Abstract
Background
Telomerase is expressed in most of malignant cells, including lung cancer cells. The success of small interfering RNA (siRNA) in silencing of the telomerase catalytic subunit depends upon carriers ability to efficiently deliver therapeutic agent to cells with minimal toxicity and most biocompatibility. In this study, a potential carrier for efficient delivery was assessed by siRNA encapsulating into Iron MNPs modified with biodegradable polyester nanoparticles consisting of PLGA and PEG.
Results
Data analysis shows that the self-assemble diblock copolymers were synthesized, and then the siRNA designed against hTERT catalytic subunit was encapsulated. Also, the rate of telomerase gene expression in equivalent with magnetic copolymers/siRNA was lower than that of free siRNA (P = 0.001).
Conclusions
In conclusion, regarding the enhancing of siRNA stability by magnetic copolymer, the expression of telomerase gene was significantly lower in the cells treated with siRNA-magnetic copolymers than those treated with free siRNA.
| Abbreviations | ||
| SiRNA | = | Small interfering RNA |
| Fe3O4 | = | Iron oxide magnetic nanoparticles |
| PLGA | = | Poly (dl-lactic/glycolic acid) |
| PEG | = | Poly ethylene glycol |
| MNPs | = | Magnetic nanoparticles |
| TERT | = | Telomerase reverse transcriptase |
| TEM | = | Transmission electron microscope |
| FTIR | = | Fourier transform infrared spectroscopy |
Background
Telomerase is a ribonucleoprotein enzyme that synthesizes the telomeric repeats at the ends of linear chromosomes and replaces the progressively lost end sequences during each cell cycle, allowing the cells to escape mortality and continue to proliferate (CitationArmanios and Greider 2005, CitationPhatak and Burger 2007, CitationBlackburn 2005). Therefore, reactivity of telomerase in more than 95% is one of the cancer hallmarks. The human telomerase consists of both a catalytic protein component human telomerase reverse transcriptase (hTERT) and a 451-bp integral RNA that are essential for telomerase activity. It has been shown that the expression of hTERT in cultured human primary cells reconstitutes telomerase activity and allows immortal cell growth (CitationBodnar et al. 1998, CitationWeinrich et al. 1997). There are reports indicating that RNA interference (RNAi) against hTERT could successfully inhibit telomerase activity in several cancer cell lines (CitationMo et al. 2003, CitationNakamura et al. 2005, CitationNatarajan et al. 2004, CitationZhang et al. 2006). Gene silencing by RNAi offers new opportunities for the treatment of lung diseases such as lung cancer (CitationScherer and Rossi 2004). Small interfering RNAs (SiRNAs) are short (usually 21 nt) double stranded (dsRNA) with two nucleotides overhangs, which is a key molecule in the RNAi pathway (CitationLee and Sinko 2006, CitationQaddoumi et al. 2004). One of the major limiting factors in successful targeted siRNA delivery is the lack of suitable delivery systems that have ability to target cancer cells and carry out specific delivering of siRNA to the cells.
For this purpose, in the present study, magnetic nanostructures assembling siRNA composed of biocompatible iron oxide magnetic nanoparticles (Fe3O4) core, poly (dl-lactic/glycolic acid) (PLGA)- poly ethylene glycol (PEG) biodegradable block copolymers as shell was designed. PLGA nanoparticles have ability to entrap some water-soluble and water-insoluble molecules, in the process of extensive evaluation for the delivery of drugs, genetic materials, and proteins in cultured cells and experimental animals. These nanoparticles are endocytosis rapidly followed by the release of their therapeutic payload with passive diffusion and slow degradation (CitationHu et al. 2003, CitationCartiera et al. 2009). Also, PEG presents unique physicochemical and biological properties including biocompatibility, low immunogenicity, and water solubility; furthermore, it can be eliminated from the animal's body when the molar mass is less than 30,000 (CitationHu et al. 2003, CitationAkbarzadeh et al. 2012a, CitationAkbarzadeh et al. 2012b, CitationAkbarzadeh et al. 2012c). The main aim of this work was to study the capability of designed Fe3O4-PLGA-PEG nanoparticles-siRNA against hTERT gene for silencing of expression as an effective anticancer agent.
