).
Nuclear magnetic resonance studies
The magnetic properties of certain atomic nuclei were explained by nuclear magnetic resonance (NMR) technique. Physical and chemical properties of nanogels as well as the structure, reaction state, and chemical environments of the molecule and electronic structure of molecules can be determined by NMR. Aqueous-based cyclodextrin nanogels are characterized by 1H-NMR and 13C-NMR spectra, which was recorded with Varian Mercury 300 NMR spectrometer operating at 300 MHz (CitationKettel et al. 2011). Cationic nanogel was formed by cross-linking between poly (ethylene oxide) (PEO) and poly ethyleneimine (CitationSun et al. 2005) where the quantity and ratio was determined by 1H-NMR spectra (CitationVinogradov et al. 2002). In another example, a self-assembled nano suspension of β- cyclodextrin (β-CD) polymer and dextran with hydrophobic lauryl side chains solution was prepared by the solvent evaporation method. In this nano suspension formulation, β-CD content and substitution in dextran-bearing hydrophobic lauryl side chains solution were determined by 1H NMR spectroscopy. The 1H NMR spectra were performed at 25°C on an Inova Varian spectrometer operating at frequencies of 400 MHz, using a 5-mm H X-probe. The spectra were recorded with a flip angle of 90°, a spectral width of 4000 Hz and 256 scans of 16 K points, a repartition time of 15 s at 25, 30, 35, and 40°C (CitationGref et al. 2006). Inverse microemulsion method was used to develop a pH-sensitive nanogel of p-nitro phenol acrylate (NPA) and N-isopropylacrylamide (NIPA) in the presence of an aerosol (surfactant) and ethylene glycol dimethacrylate (cross-linking agent). The H1NMR spectra show that the copolymer contains both NPA and NIPA monomers. The NMR spectra of nano hydrogels were obtained using a solvent CDCl3 in a Bruker ACE instrument (250 MHz) at 20°C; chemical shifts were measured relative to chloroform (ı = 7.26) (CitationGuerrero-Ramírez et al. 2008).
RAMAN spectra
It is the shift in wavelength of the inelastically scattered radiation that helps to give information about the chemical and structural data of the formulation. It is used to determine chemical bonds, symmetry of molecules, and crystallographic orientations of a sample. RAMAN spectra is generally used to determine structural information of cyclodextrin-based aqueous nanogel where RAMAN spectra were recorded with a Bruker RFS 100/s (spectral disintegration 4 cm–1) (CitationKettel et al. 2011)
FTIR techniques
FTIR techniques are used to confirm the structure of the major functional group in the nanogel formulation. Absorption, emission, and photo conductivity of the nanogel were determined by the FTIR method. An FTIR spectrometer collects spectral data in a wide spectral range of the given sample. FTIR spectrophotometer (Nicolet 6700) was used to obtain spectra data of copolymeric nanohydrogels. Guerrero-Ramírez et al. used FTIR to characterize copolymeric nanohydrogel of p-nitro phenol acrylate (NPA) and N-isopropylacrylamide (NIPA) by inverse microemulsion polymerization method (CitationGuerrero-Ramírez et al. 2008). The nano hydrogels spectrums of FTIR were collected using Attenuated Total Reflectance Smart Orbit accessory. Kettel et al. used FTIR to confirm polymerization in the nanogel of doxorubicin. They also used FTIR for cyclodextrin-based aqueous nanogels. Cyclodextrin content in nanogels has been measured by quantitative analysis of the band at 1032 cm− 1. The integrals of this band measured for the nanogel samples were compared with a calibration curve obtained by mixing different amounts of β-CD with KBr (CitationKettel et al. 2011).
Structural and morphological determination
Scanning electron microscope
Scanning electron microscop (SEM) is a type of electron microscopical technique that produces an image of a sample by scanning it with a focused beam of electrons. SEM is able to detect the surface topography of nanogels, composition, three-dimensional structure, chemical composition, crystalline structure, and orientation of the formulation. SEM can achieve a resolution better than 1 nanometer (CitationKataria et al. 2014). CitationJayakumar et al. (2012) studied doxorubicin-loaded pH-responsive chitin nanogel for drug delivery; they determine the particle size and shape by the SEM method. Kettle et al. (2011) also used SEM to determine particle size range of the nanogel.
