?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Introduction
In a recent study, we confirmed good chemical and physical compatibility of microencapsulated pancreatic β-cells using a novel formulation of low viscosity sodium alginate (LVSA), Poly-L-Ornithine (PLO), and the tertiary bile acid, ursodeoxycholic acid (UDCA). This study aimed to investigate the effect of UDCA on the morphology, swelling, stability, and size of these new microcapsules. It also aimed to evaluate cell viability in the microcapsules following UDCA addition.
Materials and methods
Microencapsulation was carried out using a Büchi-based system. Two (LVSA-PLO, control and LVSA-PLO-UDCA, test) pancreatic β-cells microcapsules were prepared at a constant ratio of 10:1:3, respectively. The microcapsules’ morphology, cell viability, swelling characteristics, stability, mechanical strength, Zeta potential, and size analysis were examined. The cell contents in each microcapsule and the microencapsulation efficiency were also examined.
Results
The addition of UDCA did not affect the microcapsules’ morphology, stability, size, or the microencapsulation efficiency. However, UDCA enhanced cell viability in the microcapsules 24 h after microencapsulation (p < 0.01), reduced swelling (p < 0.05), reduced Zeta potential (− 73 ± 2 to − 54 ± 2 mV, p < 0.01), and increased mechanical strength of the microcapsules (p < 0.05) at the end of the 24-h experimental period.
Discussion and conclusion
UDCA increased β-cell viability in the microcapsules without affecting the microcapsules’ size, morphology, or stability. It also increased the microcapsules’ resistance to swelling and optimized their mechanical strength. Our findings suggest potential benefits of the bile acid UDCA in β-cell microencapsulation.
Introduction
Diabetes mellitus is a chronic disorder characterized by persistent hyperglycemia and is categorized as either type 1 diabetes (T1D) or type 2 diabetes (T2D) (CitationStumvoll et al. 2005, CitationCodario 2011). T1D is an autoimmune disease whereby there is a complete destruction of pancreatic β-cells by the host's immune system, whereas T2D manifests as the result of genetic and environmental factors, culminating as insulin resistance with gradual loss of functional β-cells over time (CitationNolan et al. 2011, CitationTaylor 2013). The loss of viable β-cells in T2D is mainly due to excessive β-cell stimulation, which is caused by the long-term use of antidiabetic drugs such as sulfonylureas or from diabetes-associated inflammation (CitationBeck et al. 2007).
Given insulin's narrow therapeutic index and inconvenient route of administration, there is a real need to replenish the dysfunctional pancreatic β-cells. This can be achieved through transplanting viable β-cells, which produce sufficient insulin to control glycaemia. If such an approach is successful, it will be of great benefit to both T1D and T2D patients who need exogenous insulin (CitationBeck et al. 2007, CitationHarlan et al. 2004). However, to date, β-cells transplantation has not been that successful, due to many challenges (CitationPuri and Hebrok 2012, CitationClaiborn and Stoffers 2008).
A major challenge with β-cells transplantation is the host's immune response and subsequent inflammation (CitationRokstad et al. 2014). Thus, creating a new immunostatic matrix, which can encapsulate and protect the cells and maintain their biological functions, should overcome this challenge. One method for cell encapsulation is by using artificial cell microencapsulation (ACM) technology.
ACM has been used since the 1960s for cellular and drug delivery (CitationPal and Nayak 2011, CitationUrbanska et al. 2007, CitationWong and Chang 1991). ACM of β-cells using an appropriate polymer-based formulation has an advantage over other delivery systems as it creates a biologically sustainable ecosystem capable of cell proliferation and activity without the need for immunosuppressive regimens (CitationRokstad et al. 2014, CitationWeir 2013). The polymer most frequently used is the natural hydrogel low viscosity sodium alginate (LVSA) (CitationNafea et al. 2011, CitationRokstad et al. 2013). Unlike synthetic polymers, LVSA is biocompatible and favorable to cellular proliferation (CitationRokstad et al. 2014). However, when used alone, it has many limitations such as compromised mechanical strength and being prone to easy rupture and degradation under stress (CitationNafea et al. 2011, CitationBhatia et al. 2005). Thus, LVSA is often co-formulated with a polyelectrolyte such as Poly-L-Ornithine (PLO), resulting in ionic complexation and enhanced stability of the microcapsules, with reduced sudden rupture after administration (CitationThanos et al. 2007a, Citation2007b). Despite the relatively common use of the LVSA-PLO microencapsulation system, there are still significant drawbacks such as low cell viability and limited membrane mechanical strength (CitationDusseault et al. 2005, CitationSchneider et al. 2001). We have shown, in published work investigating potential antidiabetic applications of bile acids (CitationAl-Salami et al. 2008, Citation2009, Citation2012, CitationCalasan et al. 2012, CitationLalic-Popovic et al. 2013, CitationMikov et al. 2008, CitationMikov et al. 2006, CitationNegrulj et al. 2013, CitationMooranian et al. 2014a, Citation2014b), that their incorporation into LVSA-microcapsules exerts membrane-stabilizing effects and optimizes the rheological, physical and chemical parameters, uniformity, and structural morphology of these microcapsules.