Results and discussion
Nanoparticles synthesis
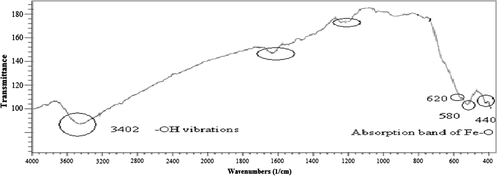
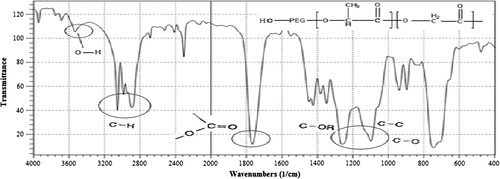
Size and shape of the magnetic nanoparticles (MNPs) were determined by transmission electronic microscopy (TEM) (). In addition, the iron magnetic nanoparticles were characterized by Fourier transform infrared (FTIR) spectroscopy with Perkin Elmer series. shows the absorption peaks at 440, 580, and 620 cm− 1 belonging to the stretching vibration mode of Fe–O bonds in Fe3O4, and the peaks at 3402 cm− 1 belonging to –OH vibrations. FTIR spectra for PLGA-PEG copolymers were also recorded (). A strong band at 1762.6 cm− 1 belonging to C = O stretch in lactide and glycolide structure, and an absorption band at 3509.9 cm− 1 was assigned to terminal hydroxyl groups in the copolymer from which PEG homopolymer has been removed (CitationZablotskaya et al. 2010, CitationAkbarzadeh et al. 2013a).

MTT assay results
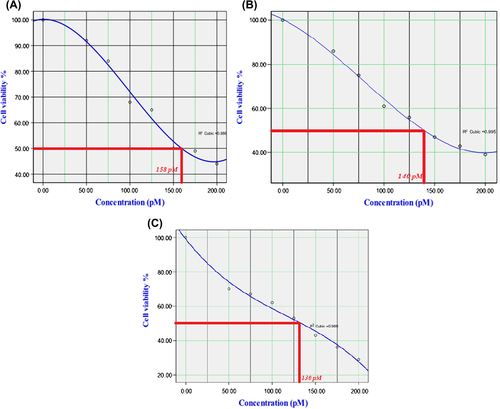
In this research, appropriate concentration of the encapsulated siRNA that does not have cytotoxicity effect on the cells was required. Therefore, Calu6 cells were exposed to varying concentrations of siRNA complex for three different times, and cell viability was demonstrated by MTT assay. Data analysis showed that high concentrations of MNPs have cytotoxicity effect on Calu6 cells. IC50 of encapsulated siRNA has been shown and its graph was drawn by SPSS 18 (). The results showed that the effect of synthesized complex on Calu6 cells is time-dependent, and less than 150 pM of siRNA complex for hTERT gene silencing indicates efficiency of designed siRNA in gene silencing.