Atomic force microscopy
Atomic force microscopy (AFM), also known as Scanning Force Microscopy (SFM), is a very high-resolution type of Scanning Probe Microscopy (SPM). This method is 1000 times superior to the optical diffraction limit. AFM is generally used to determine the size and structure of nanogel. CitationJayakumar et al. (2012) determined that doxorubicin-loaded chitin nanogels contain spherical shaped nanoparticles. CitationShen et al. (2011) synthesized biocompatible and thermo-sensitive core–shell nanogel in which AFM was used to determine the size and structure of a nanogel on a Shimadzu SPM-9600 in the tapping mode.
Transmission electron microscopy
Transmission Electron Microscopy (TEM) is a technique in which a beam of electrons is transmitted through an ultra-thin specimen, interacting with the given sample. A black and white high-resolution image is formed by the TEM when the sample and electrons are interacting. TEMs are able to give information of surface features, shape, size, and structure of the nanogel. Flaws, fractures, and damages occurring in the nanogel formulation can also be determined by TEM. Peng et al. also used TEM to analyze the morphology of dual-responsive cisplatin nanogel (CitationPeng et al. 2013). CitationGref et al. (2006) determined the structural formation of self-assembled nanogel formed by β-cyclodextrin polymers (pβ-CD) and Dextran bearing hydrophobic lauryl side chains.
Mean diameter and size distribution determination technique
Dynamic light scattering
Dynamic light scattering (DLS) is also known as Quasi- Elastic Light Scattering (QELS), or Photon Correlation spectroscopy (PCS). This technique is generally used to determine the size and distribution of the nano molecules. Jayakumar et al. determined the particles size with the help of DLS in a doxorubicin–chitin nanogel (CitationJayakumar et al. 2012). Shen et al. prepared biocompatible and thermo-sensitive core–shell nanogel that was synthesized by RAFT aqueous dispersion polymerization. They prepared two types of nanogels: one is a linear poly (ethylene glycol) s (l-PEGs) and another is a nonlinear analogu as polymers derived from oligo (ethylene glycol) (meth) acrylates (g-PEGs); they are characterized by DLS for their particles structure and size distribution in the absence of surfactant. DLS was performed by a Malvern Zetasizer 3000 HSA at 25°C (CitationShen et al. 2011). Daoud-Mahammed et al. synthesized nanogel by the spontaneous addition of β-cyclodextrin and modified dextran (modified with alkyl chain) with hydrophobic Benzophenone (guest molecules). In this kind of nanogel, DLS is used to determine the mean diameter and size distribution using a Coulter Nanosizer (Model N4MD, Coultronic, France) (Daoud- Mahammed et al. 2009). Inomoto et al. determined the structure of hydrogel nanoparticles, formed by self-aggregation of cholesterol-bearing pullulan (CHP); this was studied by dynamic light scattering (DLS). DLS measurements were performed by a static/Dynamic Compact Goniometer (DLS/SLS-5000), which determined that a system has unimodel distribution (CitationInomoto et al. 2009).
Small-angle neutron scattering
Various structures of the nano substances (size between 1 and100 nm) are investigated by SANS technique. Inomoto et al. determined the structural properties of CHP nanogels and the interaction of CHP nanogel with cyclodextrin, or chaperone-like function by SANS. SANS also shows that, as the concentration of CD increases, the scattering intensity of the CHP nanogel decreases (CitationInomoto et al. 2009).
Drug entrapment
UV spectroscopy
Drug entrapment in nanogel is determined by UV-Vis spectroscopy method. UV-Vis spectroscopy method refers to absorption spectroscopy or reflectance spectroscopy in the Ultraviolet-Visible spectral region (CitationGoyal et al. 2013). CitationDaoud-Mahammed et al. (2009) determined the entrapment efficiency by UV spectroscopy of two hydrophobic molecules (benzophenone and tamoxifen). CitationWang et al. (2008) also determined the entrapment efficiency by UV spectroscopy method in gelatinized thermo-sensitive nanogels.
Drug release
Equilibrium dialysis technique
Equilibrium dialysis is a very simple technique that is used to study the interaction between molecules (CitationGagandeep et al. 2014). Equilibrium dialysis is an inexpensive method and easy to perform. Nanosized cationic hydrogel was synthesized by the cross-linking between poly(ethylene oxide) (PEO) and polyethyleneimine (CitationSun et al. 2005). PEO-cl-PEI nanogel allowed for immobilization of negatively charged, biologically active compounds such as Retinoic acid, Indomethacin and oligonucleotides or hydrophobic molecules. Release kinetics of the drug from the nanogel was evaluated using the equilibrium dialysis technique, which shows that 17.5% of the drug is released in the first hour and 82% of indomethacin was released in 24 h. This shows that releasing of immobilized biological agent from nanogel system may be useful for the drug delivery system (CitationVinogradov et al. 2002).