It is worth stating that current systems for β-cell microencapsulation failed in the clinic to replace insulin use, including simple alginate-PLO microcapsules. The lack of cell viability as well as immune-matrix chemical interaction are major obstacles. Our findings showed better viability (in vitro), which remained to be examined, in vivo, in our future work.
Thus, in this study, we aimed to investigate the effects of the bile acid, ursodeoxycholic acid (UDCA), on our LVSA-PLO microcapsules containing β-cells, in terms of the microcapsules’ morphology, swelling properties, mechanical strength, stability, and cell viability following microencapsulation.
Materials and methods
Cell culture
BRIN-BD11 cells were cultured on T-75 cm2 tissue culture flasks (Thermo Fisher Scientific®, Australia) and fed with Roswell Park Memorial Institute (RPMI) 1640 media (Gibco, Life Technologies, USA) supplemented with 5.5 mmol glucose (Sigma Chemical Co, USA), 10% fetal bovine serum (Thermo Fisher Scientific, Australia), and 5% penicillin-streptomycin (Thermo Fisher Scientific, Australia). The β-cells, BRIN-BD11, were incubated in an environment of 5% CO2 in humidified air at 37°C using a Nuaire NU-8500 Water Jacket CO2 Incubator (Nuaire, USA) and the medium was changed every 48 h. The cells were subcultured weekly by incubating the BRIN-BD11 monolayer with 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) (Thermo Fisher Scientific, Australia) for 4–5 min under the same environmental conditions used to culture the cells. The trypsinized cells were added to the equivalent volume of freshly prepared media and centrifuged at 1500 rpm for 5 min at 20°C using a Beckman Coulter Allegra X-12 centrifuge (Beckman Coulter, USA). The supernatant was then discarded, and cells were resuspended in fresh media ready for microencapsulation under sterile conditions.
Formulation preparation
LVSA (≥ 99%, sterile and suitable for cell culture, A1112), sterile PLO (P2533), and UDCA (99%) were all purchased from Sigma Chemical Co, USA. Calcium chloride dihydrate (CaCl2.2H20, 98%) was obtained from Scharlab S.L, Australia.
Stock solutions of LVSA and 10% CaCl2 were prepared using UltraPure™ distilled water (Life Technologies, USA). The PLO solution was added to the stock solution of LVSA to give a LVSA-PLO mixture ratio of 10:1, respectively. This ratio was found to exhibit the best morphology and consistency. From this stock solution of LVSA-PLO, a proportion was removed and UDCA powder was added to this in order to form the LVSA-PLO-UDCA mixture (ratio of 10:1:3, respectively) containing 4mg/ml of UDCA in the LVSA-PLO mixture.
All solutions were mixed for 4 h using sterilized laboratory equipment in a Gelaire BH-EN Class II biological safety cabinet (Gelaire Company, Australia), with independently certified sterile working conditions, in a physical containment level 2 laboratory (PC2).
Sterilization protocol
All reagents were sterile upon purchase, endotoxin tested, and suitable for cell culture. All subsequent solutions and formulations made in our laboratory were sterilized by filtration (0.22μm) before their use, and all mixtures/solution were prepared in the utmost sterile working conditions. Random samples were collected and analyzed to confirm sterility throughout the experiment. Reusable laboratory items, equipment, and labware were thoroughly cleaned and sterilized via a Getinge laboratory autoclave oven (model 92222, Getinge Cooperation, Sweden).
Microencapsulation by vibrating-jet flow method
Microencapsulation was carried out using a Büchi- microencapsulation system established in our laboratory. Briefly, this method employs the use of the Büchi- microencapsulator, where microcapsules are made via the vibrating-jet flow method (VJFM) using polymer solutions and set parameters (voltage, frequency, liquid flow rate, and air pressure) suitable for producing microcapsules of the desired physico-chemical properties (CitationMooranian et al. 2014a, Citation2014b).