Real-time PCR
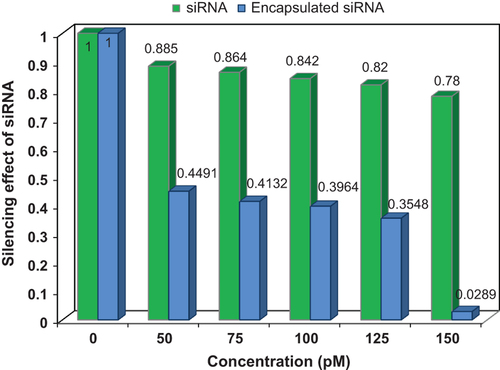
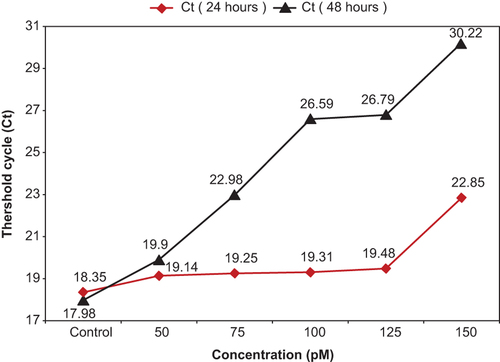
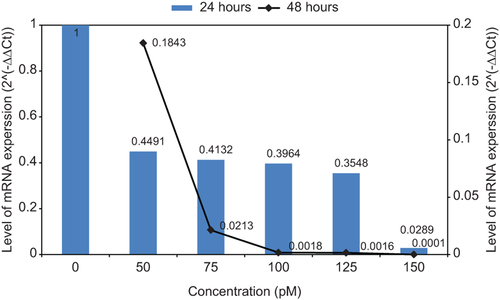
Average threshold cycle (Ct) for each sample in 24 h and 48 h was obtained and compared (). All duplicate amplifications showed very similar results. With increase in the amount of concentration and treated time, the amount of Ct was increased. Also the amounts of ΔCt and ΔΔCt for different times as well as the differences of 2–ΔΔCt values were calculated. Expression level of mRNA was reduced with increase in the amount of ΔCt (). In this study, pure siRNA was transfected, but showed weak effect on gene silencing in compared with the siRNA-loaded MNPs. Gene expression curves for siRNA and siRNA-loaded MNPs are shown (). The value of hTERT mRNA gene expression was decreased for both curves by increase in the concentration of siRNA. By increase of concentration from 50 to 150 pM, for siRNA, the hTERT gene expression was decreased from 0.885 to 0.78, and for siRNA-loaded MNPs, it was decreased from 0.4491 to 0.0289.
Materials and methods
SiRNA preparation
The siRNA sequences against two isoforms of hTERT mRNA were designed by a commercial software (Applied Biosystem/Ambion, Austin, TX). This siRNA sense sequence is 5′ CUGUUCAGCGUGCUCAACU (dTdT) 3′, and the anti-sense sequence is 5′ AGUUGAGCACGCUGAACAG (dTdT) 3′. In order to confirm the specificity of designed siRNA, the sequences were submitted to http://www.ncbi.nlm.nih.gov/blast/. Targeting siRNA and siCONTROL were purchased from Bioneer Corporation (Lot No≠ R111018-26; South Korea). 500 μl of DEPC-DW were added to each siRNA tube to obtain 20 μM final concentrations according to the manufacturer's instructions.
Cell culture
Calu6 lung adenocarcinoma cell line was purchased from Cell Bank of Pasteur Institute, Iran (Code: C203) and the cells were incubated in RPMI 1640 culture medium with 10% fetal bovine serum (FBS) at 37°C and humidified air containing 5% CO2. All culture medium and reagents were purchased from Gibco, Invitrogen, UK.
Iron magnetic nanoparticles and copolymer
Iron magnetic nanoparticles were prepared using a convenient chemical coprecipitation method (CitationKedar et al. 1997). Briefly, 7.5684 g of ferric chloride hexahydrate (FeCl3 + 6H2O) and 3.1736 g of Ferrous chloride tetrahydrate (FeCl2 + 4H2O) (Fluka, Switzerland) were dissolved in de-ionized water so that the Fe2+/Fe3+ ratio reached 0.57. Then, the solution was mixed under nitrogen at 80°C for 1 h. After injection of 40 ml NH3 + H2O, the mixture was stirred under nitrogen for another hour and the particles were precipitated at room temperature. Finally, the product was washed with hot water and dried under vacuum at 70°C. PLGA-PEG6000 copolymers were synthesized by ring-opening polymerization based on a previous report (CitationAkbarzadeh et al. 2012d).