Franz-diffusion cells
Drug release pattern is also determined by the Franz- diffusion cells (CitationAbu Samah and Heard 2013).
Swelling ratio
Equation of Crowther and Vincent
The swelling ratio (CitationBencherif et al. 2009) of the nanogels was calculated by the equation:
Here, Vswollen and Vshrunken are the volumes of nanogel particles in swollen status at 20°C and in shrunken status at 50°C, respectively. D20 and D50 are the average diameters of the nanogels particles at 20°C and 50°C, respectively.
Stability studies
Freeze drying
Stability study of biocompatible, thermosensitive core–shell nanogel was done by freeze drying method. Two types of nanogel, l-nanogel and g-nanogel, were compared for their ability to survive in freeze-drying condition (CitationSingh et al. 2014, CitationParnami et al. 2013). The g-nanogels size was unchanged after freeze drying, whereas l-nanogels increased in size and polydispersity with freeze drying. Partial crystallization of l-PEGs nanogels occurred during freezing and resistant to redispersion of nanoparticles. In contrast, g-PEGs with multiple side chains oligo (ethylene glycol) with a rich collection of conformations, which prevents the crystallization process during freeze-drying condition and thus a better stabilizing effect is achieved (CitationShen et al. 2011).
Flocculation in biological medium
Stability of nanogel can also be determined by flocculation method. Aqueous dispersion of core–shell nanogels (l-nanogel and g-nanogel) occurred in different medium like 1.5M NaCl solution, 1% bovine serum albumin (BSA), and 100% fetal bovine serum (FBS). Both l- and g-nanogels show long-term stability (3 months) in NaCl solution. Both l- and g-nanogels shrunk when they transferred from water to NaCl solution due to dehydration. Both l- and g-nanogels remained stable in BSA solution for a week but flocculated afterward. In FBS, g-nanogels showed stability more than 4 days, while l-nanogels started to aggregate on the third day. All studies show that g-nanogel is more stable than l-nanogels (CitationShen et al. 2011)
UV-Vis (aminolysis and hydrolysis of the main transfer agents, chain transfer agent CTAs)
CitationShen et al. (2011) synthesized nanogels (which mainly composed of l-PEGs and/or g-PEGs) by the RAFT polymerization method. The stability studies were done by aminolysis and the hydrolysis process of the main transfer agents and chain transfer agent. During the aminolysis process, g-nanogels were completely aminolyzed at 4°C. A drastic reduction in absorption was seen at 37°C within the first 7 h for l-nanogel and complete aminolysis occurred after 52 h. During the aminolysis process, the nanogel sample was colored due to the presence of the CTA, which resulted in hydrolytic stability of the CTAs in the nanogels due to the steric hindrance (CitationShen et al. 2011).
These results indeed pointed to the enhanced hydrolytic stability of the CTAs in the nanogels due to the steric hindrance. Dithioester group in the g-nanogels in the presence of UV-Vis absorbance show attenuated conditions for a period of 22 days, but the dithioester group in l-nanogel started to degrade from the eighth day and a significant decrease (25%) in the absorbance was detected within 22 days.
Thermosensitivity
1H-NMR
Change in the polymer structure is an integral part of temperature responsivity and can be measured by structural characterization techniques known as H1 NMR. A thermo-sensitivity test is done on doxorubicin nanogels and variation in the intensity or a chemical shift of the characteristic NMR peak in a polymer is determined, which indicates structural variation with different temperature response.
Applications
Applications in cancer therapy
Nanogels are used for cancer treatment by incorporating the following drugs: doxorubicin, cisplatin, 5-flurouracil, temozolomide, heparin, etc. Doxorubicin-loaded nanogels are frequently used in the cancer treatment in formulation like pH- and temperature-responsive nanogels in the presence of maleic acid poly-(N-isopropylacrylamide) polymer, where doxorubicin is released at a specific pH and temperature. Chitin-polymerized Doxorubicin nanogels are used for treatment of prostate, breast, lungs, and liver cancer (CitationGarg et al. 2012a, CitationKaur et al. 2014). The different nanogel formulations in cancer therapy are listed below in .