Parameters were set in a frequency range of 1000–1500Hz and a flow rate of 4 ml/min under a consistent air pressure of 300 mbar, based on previously published work (Mooranian et al. 2014). Microcapsules were generated over a period of 15 min and left in the gelation bath for 5 min and washed with buffer, for 2 min.
Post-microencapsulation, the microcapsules were stored in T-25 cm2 flasks (Thermo Fisher Scientific, Australia) containing 5ml of media and incubated under sterile conditions (37°C with 5% CO2 in the incubator) for 24 h prior to use.
Characterization of loaded microcapsules
Optical microscopy
Morphological characteristics and particle size analysis were determined with optical microscopy (OM); utilizing Nikon YS2-H mounted with ToupTek photonics FMA050 fixed calibrated microscope adaptor (Japan). Sample analysis was carried out in triplicates. Briefly, pre-determined quantities (10 microcapsules from each formulation) of freshly prepared microcapsules were loaded onto a glass slide mounted to a calibrated scale. OM software (ToupTek Digital, Japan) capable of particle size analysis, microcapsule characterization, and morphological assessments was utilized to determine basic characteristics of the microcapsules.
Microencapsulated cell count and microencapsulation efficiency
To determine the total number of cells used for microencapsulation following resuspension post centrifugation, a 40-μl aliquot was removed and placed in a 65-μl Eppendorf tube (Eppendorf South Pacific, Australia) to which 10 μl of trypan blue solution 0.4% was added (Sigma Chemical CO, Australia). The mixture was mixed well and two lots of 10 μl were removed from the mixture and each placed on opposite sides of a Countess Cell Counter chamber slide (Invitrogen, Korea), and the slide was then placed into the slide port of a Countess Automated Cell Counter (Invitrogen, Korea) for analysis. This instrumentation is capable of reporting the total cell count, cell size, and details regarding cell viability. Taking account of the dilution factor, we were able to then calculate the total cell count in the culture flask, the actual number microencapsulation, and the efficiency of the process. These microencapsulation performance indicators were only based on live cells used in the process, not the total cell population.
To determine the number of cells and their viability following microencapsulation, a predetermined number of microcapsules (n = 10) from both formulations was placed in a T-25 cm2 flask. To ensure a gradual rupture of the microcapsule membrane without adverse effects on the cells, 0.1M NaOH (VWR, Australia) was added to the media to raise the pH from 6.9 to 7.3, and 5 ml of the newly made alkaline media were then placed in the flask containing the microcapsules and allowed to incubate for 24 h at 37°C with 5% CO2. The exact same protocol was performed using empty microcapsules from both formulations to serve as controls. At the end of 24 h, the microcapsules were removed and a 40-μl aliquot of the medium was removed and placed in a 65-μl Eppendorf tube to which 10 μl of trypan blue (0.4%) were added and a cell count with viability was performed.
MTT assay
The MTT assay for cellular mitochondrial activity is a common and an important analytical technique for determining the degree of cell viability and biological activity. A well validated method was deployed, based on the work of Uludag and Sefton (CitationUludag and Sefton 1990) with slight modifications. Briefly, MTT was prepared as 5mg/ml stock solution (Sigma Chemical CO, USA) in phosphate buffer, at pH 7.4 (Thermo Fisher Scientific, Australia). The undissolved residues were removed by sterile filtration. The stock solution was stored in a sterile environment at 4°C in the dark and used within 7 days of preparation. For the MTT assay protocol, 20μl of MTT from the stock solution were added into each well of 96-well plates (Thermo Fisher Scientific, Australia) containing freshly made microcapsules that had been placed in 200μl of media (pH 7.4) for 24 h and incubated at 37°C with 5% CO2 in humidified air. The MTT conversion to formazan was removed from the incubator after 4 h by washing the microcapsules with MilliQ water for 5 min in order to remove spectroscopic interference. Formazan was dissolved in 100 μl of dimethyl sulfoxide (DMSO) (Sigma Chemical CO, USA) via reverse pipetting and the resultant purple solution was analyzed photometrically at 550nm. Empty microcapsules from each formulation (LVSA-PLO and LVSA-PLO-UDCA) were also analyzed and served as negative controls, together with non- microencapsulated cells grown in the 96-well plates using the same alkaline medium to serve as positive controls. This was necessary to ensure no spectrophotometric interference occurred due to the polymer, polyelectrolyte, or bile acid. In addition, the number of cells was consistent across both microencapsulated and non-microencapsulated formulations, as described elsewhere (CitationUludag and Sefton 1990).