Encapsulation in nanoparticles modified by copolymers
SiRNA complex were prepared using the double emulsion method with iron magnetic nanoparticles and copolymers. For this purpose, 100 μl of each siRNA solution was emulsified in 10 ml chloroform, in which 120 mg of the PLGA-PEG copolymer and 10 mg iron magnetic nanoparticles had been dissolved using a probe homogenizer at 20,000 rpm for 3 min (Heidolph Instruments GmbH, Schwabach, Germany). In order to prevent excessive heat generation, the system was placed in ice bath. Then 20 ml of the aqueous solution of polyvinyl alcohol 1% was added to water-in-oil (w/o) emulsion and the mixture was homogenized at 72,000 rpm for 5 min. The emulsion was stirred at room temperature to evaporate the organic phase (Rotary Evaporators, Heidolph Instruments, Hei-VAP series) (CitationZablotskaya et al. 2010, CitationAkbarzadeh et al. 2013a).
Cytotoxicity determination by the MTT assay
200 μl of the medium containing a density of 1.5 × 104 Calu6 cells was seeded in 96-well plates and incubated 24 h before the transfection. The nanoparticles suspension was diluted serially in seven concentrations with RPMI/10% fetal bovine serum. After removal of the cell culture medium, 200 μl of RPMI with 20 μl of MTT (3-(5-dimethylthiazol-2-yl)-2, 5-dephenyltetrazolium bromide) solution was added to each well and incubated. The MTT medium was removed after 4 h and formazan was dissolved using 200 μl DMSO, and the absorbance of each well was read in 570 nm using ELx800 Microplate Absorbance Reader (Bio-Tek Instruments) immediately. In order to adjust the final pH, 25 μl of Sorensen's glycine buffer (0.1 M glycine and 0.1 M NaCl, pH 10.5) was added to all the wells. Each experimental condition was carried out in quadruplicate and is presented as a mean of four measurements.
Real-time PCR
Cells were cultured in 6-well plates with 2.5 ml growth medium to reach exponential phase. The cells were exposed to different concentrations of siRNA complex and incubated for 24, 48, and 72 h. Then TRIzol reagent was used for the isolation of total RNA. After RNA extraction, its concentration was determined by nanodrop. Silencing ability of the encapsulated siRNA was calculated using the Syber Green-I (Roche, Germany) by the Rotor-GeneTM 6000 machinery (Corbett research, Australia). Genbank accession for hTERT and beta actin primers were (NM_198255, bp 2165-2362) and (NM_001101, bp 787-917). The forward/reverse primers of hTERT and beta actin genes were (5′CCGCCTGAGCTGTACTTTGT3′/5′CAGGTGAGCCACGAACTGT3′) and (5′TCCCTGGAGAAGAGCTACG3′/5′GTAGTTTCGTGGATGCCACA3′) respectively. The amplicons were generated in a 25-μl reaction mixture containing 5 pm of 2X PCR Master Mix Syber Green I and 2 μl of the cDNA.
Discussion
Telomerase inhibitors are considered potential anticancer agents, with this expectancy that the reducing of telomerase expression in normal somatic cells can result in a highly specific treatment (CitationShay and Gazdar 1997). Many drugs such as curcumin, helenaline or other agents such as nucleoside analogs, small non-nucleosidic synthetic compounds and peptides derived from TERT can inhibit telomerase gene expression (CitationKazemi et al. 2012, CitationYallapu et al. 2010, CitationEl Daly and Martens 2007, CitationLi et al. 2007). For instance, 3′-azido-2′, 3′-dideoxythymine (AZT) can be effective in targeting the active site of TERT, but this approach lacks the desired selectivity of many other approaches (CitationFletcher et al. 2001, CitationChen et al. 2009).