Table II. Applications of nanogels in the cancer therapy.
Applications of nanogels in gene delivery, enzymology, and protein folding
Nanogel formulations are also used for the delivery of protein, enzymes, and gene at a targeted site. Artificial chaperon is one of the methods that are used to deliver different proteins and enzymes. Modified polymers are used in the artificial chaperon method. Different formulations of nanogels are used in enzymology; protein folding and gene delivery systems are listed below in .
Table III. Applications of nanogels in gene delivery, enzymology, and protein folding.
Nanogel applications in gastrointestinal disorder
Nanogels are also used in gastrointestinal disorders, either in a form of conjugated nanogel or in some other formulation of nanogels (CitationChang 2007). Nanogel applications in gastrointestinal disorder are listed in .
Table IV. Nanogel applications in gastrointestinal disorder.
Marketed formulations
Many nanogels formulations are available on the market. Most of the marketed formulations are cosmetic remedies, a number of tooth paste formulation available that reduce tooth decay problems, and several formulations are personal care products that are widely used for skin care problems. A list of various marketed nanogel formulations are given in .
Table V. Marketed formulation of nanogel.
Conclusion
Nanogel systems have been studied for both theoretical and practical aspects. They are widely used for controlled delivery system, targeted delivery system, coatings purpose, and for the cosmetics products. Nanogels show promising future developments, widening the prospects for drug delivery. Every new analysis entails discovery of recent polymer and mechanistic approaches with a promising role in therapies and innovation on fabrication of nanogels design. The use of nanogels permits the advance of biopharmaceutical parameters of an entrapped drug. We increase the use of these materials in many fields or other delivery systems. Nanogels are probably one of the better drug delivery systems to provided controlled or sustained release of the drug. However, we need to design a perfect delivery system that gives proper knowledge of the interaction between the drug and the carrier and the effect of size and drug loading on drug release.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abu Samah NH, Heard CM. 2013. Enhanced in vitro transdermal delivery of caffeine using a temperature- and pH-sensitive nanogel, poly(NIPAM-co-AAc). Int J Pharm. 453:630–640.
- Akiyoshi K, Kobayashi S, Shichibe S, Mix D, Baudys M, Wan Kim S, Sunamoto J. 1998. Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: complexation and stabilization of insulin. J Control Release. 54:313–320.
- Alles N, Soysa NS, Hussain MD, Tomomatsu N, Saito H, Baron R, et al. 2009. Polysaccharide nanogel delivery of a TNF-alpha and RANKL antagonist peptide allows systemic prevention of bone loss. Eur J Pharm Sci. 37:83–88.
- Alvarez‐Lorenzo C, Moya‐Ortega MD, Loftsson T, Concheiro A, Torres‐Labandeira JJ. 2011. Cyclodextrin‐Based Hydrogels. Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine: Current and Future Industrial Applications New York: John Wiley & Sons, pp. 297–321.
- Bae BC, Na K. 2010. Self-quenching polysaccharide-based nanogels of pullulan/folate-photosensitizer conjugates for photodynamic therapy. Biomaterials. 31:6325–6335.
- Bae KH, Mok H, Park TG. 2008. Synthesis, characterization, and intracellular delivery of reducible heparin nanogels for apoptotic cell death. Biomaterials. 29:3376–3383.
- Ballal NV, Tweeny A, Baumgartner JC, Ginjupalli K, Saraswathin V. 2013. Effect of maleic acid and ethylenediaminetetraacetic acid on the shear bond strength of RealSeal SE sealer to root canal dentin. Eur J Prosthodont Restor Dent. 21:152–156.
- Bencherif SA, Siegwart DJ, Srinivasan A, Horkay F, Hollinger JO, Washburn NR, Matyjaszewski K. 2009. Nanostructured hybrid hydrogels prepared by a combination of atom transfer radical polymerization and free radical polymerization. Biomaterials. 30:5270–5278.
- Booth C, Attwood D. 2000. Effects of block architecture and composition on the association properties of poly (oxyalkylene) copolymers in aqueous solution. Macromol Rapid Commun. 21:501–527.