Swelling studies
To determine the swelling properties of the microcapsules, 50mg of dry microcapsules were weighed and placed in 20 ml of phosphate buffer of pH 7.8 (Mooranian et al. 2014) at a temperature of 37°C for 6 h. The swollen microcapsules were then removed at periodically predetermined intervals (hourly). The net weight of the swollen microcapsules was determined by blotting them with filter paper to remove moisture adhering to the surface, immediately followed by weighing on an electronic balance. All experiments were performed in triplicate. The swelling index of the microcapsules was calculated from the following formula (CitationPal and Nayak 2012, CitationAwasthi and Kulkarni 2013):
Stability studies
The stability test was carried out by placing predetermined amounts of freshly prepared microcapsules onto sterile petri dishes (30 microcapsules in each) and storing them in thermostatically controlled ovens (environmental stability chambers) at − 20°C, 5°C, 25°C, and 40°C with relative humidity set at 35% for 3 days. The experiment was conducted using a stability chamber (Angelantoni Environmental and Climatic Test Chamber, Italy). A temperature and humidity regulator was used to ensure constant experimental conditions. At the end of the experiment, the microcapsules were analyzed for any changes in appearance and morphology.
Zeta potential and size analysis
To determine the electrokinetic stability and size uniformity of the microcapsules in the dispersion system, the Zeta potential and size distribution for the microencapsulated formulation of LVSA-PLO and LVSA-PLO-UDCA were measured by photon correlation spectroscopy using a Zetasizer 3000HS (Malvern Instruments, Malvern, UK) and by Mie and Fraunhofer scattering technique using a Mastersizer 2000 (Malvern Instruments, Malvern, UK). The measurements were performed at 25°C with a detection angle of 900, and the raw data were subsequently correlated to Z average mean size using a cumulative analysis via an OmniSEC-Zetasizer software package. All analyses were performed on samples appropriately diluted with filtered deionized water. All determinations were performed in triplicate. Results are reported as mean ± SD.
Mechanical resistance
In order to test the mechanical stability of the microcapsules, a mechanical resistance testing was undertaken using a Boeco Multishaker PSU 20 (Boeco Company, Germany) as described elsewhere (CitationRokstad et al. 2013, CitationJiin 2000). Briefly, vials containing 20 microcapsules in 20 mL of phosphate buffer (pH 7.4) were placed in a shaker and stirred at a frequency of 150 rpm for a period of 24 h. At various time intervals, the number of fractured or damaged microcapsules was counted. The mechanical strength of the microcapsules was calculated as follows (CitationJiin 2000):
Statistical analysis
The results are expressed as mean ± standard deviation, recoded in triplicates. For statistical analysis, two-way ANOVA with Tukey post-hoc analysis was conducted, setting the level of significance at p < 0.05. All the statistical analysis was calculated using GraphPad Prism version 6.0. The results for the p value were only reported where significance was noted.
Results and discussion
Optical microscopy
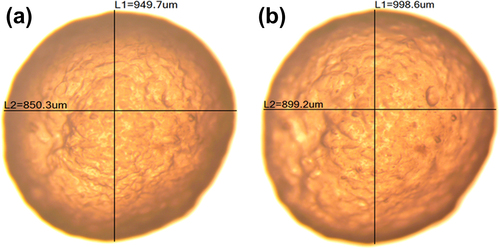
Morphological assessments of the microcapsules from both formulations (LVSA-PLO and LVSA-PLO-UDCA) revealed spherical microcapsules of homogenous shape and size distribution (), with no difference noted between both types of microcapsules.
Microencapsulation efficiency and cell count
To be clinically beneficial, cell viability following microencapsulation is critical (CitationNafea et al. 2011). The test for microencapsulated cell content and efficiency was performed using a 0.4% trypan blue solution and analysis of microencapsulated live cells and microencapsulation efficiency was carried out. shows that, 24 h after microencapsulation, there were more live cells in the microcapsules containing UDCA compared to microcapsules without UDCA (p < 0.01). In addition, the microencapsulation efficiency was similar for both formulations. The positive effect of UDCA on cell proliferation post-microencapsulation is possibly due to either minimizing cell death or enhancing the metabolic and growth activities of the cells. Previous work has shown that when UDCA was added into hepatocytes, there was no direct effect on cell proliferation (CitationRudi et al. 1995, CitationMartinez et al. 1998), while UDCA exerted a positive effect on bile duct cellular proliferation in bile duct ligated rats (CitationFrezza et al. 1993). A possible explanation is that UDCA maintained cell viability in the microcapsules, 24 h post-microencapsulation, rather than directly inducing proliferation. This is supported further by results from the MTT assay ().