Our results demonstrated that the designed siRNA is effective agent for hTERT gene silencing. SiRNA is based on the ability of short dsRNA molecules to form the RNA- induced silencing complex (RISC), which can then hybridize with specific mRNA and cleave it; thereby, gene expression becomes silent (CitationAgrawal et al. 2003). This technology has numerous potential applications against diseases, but the main technical hurdle that prevents the advancement of siRNA-based cancer therapy is reliable tools for its delivery. Due to its large molecular weight (∼13 kDa) and strong anionic charge of the phosphodiester backbone (∼40 negative phosphate charges), naked siRNA does not freely cross the cell membrane. Cellular exclusion resulting from the inability of siRNA to passively diffuse through the cell membrane and electrostatic repulsion from the anionic cell membrane surface is one of the major obstacles to be overcome. Therefore, delivery systems are required to facilitate the access of siRNA to intracellular sites of action as known in the case of oligonucleotide antisense delivery (CitationKim and Kim 2009). Biodegradable PLGA polyester is strongly hydrophobic, and this has caused some limitations in practical drug formulations. In order to increase hydrophilic and other physico-chemical properties, PEG has been incorporated into PLGA as biodegradable polyester which is nontoxic, water-soluble and has proven biocompatibility.
In the present study, siRNA complex with modified MNPs by PLGA-PEG copolymers enhanced siRNA delivery can lead to efficient telomerase post transcription gene silencing. In the present study PLGA-PEG provide controlled delivery of siRNA. Fe3O4 caused the targeted delivery of designed siRNA absorbing onto the surface of nanoparticles, and drugs are then released on the desired area over a period of time. This local therapy could improve the efficiency of the treatment, reduce systemic toxicity, and ensure selective treatment of tumors in organs (CitationAkbarzadeh et al. 2012a, CitationValizadeh et al. 2012, CitationKalambur et al. 2005, CitationWidder et al. 1978). In addition, targeting of drugs by nanoparticles is intended to reduce drug wastage, such that a very low dose of siRNA-NPs complex solution (< 150 pM) was used for gene silencing in this study (CitationAkbarzadeh et al. 2013b, CitationAkbarzadeh et al. 2014, CitationAkbarzadeh et al. 2013c, CitationAkbarzadeh et al. 2014, CitationMollazadeh et al. 2013, CitationNejati-Koshki et al. 2012, CitationNejati-Koshki et al. 2013).
Conclusion
This study demonstrated the synthesis of biocompatible, biodegradable distinct magnetic nanoparticles and PLGA-PEG copolymers for siRNA delivery. Also due to insolubility, low dosage and gene silencing potential of the designed siRNA, it can be considered an efficient drug against lung cancer cells by targeting the TERT component of telomerase. Furthermore, the mentioned delivery system can be used for targeted delivery in magnetic fields. Therefore siRNA complex with modified MNPs by PLGA-PEG copolymers can enhance siRNA delivery to efficient telomerase post transcription gene silencing.
Authors’ contributions
AA conceived of the study and participated in its design and coordination. NZ participated in the sequence alignment and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors thank Department of Medical Nanotechnology, Faculty of Advanced Medical Science of Tabriz University for all supports provided.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
This work is funded by Lung Research Center of Tabriz University of Medical Sciences, Tabriz.
References
- Agrawal N, Dasaradhi P, Mohmmed A, Malhotra P, Bhatnagar R, Mukherjee S. 2003. RNA interference: biology, mechanism, and applications. Microbiol Mol Biol Rev. 67:657–685.
- Akbarzadeh A, Mikaeili H, Zarghami N, Rahmati M, Barkhordari A, Davaran S. 2012d. Preparation and in vitro evaluation of doxorubicin loaded Fe3O4 magnetic nanoparticles modified with biocompatible copolymers. Int J Nanomedicine. 7:511–526.
- Akbarzadeh A, Nejati-Koshki K, Mahmoudi Soghrati M, et al. 2013b. In vitro studies of NIPAAM-MAA-VP copolymer coated magnetic nanoparticles for controlled anticancer drug release. JEAS. 3: 108–115.