- Castro E, Barbosa S, Juarez J, Taboada P, Katime IA, Mosquera V. 2008. Influence of external factors on the micellization process and aggregate structure of poly(oxy)styrene-poly(oxy)ethylene block copolymers. J Phys Chem B. 112:5296–5304.
- Chang T. 2007. Artificial Cells: Biotechnology, Nanotechnology, Regenerative Medicine, Blood Substitutes Bioencapsulation and Cell/Stem Cell Therapy. Singapore: World Scientific/Imperial press, p. 454.
- Cheng G, Mi L, Cao Z, Xue H, Yu Q, Carr L, Jiang S. 2010. Functionalizable and ultrastable zwitterionic nanogels. Langmuir. 26: 6883–6886.
- Choi JH, Jang JY, Joung YK, Kwon MH, Park KD. 2010. Intracellular delivery and anti-cancer effect of self-assembled heparin-Pluronic nanogels with RNase A. J Control Release. 147:420–427.
- Daoud-Mahammed S, Couvreur P, Bouchemal K, Chéron M, Lebas G, Amiel C, Gref R. 2009. Cyclodextrin and polysaccharide-based nanogels: entrapment of two hydrophobic molecules, benzophenone and tamoxifen. Biomacromolecules. 10:547–554.
- Denkova AG, Mendes E, Coppens MO. 2008. Effects of salts and ethanol on the population and morphology of triblock copolymer micelles in solution. J Phys Chem B. 112:793–801.
- Donini C, Robinson DN, Colombo P, Giordano F, Peppas NA. 2002. Preparation of poly(methacrylic acid-g-poly(ethylene glycol)) nanospheres from methacrylic monomers for pharmaceutical applications. Int J Pharm. 245:83–91.
- Gagandeep Garg T, Malik B, Rath G, Goyal AK. 2014. Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur J Pharm Sci. 53:10–16.
- Garg T, Arora S, Murthy R, Goyal AK. 2012a. Development, optimization & evaluation of porous chitosan scaffold formulation of gliclazide for the treatment of type-2 diabetes mellitus. Drug Delive Lett. 2:251–261.
- Garg T, Goyal AK. 2014. Biomaterial-based scaffolds – current status and future directions. Expert Opin Drug Deliv. 11:767–789.
- Garg T, Rath G, Goyal AK. 2014. Novel technology to improve drug loading in polymeric nanofibers. Drug Deliv Lett. 4:79–86.
- Garg T, Singh O, Arora S, Murthy R. 2012b. Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst. 29:1–63.
- Garg T, Singh S, Goyal AK. 2013. Stimuli-sensitive hydrogels: an excellent carrier for drug and cell delivery. Crit Rev Ther Drug Carrier Syst. 30:369–409.
- Gong Y, Fan M, Gao F, Hong J, Liu S, Luo S, et al . 2009. Preparation and characterization of amino-functionalized magnetic nanogels via photopolymerization for MRI applications. Colloids Surf B Biointerfaces. 71:243–247.
- Goyal G, Garg T, Malik B, Chauhan G, Rath G, Goyal AK. 2013. Development and characterization of niosomal gel for topical delivery of benzoyl peroxide. Drug Deliv.
- Gratton SE, Pohlhaus PD, Lee J, Guo J, Cho MJ, Desimone JM. 2007. Nanofabricated particles for engineered drug therapies: a preliminary biodistribution study of PRINT nanoparticles. J Control Release. 121:10–18.
- Gref R, Amiel C, Molinard K, Daoud-Mahammed S, Sebille B, Gillet B, et al . 2006. New self-assembled nanogels based on host-guest interactions: characterization and drug loading. J Control Release. 111:316–324.
- Gu Z, Aimetti AA, Wang Q, Dang TT, Zhang Y, Veiseh O, et al . 2013. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano. 7:4194–4201.
- Guerrero-Ramírez L, Nuño-Donlucas S, Cesteros L, Katime I. 2008. Smart copolymeric nanohydrogels: Synthesis, characterization and properties. Mater Chem Phys. 112:1088–1092.
- Hasegawa U, Sawada S, Shimizu T, Kishida T, Otsuji E, Mazda O, Akiyoshi K. 2009. Raspberry-like assembly of cross-linked nanogels for protein delivery. J Control Release. 140:312–317.
- Hayashi H, Iijima M, Kataoka K, Nagasaki Y. 2004. pH-sensitive nanogel possessing reactive PEG tethered chains on the surface. Macromolecules. 37:5389–5396.