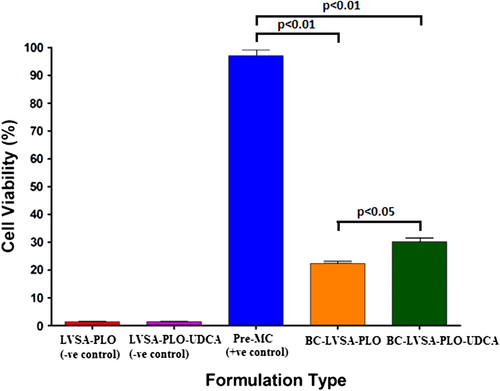
Table I. Microencapsulated cell content and efficiency of the microencapsulation process for the two formulations containing β-cells.
MTT assay analysis
represents the assessment of cell metabolic (mitochondrial) activity of the test (BC-LVSA-PLO-UDCA: β-Cells in LVSA-PLO-UDCA) microcapsules, control (BC-LVSA-PLO: β-Cells in LVSA-PLO) microcapsules, positive control (Pre-MC: pre-microencapsulation β-Cells), negative control without cells but with UDCA (LVSA-PLO-UDCA), and negative control without the cells or UDCA (LVSA-PLO). shows that the β-cell count is reduced after microencapsulation (p < 0.01), and the cell count was higher in microcapsules containing UDCA compared with microcapsules not containing UDCA (p < 0.05).
LVSA-PLO-UDCA microcapsules containing β-cells displayed greater mitochondrial activity than LVSA-PLO microcapsules. The enhanced mitochondrial activity represents greater cellular metabolic activity and viability, suggesting that the incorporation of UDCA resulted in microcapsules with more desired membrane characteristics and a more hospitable ecosystem. The results are in line with our data showing a strong membrane-stabilizing effect of UDCA on empty and drug-containing LVSA-microcapsules (manuscript under review). However, and to the best of our knowledge, the effects of UDCA on LVSA-PLO microencapsulated β-cells have not been studied, and this clearly demonstrates the novelty of our findings.
Published work examining the effects of UDCA, focusing on cell biochemical reactions, has shown anti-inflammatory and antioxidant effects, in vivo (CitationMartinez-Moya et al. 2013). Such effects may support our findings (). However, other published work showed an inhibitory effect of UDCA on proliferation and differentiation of human subcutaneous adipocytes (CitationMalisova et al. 2013). The in vivo impact of UDCA on β-cell biochemical and bioinflammatory reactions is outside the scope of this study, but transplantation of our microencapsulated LVSA-PLO-UDCA microcapsules into Type 1 diabetic animals should provide more insight into the efficacy and long term effects on glycemic control and inflammation.
Swelling studies
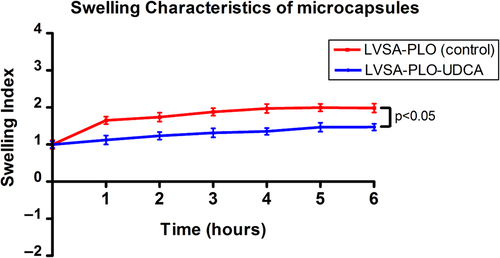
As shown below (), addition of UDCA resulted in a reduction in the swelling of the microcapsules at pH 7.4 and temperature 37°C (p < 0.05), most likely due to reinforcement of the calcium-alginate membrane (CitationTakka and Cali 2012).
Stability studies
At constant humidity (35%), a change in temperature had a profound effect on the morphology, appearance, and size of both types of microcapsules (LVSA-PLO and LVSA-PLO-UDCA). At − 20°C, both β-cells-containing microcapsules retained their original shape (spherical), morphology, color (milky-white), and texture (soft and flexible). At 5°C, both experienced a 10% reduction in size without change in color or appearance. However, at 25°C and 40°C, there was a significant reduction in microcapsule size (by up to 50% at 40°C), with more reduction noticed in the LVSA-PLO microcapsules, which is consistent with the swelling findings, () that show a positive effect of UDCA in maintaining the integrity of the microcapsules. Moreover, the color of the microcapsule surface changed from milky-white to yellow (25°C) or orange (40°C) for both formulations. In addition, both microcapsules had changed their texture and had become harder and more brittle, especially at 40°C. The changes that occur with increasing temperatures can be explained by the loss of moisture content and dehydration that results in shrinkage of the microcapsules and alteration of the surface texture, causing them to become rough, brittle, and hard (CitationBhatia et al. 2005). These results suggest that the incorporation of UDCA does provide some degree of added stability during accelerated stability studies, especially at higher temperatures.