- Akbarzadeh A, Nejati-Koshki K, Mahmoudi Soghrati M, et al. 2013c. In vitro studies of NIPAAM-MAA-VP copolymer coated magnetic nanoparticles for controlled anticancer drug release. JEAS. 3:108–115.
- Akbarzadeh A, Rezaei A, Nejati-Koshki K, et al. 2014. Synthesis and physicochemical characterization of biodegradable star-shaped poly lactide-co-glycolide– β -cyclodextrin copolymer nanoparticles containing albumin. J Adv Nanoparticles. 3:1–9.
- Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, et al. 2013a. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 8:102.
- Akbarzadeh A, Samiei M, Davaran S. 2012c. Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res Letrs. 7:144.
- Akbarzadeh A, Samiei M, Joo SW, Anzaby M, Hanifehpour Y, Nasrabadi HT, Davaran S. 2012a. Synthesis, characterization and in vitro studies of doxorubicin-loaded magnetic nanoparticles grafted to smart Copolymers on A549 lung cancer cell line. J Nanobiotechnology. 10:46.
- Akbarzadeh A, Zarghami N, Mikaeili H, Asgari D, Goganian AM, Khaksar Khiabani H, et al. 2012b. Synthesis, characterization and in vitro evaluation of novel polymer-coated magnetic nanoparticles for controlled delivery of doxorubicin. Nanotechnol Sci Appl. 5:1–13.
- Armanios M, Greider CW. 2005. Telomerase and cancer stem cells. Cold Spring Harbor Symp Quant Biol. 70:205–208.
- Blackburn EH. 2005. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 579:859–862.
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, et al. 1998. Extention of life-span by introduction of telomerase into normal human cells. Science. 279:349–352.
- Cartiera MS, Johnson KM, Rajendran V, Caplan MJ, Saltzman WM. 2009. The uptake and intracellular fate of PLGA nanoparticles in epithelial cells. Biomaterials. 30:2790–2798.
- Chen H, Li Y, Tollefsbol TO. 2009. Strategies targeting telomerase inhibition. Mol Biotechnol. 41:194–199.
- El Daly H, Martens U. 2007. Telomerase inhibition and telomere targeting in hematopoietic cancer cell lines with small non- nucleosidic synthetic compounds (BIBR1532). Methods Mol Biol. 405:47–60.
- Fletcher T, Cathers B, Ravikumar K, Mamiya B, Kerwin S. 2001. Inhibition of human telomerase by 7-deaza-2′-deoxy-guanosine nucleoside triphosphate analogs: Potent inhibition by 6-thio- 7-deaza-2′-deoxyguanosine 5′-triphosphate. Bioorg Chem. 29:36–55.
- Hu Y, Jiang X, Ding Y, Zhang L, Yang C, Zhang J, et al. 2003. Preparation and drug release behaviors of nimodipine-loaded poly(caprolactone)-poly(ethylene oxide)-polylactide amphiphilic copolymer nanoparticles. Biomaterials. 24:2395–2404.
- Kalambur V, Han B, Hammer BE, Shield TW, Bischof JC. 2005. In vitro characterization of movement, heating and visualization of magnetic nanoparticles for biomedical applications. Nanotechnology. 16:1221.
- Kazemi F, Zarghami N, Fekri S, Monfaredan A. 2012.β-Cyclodextrin-curcumin complex inhibit telomerase gene expression in T47-D breast cancer cell line. Afr J Biotechnol. 10:19481–19488.
- Kedar E, Algi O, Golod G, Babai I, Barenholz Y. 1997. Delivery of cytokines by liposomes. III. Liposome-encapsulated GM-CSF and TNF-alpha show improved pharmacokinetics and biological activity and reduced toxicity in mice.J Immunother. 20:180–193.