- Hirakura T, Yasugi K, Nemoto T, Sato M, Shimoboji T, Aso Y, et al . 2010. Hybrid hyaluronan hydrogel encapsulating nanogel as a protein nanocarrier: new system for sustained delivery of protein with a chaperone-like function. J Control Release. 142:483–489.
- Hong J, Xu D, Gong P, Yu J, Ma H, Yao S. 2008. Covalent-bonded immobilization of enzyme on hydrophilic polymer covering magnetic nanogels. Microporous Mesoporous Mater. 109:470–477.
- Inomoto N, Osaka N, Suzuki T, Hasegawa U, Ozawa Y, Endo H, et al . 2009. Interaction of nanogel with cyclodextrin or protein: study by dynamic light scattering and small-angle neutron scattering. Polymer. 50:541–546.
- Jayakumar R, Nair A, Rejinold NS, Maya S, Nair S. 2012. Doxorubicin-loaded pH-responsive chitin nanogels for drug delivery to cancer cells. Carbohydr Polym. 87:2352–2356.
- Kabanov AV, Alakhov VY. 2002. Pluronic block copolymers in drug delivery: from micellar nanocontainers to biological response modifiers. Crit Rev Ther Drug Carrier Syst. 19:1–72.
- Kabanov AV, Nazarova IR, Astafieva IV, Batrakova EV, Alakhov VY, Yaroslavov AA, Kabanov VA. 1995. Micelle formation and solubilization of fluorescent probes in poly (oxyethylene-b-oxypropylene-b-oxyethylene) solutions. Macromolecules. 28:2303–2314.
- Kabanov AV, Vinogradov SV. 2009. Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew Chem Int Ed Engl. 48:5418–5429.
- Kataria K, Garg T, Goyal AK, Rath G. 2014. Novel technology to improve drug loading in polymeric nanofibers. Drug Deliv Lett. 4:79–86.
- Kaur M, Garg T, Rath G, Goyal AK. 2014. Current nanotechnological strategies for effective delivery of bioactive drug molecules in the treatment of tuberculosis. Crit Rev Ther Drug Carrier Syst. 31:49–88.
- Kettel MJ, Dierkes F, Schaefer K, Moeller M, Pich A. 2011. Aqueous nanogels modified with cyclodextrin. Polymer. 52:1917–1924.
- Kim S, Park KM, Ko JY, Kwon IC, Cho HG, Kang D, et al. 2008. Minimalism in fabrication of self-organized nanogels holding both anti-cancer drug and targeting moiety. Colloids Surf B Biointerfaces. 63:55–63.
- Kohli E, Han HY, Zeman AD, Vinogradov SV. 2007. Formulations of biodegradable Nanogel carriers with 5’-triphosphates of nucleoside analogs that display a reduced cytotoxicity and enhanced drug activity. J Control Release. 121:19–27.
- Kriwet B, Walter E, Kissel T. 1998. Synthesis of bioadhesive poly(acrylic acid) nano- and microparticles using an inverse emulsion polymerization method for the entrapment of hydrophilic drug candidates. J Control Release. 56:149–158.
- Landfester K, Willert M, Antonietti M. 2000. Preparation of polymer particles in nonaqueous direct and inverse miniemulsions. Macromolecules. 33:2370–2376.
- Lee H, Mok H, Lee S, Oh Y-K, Park TG. 2007. Target-specific intracellular delivery of siRNA using degradable hyaluronic acid nanogels. J Control Release. 119:245–252.
- Lee JI, Kim HS, Yoo HS. 2009. DNA nanogels composed of chitosan and Pluronic with thermo-sensitive and photo-crosslinking properties. Int J Pharm. 373:93–99.
- Lee JI, Yoo HS. 2008. Pluronic decorated-nanogels with temperature-responsive volume transitions, cytotoxicities, and transfection efficiencies. Eur J Pharm Biopharm. 70:506–513.
- Lin Y, Alexandridis P. 2002. Cosolvent effects on the micellization of an amphiphilic siloxane graft copolymer in aqueous solutions. Langmuir. 18:4220–4231.
- Luisi PL, Straub B. 1984. Reverse Micelles: Biological and Technological Relevance of Amphiphilic Structures in Apolar Media New York: Plenum Pub Corp.