Mechanical resistance
In line with published work (CitationJiin 2000), the analysis of mechanical strength (using the shaker) provided further confirmation of the membrane stabilizing effects of UDCA and its role in reinforcement of the calcium-alginate matrix (). As shown below, when both microcapsules were placed in a shaker, more LVSA-PLO-UDCA microcapsules remained intact compared with LVSA-PLO microcapsules at 16, 20, and 24 h (p < 0.05). The findings support the swelling studies () in that the addition of UDCA enhanced the stability and coherence of the microcapsules containing β-cells, conferring potential benefits, especially when it also brought about better cell viability ( and ).
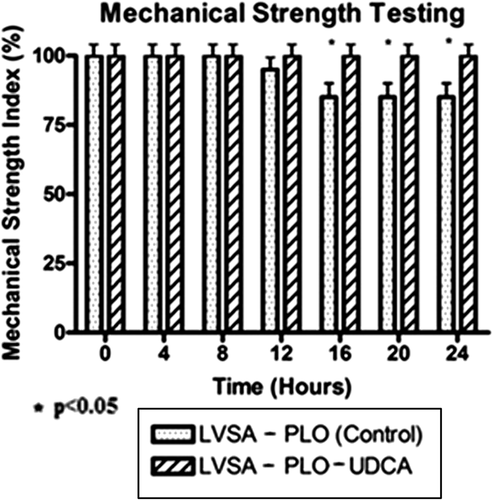
Accordingly, it seems that UDCA has an important role in the formulation used for microencapsulation of β-cells in terms of microcapsule stability and cell viability, which results in an optimized delivery platform.
Zeta potential and size analysis
Zetasizer analysis of both formulations revealed that UDCA did not change the particle size distribution of the β-cells containing microcapsules, with the size being similar to that shown in . The dispersion system created by the LVSA-PLO mixture had a zeta potential of − 70 ± 2 mV and the LVSA-PLO-UDCA mixture had a zeta potential of − 51 ± 2 mV. A negative charge is anticipated to exert a greater stability of microcapsules when delivered in vivo, as positive surface charges adsorb proteins and result in the activation of the immune system (CitationRokstad et al. 2013, Citationde Vos et al. 2007). Thus, both formulations have good stability but the addition of UDCA reduced the overall surface charge of the mixture.
Conclusion
The incorporation of the bile acid, UDCA, into our LVSA-PLO microencapsulated β-cells optimized the stability and cell viability, and may provide an added benefit, in vivo. However, and despite improvement of cell viability after microencapsulation, it remains limited. Thus, a future study may test different bile acid-formulations aiming to improve further cell viability post-microencapsulation.
Notice of Correction
The version of this article published online ahead of print on 11th July 2014 contained an error in the Acknowledgements section. The error has been corrected for this version
Acknowledgments
The authors acknowledge the CHIRI at Curtin University and the Curtin-seeding grant for support, and also acknowledge the use of equipment, scientific and technical assistance of the Curtin University Electron Microscope Facility, which has been partially funded by the University, State, and Commonwealth Governments. The authors also acknowledge the Pharmaceutical Technology Laboratory for their valuable assistance (Curtin School of Pharmacy) and the School of Biomedical Science, Curtin University, specifically, Dr Kevin Keane for the training and Professor Philip Newsholme for providing the BRIN BD-11 cells (clonal rat pancreatic β-cells and originally sourced from the European Collection of Cell Cultures (ECACC) and CellBank Australia.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Al-Salami H, Butt G, Tucker I, Fawcett PJ, Golocorbin-Kon S, Mikov I, Mikov M. 2009. Gliclazide reduces MKC intestinal transport in healthy but not diabetic rats. Eur J Drug Metab Pharmacokinet. 34:43–50.