- Kim WJ, Kim SW. 2009. Efficient siRNA Delivery with non-viral polymeric vehicles. Pharm Res. 26:657–666.
- Lee SH, Sinko PJ. 2006. siRNA—Getting the message out. Eur J Pharm Sci. 27:401–410.
- Li H, Katik I, Liu J. 2007. Uses of telomerase peptides in anti-tumor immune therapy. Methods Mol Biol. 405:61–86.
- Mo Y, Gan Y, Song S, Johnston J, Xiao X, Wientjes MG, Au JL. 2003. Simultaneous targeting of telomeres and telomerase as a cancer therapeutic approach. Cancer Res. 63:579–585.
- Mollazadeh M, Nejati-Koshki K, Akbarzadeh A, Zarghami N, Nasiri M, Jahanban-Esfahlan R, Alibakhshi A. 2013. PAMAM dendrimers arugment inhibitory effect of curcumin on cancer cell proliferation: possible inhibition of telomerase. Asian Pac J Cancer Prev.14:6925–6929.
- Nakamura M, Masutomi K, Kyo S, Hashimoto M, Maida Y, Kanaya T, et al. 2005. Efficient inhibition of human telomerase reverse transcriptase expression by RNA interference sensitizes cancer cells to ionizing radiation and chemotherapy. Hum Gene Ther. 16:859–868.
- Natarajan S, Chen Z, Wancewicz EV, Monia BP, Corey DR. 2004. Telomerase reverse transcriptase (hTERT) mRNA and telomerase RNA (hTR) as targets for downregulation of telomerase activity. Oligonucleotides. 14:263–273.
- Nejati-Koshki K, Akbarzadeh A, Pourhasan-Moghadam M, et al. 2013. Inhibition of leptin and leptin receptor gene expression by silibinin- curcumin combination. Asian Pac J Cancer Prev. 14: 6595–6599.
- Nejati-Koshki K, Zarghami N, Pourhassan-Moghaddam M, et al. 2012. Inhibition of leptin gene expression and secretion by silibinin: possible role of estrogen receptors. Cytotechnology. 64:719–726.
- Phatak P, Burger AM. 2007. Telomerase and its potential for therapeutic intervention. Br J Pharmacol. 152:1003–1011.
- Qaddoumi MG, Ueda H, Yang J, Davda J, Labhasetwar V, Lee VH. 2004. The characteristics and mechanisms of uptake of PLGA nanoparticles in rabbit conjunctival epithelial cell layers. Pharm Res. 21:641–648.
- Scherer L, Rossi J. 2004. RNAi applications in mammalian cells. BioTechniques. 36:557–561.
- Shay JW, Gazdar AF. 1997. Telomerase in the early detection of cancer. J Clin Pathol. 50:106–109.
- Valizadeh A, Mikaeili H, Samiei M, Farkhani SM, Zarghami N, Kouhi M, et al. 2012. Quantum dots: synthesis, bioapplications, and toxicity Nanoscale. Res Lett. 7:480.
- Weinrich SL, Pruzan R, Ma L, Ouellette M, Tesmer VM, Holt SE, et al. 1997. Reconstitution of human telomerase with the catalytic protein subunit hTRT. Nat Genet. 17:498–502.
- Widder KJ, Senyel AE, Scarpelli GD. 1978. Magnetic microspheres: a model system of site specific drug delivery in vivo. Proc Soc Exp Biol Med. 58:141.
- Yallapu MM, Jaggi M, Chauhan SC. 2010. beta-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf B Biointerfaces. 79:113–125.
- Zablotskaya A, Segal I, Lukevics E. 2010. Iron oxide-based magnetic nanostructures bearing cytotoxic organosilicon molecules for drug delivery and therapy. Appl Organometa Chem. 24: 150–157.
- Zhang PH, Zou L, Tu ZG. 2006. RNAi-hTERT inhibition hepatocellular carcinoma cell proliferation via decreasing telomerase activity. J Surg Res. 131:143–149.