- Ma Q, Remsen EE, Kowalewski T, Wooley KL. 2001. Two-dimensional, shell-cross-linked nanoparticle arrays. J Am Chem Soc. 123: 4627–4628.
- Marek SR, Conn CA, Peppas NA. 2010. Cationic nanogels based on diethylaminoethyl methacrylate. Polymer. 51:1237–1243.
- Mok H, Park TG. 2006. PEG-assisted DNA solubilization in organic solvents for preparing cytosol specifically degradable PEG/DNA nanogels. Bioconjug Chem. 17:1369–1372.
- Nayak S, Lyon LA. 2005. Soft nanotechnology with soft nanoparticles. Angew Chem Int Ed Engl. 44:7686–7708.
- Nomura Y, Ikeda M, Yamaguchi N, Aoyama Y, Akiyoshi K. 2003. Protein refolding assisted by self-assembled nanogels as novel artificial molecular chaperone. FEBS Lett. 553:271–276.
- Nukolova NV, Oberoi HS, Cohen SM, Kabanov AV, Bronich TK. 2011. Folate-decorated nanogels for targeted therapy of ovarian cancer. Biomaterials. 32:5417–5426.
- Oh JK, Drumright R, Siegwart DJ, Matyjaszewski K. 2008. The development of microgels/nanogels for drug delivery applications. Prog Polym Sci. 33:448–477.
- Oh JK, Tang C, Gao H, Tsarevsky NV, Matyjaszewski K. 2006. Inverse miniemulsion ATRP: a new method for synthesis and functionalization of well-defined water-soluble/cross-linked polymeric particles. J Am Chem Soc. 128:5578–5584.
- Oh NM, Oh KT, Baik HJ, Lee BR, Lee AH, Youn YS, Lee ES. 2010. A self-organized 3-diethylaminopropyl-bearing glycol chitosan nanogel for tumor acidic pH targeting: in vitro evaluation. Colloids Surf B Biointerfaces. 78:120–126.
- Park W, Park SJ, Na K. 2010. Potential of self-organizing nanogel with acetylated chondroitin sulfate as an anti-cancer drug carrier. Colloids Surf B Biointerfaces. 79:501–508.
- Parnami N, Garg T, Rath G, Goyal AK. 2013. Development and characterization of nanocarriers for topical treatment of psoriasis by using combination therapy. Artif Cells Nanomed Biotechnol.
- Peng J, Qi T, Liao J, Chu B, Yang Q, Li W, et al. 2013. Controlled release of cisplatin from pH-thermal dual responsive nanogels. Biomaterials. 34:8726–8740.
- Raemdonck K, Naeye B, Hogset A, Demeester J, De Smedt SC 2010. Prolonged gene silencing by combining siRNA nanogels and photochemical internalization. J Control Release. 145:281–288.
- Riess G. 2003. Micellization of block copolymers. Progr Polym Sci. 28:1107–1170.
- Samah NA, Williams N, Heard CM. 2010. Nanogel particulates located within diffusion cell receptor phases following topical application demonstrates uptake into and migration across skin. Int J Pharm. 401:72–78.
- Sasaki Y, Asayama W, Niwa T, Sawada SI, Ueda T, Taguchi H, Akiyoshi K. 2011. Amphiphilic Polysaccharide Nanogels as Artificial Chaperones in Cell‐Free Protein Synthesis. Macromol Biosci. 11:814–820.
- Sasaki Y, Nomura Y, Sawada S-I, Akiyoshi K. 2010. Polysaccharide nanogel–cyclodextrin system as an artificial chaperone for in vitro protein synthesis of green fluorescent protein. Polym J. 42:823–828.
- Sawada S-I, Sasaki Y, Nomura Y, Akiyoshi K. 2011. Cyclodextrin- responsive nanogel as an artificial chaperone for horseradish peroxidase. Colloid Polym Sci. 289:685–691.
- Shen W, Chang Y, Liu G, Wang H, Cao A, An Z. 2011. Biocompatible, antifouling, and thermosensitive core− shell nanogels synthesized by RAFT aqueous dispersion polymerization. Macromolecules. 44:2524–2530.
- Shin Y, Chang JH, Liu J, Williford R, Exarhos GJ. 2001. Hybrid nanogels for sustainable positive thermosensitive drug release. J Control Release. 73:1–6.