- Al-Salami H, Butt G, Tucker I, Golocorbin-Kon S, Mikov M. 2012. Probiotics decreased the bioavailability of the bile acid analog, monoketocholic acid, when coadministered with gliclazide, in healthy but not diabetic rats. Eur J Drug Metab Pharmacokinet. 37:99–108.
- Al-Salami H, Butt G, Tucker I, Mikov M. 2008. Influence of the semisynthetic bile acid MKC on the ileal permeation of gliclazide in vitro in healthy and diabetic rats treated with probiotics. Methods Find Exp Clin Pharmacol. 30:107–113.
- Awasthi R, Kulkarni GT. 2013. Development of novel gastroretentive drug delivery system of gliclazide: hollow beads. Drug Dev Ind Pharm. 40:1–11.
- Beck J, Angus R, Madsen B, Britt D, Vernon B, Nguyen KT. 2007. Islet encapsulation: strategies to enhance islet cell functions. Tissue Eng. 13:589–599.
- Bhatia SR, Khattak SF, Roberts SC. 2005. Polyelectrolytes for cell encapsulation. Curr Opin Colloid Interface Sci. 10:45–51.
- Calasan J, Al-Salami H, Mikov M. 2012. Bile acids and probiotics could help treating diabetes. FEBS J. 279:267–267.
- Claiborn KC, Stoffers DA. 2008. Toward a cell-based cure for diabetes: advances in production and transplant of beta cells. Mt Sinai J Med. 75:362–371.
- Codario R. 2011. Pathophysiology of Type-2 diabetes In: Type 2 Diabetes, Pre-Diabetes, and the Metabolic Syndrome. Totowa, NJ: Humana Press, pp. 1–14.
- de Vos P, de Haan BJ, Kamps JA, Faas MM, Kitano T. 2007. Zeta- potentials of alginate-PLL capsules: a predictive measure for biocompatibility?J Biomed Mater Res A. 80:813–819.
- Dusseault J, Leblond FA, Robitaille R, Jourdan G, Tessier J, Ménard M, et al. 2005. Microencapsulation of living cells in semi-permeable membranes with covalently cross-linked layers. Biomaterials. 26:1515–1522.
- Frezza EE, Gerunda GE, Plebani M, Galligioni A, Giacomini A, Neri D, et al. 1993. Effect of ursodeoxycholic acid administration on bile duct proliferation and cholestasis in bile duct ligated rat. Dig Dis Sci. 38:1291–1296.
- Harlan DM, Rother KI, Robertson R. 2004. Islet transplantation as a treatment for diabetes. N Engl J Med. 350:2104.
- Jiin WY. 2000. Development of new polycations for cell encapsulation with alginate. Mater Sci Eng. 1:59–63.
- Lalic-Popovic M, Vasović V, Milijašević B, Goločorbin-Kon S, Al-Salami H, Mikov M. 2013. Deoxycholic Acid as a Modifier of the Permeation of Gliclazide through the Blood Brain Barrier of a Rat. J Diabetes Res. 2013:598603.
- Malisova L, Kováčová Z, Koc M, Kračmerová J, Stich V, Rossmeislová L. 2013. Ursodeoxycholic acid but not tauroursodeoxycholic acid inhibits proliferation and differentiation of human subcutaneous adipocytes. PLoS One. 8:e82086.
- Martinez JD, Stratagoules ED, LaRue JM, Powell AA, Gause PR, Craven MT, et al. 1998. Different bile acids exhibit distinct biological effects: the tumor promoter deoxycholic acid induces apoptosis and the chemopreventive agent ursodeoxycholic acid inhibits cell proliferation. Nutr Cancer. 31:111–118.
- Martinez-Moya P, Romero-Calvo I, Requena P, Hernández-Chirlaque C, Aranda CJ, González R, et al. 2013. Dose-dependent antiinflammatory effect of ursodeoxycholic acid in experimental colitis. Int Immunopharmacol. 15:372–380.
- Mikov M, Al-Salami H, Golocorbin-Kon S, Skrbic R, Raskovic A, Fawcett JP. 2008. The influence of 3alpha,7alpha-dihydroxy-12-keto-5beta-cholanate on gliclazide pharmacokinetics and glucose levels in a rat model of diabetes. Eur J Drug Metab Pharmacokinet. 33:137–142.
- Mikov M, Boni NS, Al-Salami H, Kuhajda K, Kevresan S, Golocorbin-Kon S, Fawcett JP. 2006. Pharmacokinetics and hypoglycaemic effect of 3 alpha, 7 alpha-dihydroxy-12-oxo-5beta-cholanate (MKC) in diabetic rat. FEBS J. 273:210–210.