- Singh H, Sharma R, Joshi M, Garg T, Goyal AK, Rath G. 2014. Transmucosal delivery of Docetaxel by mucoadhesive polymeric nanofibers. Artif Cells Nanomed Biotechnol.
- Singka GS, Samah NA, Zulfakar MH, Yurdasiper A, Heard CM. 2010. Enhanced topical delivery and anti-inflammatory activity of methotrexate from an activated nanogel. Eur J Pharm Biopharm. 76:275–281.
- Sun H, Yu J, Gong P, Xu D, Hong J, Zhang C, Yao S. 2006. Novel core–shell magnetic nanogels synthesized in an emulsion-free aqueous system under UV irradiation for potential targeted radiopharmaceutical applications. Int J Nanosci. 5:253–258.
- Sun H, Yu J, Gong P, Xu D, Zhang C, Yao S. 2005. Novel core–shell magnetic nanogels synthesized in an emulsion-free aqueous system under UV irradiation for targeted radiopharmaceutical applications. J Magn Magn Mater. 294:273–280.
- Takahashi H, Sawada S-I, Akiyoshi K. 2010. Amphiphilic polysaccharide nanoballs: a new building block for nanogel biomedical engineering and artificial chaperones. ACS Nano. 5:337–345.
- Tamura A, Oishi M, Nagasaki Y. 2010. Efficient siRNA delivery based on PEGylated and partially quaternized polyamine nanogels: enhanced gene silencing activity by the cooperative effect of tertiary and quaternary amino groups in the core. J Control Release. 146: 378–387.
- Tan JP, Wang Q, Tam KC. 2008. Control of burst release from nanogels via layer by layer assembly. J Control Release. 128:248–254.
- Vinogradov SV, Bronich TK, Kabanov AV. 2002. Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells. Adv Drug Deliv Rev. 54:135–147.
- Vinogradov SV, Zeman AD, Batrakova EV, Kabanov AV. 2005. Polyplex Nanogel formulations for drug delivery of cytotoxic nucleoside analogs. J Control Release. 107:143–157.
- Vintiloiu A, Leroux J-C. 2008. Organogels and their use in drug delivery—a review. J Control Release. 125:179–192.
- Wang C, Mallela J, Garapati US, Ravi S, Chinnasamy V, Girard Y, et al . 2013. A chitosan-modified graphene nanogel for noninvasive controlled drug release. Nanomedicine. 9:903–911.
- Wang NX, von Recum HA. 2011. Affinity‐Based Drug Delivery. Macromolecular Bioscience. 11:321–332.
- Wang Q, Xu H, Yang X, Yang Y. 2008. Drug release behavior from in situgelatinized thermosensitive nanogel aqueous dispersions. Int J Pharm. 361:189–193.
- Wong JE, Mu ller CB, DI ez-Pascual AM, Richtering W. 2009. Study of layer-by-layer films on thermoresponsive nanogels using temperature-controlled dual-focus fluorescence correlation spectroscopy. J Phys Chem B. 113:15907–15913.
- Wu W, Aiello M, Zhou T, Berliner A, Banerjee P, Zhou S. 2010. In-situ immobilization of quantum dots in polysaccharide-based nanogels for integration of optical pH-sensing, tumor cell imaging, and drug delivery. Biomaterials. 31:3023–3031.
- Xing Z, Wang C, Yan J, Zhang L, Li L, Zha L. 2011. Dual stimuli responsive hollow nanogels with IPN structure for temperature controlling drug loading and pH triggering drug release. Soft Matter. 7: 7992–7997.
- Xu DM, Yao SD, Liu YB, Sheng KL, Hong J, Gong PJ, Dong L. 2007. Size-dependent properties of M-PEIs nanogels for gene delivery in cancer cells. Int J Pharm. 338:291–296.
- Yan L, Tao W. 2010. One-step synthesis of pegylated cationic nanogels of poly ( N, N′-dimethylaminoethyl methacrylate) in aqueous solution via self-stabilizing micelles using an amphiphilic macroRAFT agent. Polymer. 51:2161–2167.
- Yu S, Yao P, Jiang M, Zhang G. 2006. Nanogels prepared by self-assembly of oppositely charged globular proteins. Biopolymers. 83:148–158.
- Zamurovic M, Christodoulou S, Vazaios A, Iatrou E, Pitsikalis M, Hadjichristidis N. 2007. Micellization behavior of complex comblike block copolymer architectures. Macromolecules. 40:5835–5849.