- Mooranian A, Negrulj R, Mathavan S, Martinez J, Sciarretta J, Chen-Tan N, et al. 2014a. A complex microencapsulated system: a platform for optimised oral delivery of antidiabetic drug-bile acid formulations. Pharm Dev Technol. (Epub ahead of print).
- Mooranian A, Negrulj R, Mathavan S, Martinez J, Sciarretta J, Chen-Tan N, et al. 2014b. Stability and release kinetics of an advanced gliclazide-cholic acid formulation: the use of artificial-cell microencapsulation in slow release targeted oral delivery of antidiabetics. J Pharm Innov9:150–157.
- Nafea EH, Marson A, Poole-Warren LA, Martens PJ. 2011. Immunoisolating semi-permeable membranes for cell encapsulation: focus on hydrogels. J Control Release. 154:110–122.
- Negrulj R, Mooranian A, Al-Salami H. 2013. Potentials and limitations of bile acids in Type 2 diabetes mellitus: Applications of microencapsulation as a novel oral delivery system. J Endocrinol. Diabetes Mellitus. 1:49–59.
- Nolan CJ, Damm P, Prentki M. 2011. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 378:169–181.
- Pal D, Nayak AK. 2011. Development, optimization, and anti-diabetic activity of gliclazide-loaded alginate-methyl cellulose mucoadhesive microcapsules. AAPS PharmSciTech. 12:1431–1441.
- Pal D, Nayak AK. 2012. Novel tamarind seed polysaccharide-alginate mucoadhesive microspheres for oral gliclazide delivery: in vitro-in vivo evaluation. Drug Deliv. 19:123–131.
- Puri S, Hebrok M. 2012. Diabetic beta cells: to be or not to be?Cell. 150:1103–1104.
- Rokstad AM, Lacík I, de Vos P, Strand BL. 2013. Advances in biocompatibility and physico-chemical characterization of microspheres for cell encapsulation. Adv Drug Deliv Rev. 67–68:111–130.
- Rokstad AM, Lacík I, de Vos P, Strand BL. 2014. Advances in biocompatibility and physico-chemical characterization of microspheres for cell encapsulation. Adv Drug Deliv Rev. 67–68C: 111–130.
- Rudi J, Schlenker T, Raedsch R, Waldherr R, Zorn M, Stremmel W. 1995. Effect of ursodeoxycholic acid on biochemical parameters, hepatocyte proliferation and liver histology in galactosamine hepatitis in the rat. Res Exp Med (Berl). 195:309–315.
- Schneider S, Feilen PJ, Slotty V, Kampfner D, Preuss S, Berger S, et al. 2001. Multilayer capsules: a promising microencapsulation system for transplantation of pancreatic islets. Biomaterials. 22:1961–1970.
- Stumvoll M, Goldstein BJ, van Haeften TW. 2005. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 365:1333–1346.
- Takka S, Cali AG. 2012. Bile salt-reinforced alginate-chitosan beads. Pharm Dev Technol. 17:23–29.
- Taylor R. 2013. Type 2 diabetes: etiology and reversibility. Diabetes Care. 36:1047–1055.
- Thanos CG, Bintz BE, Emerich DF. 2007a. Stability of alginate- polyornithine microcapsules is profoundly dependent on the site of transplantation. J Biomed Mater Res A. 81:1–11.
- Thanos CG, Calafiore R, Basta G, Bintz BE, Bell WJ, Hudak J, et al. 2007b. Formulating the alginate-polyornithine biocapsule for prolonged stability: evaluation of composition and manufacturing technique. J Biomed Mater Res A. 83:216–224.
- Uludag H, Sefton MV. 1990. Colorimetric assay for cellular activity in microcapsules. Biomaterials. 11:708–712.
- Urbanska AM, Bhathena J, Prakash S. 2007. Live encapsulated Lactobacillus acidophilus cells in yogurt for therapeutic oral delivery: preparation and in vitro analysis of alginate-chitosan microcapsules. Can J Physiol Pharmacol. 85:884–893.
- Weir GC. 2013. Islet encapsulation: advances and obstacles. Diabetologia. 56:1458–1461.
- Wong H, Chang TM. 1991. The microencapsulation of cells within alginate poly-L-lysine microcapsules prepared with the standard single step drop technique: histologically identified membrane imperfections and the associated graft rejection. Biomater Artif Cells Immobilization Biotechnol. 19:675–686.