Abstract
The objectives were to study the potential of Pulicaria gnaphalodes (Vent.) Boiss. aerial parts in production of nanoparticles and the effect of the extraction solvent on the produced nanoparticles. Methanol and dichloromethane extracts were prepared by percolation of the plant powder. Both the extracts of P. gnaphalodes (Vent.) Boiss. successfully produced small and polydispersed nanoparticles with low aggregates in early hours of the biotransformation. Methanol extract produced spherical and many single nanoparticles, whereas dichloromethane produced porous polyhedral and more aggregated nanoparticles. Methanol extract of this plant seems to be quiet useful for industrial scale production of nanoparticles.
Introduction
Various important applications of silver nanoparticles (NPs) have led to an increase in the need for the production of these NPs. Different methods were developed to obtain silver NPs of different morphologies and sizes, including laser ablation, gamma irradiation, electron irradiation, chemical reduction, photochemical methods, microwave processing, and thermal decomposition of silver oxalate in water and in ethylene glycol. But in recent years, phytosynthesis of silver NPs by plants was reported by researchers (CitationChahardooli et al. 2014, CitationGade et al. 2014, CitationMariselvam et al. 2014, CitationSun et al. 2014, CitationSuresh et al. 2014, CitationUmoren et al. 2014, CitationVelmurugan et al. 2014). These green methods can be considered as possible eco-friendly alternatives to chemical and physical approaches. Thus, using screened plants with high production capability and controlling the reaction conditions, well-characterized NPs can be obtained by making the synthesis rates faster or comparable with those of chemical and physical approaches. Phytosynthesis of silver NPs can potentially be used in various areas, including pharmaceuticals, cosmetics, foods, and medical applications (CitationKorbekandi and Iravani 2012, CitationIravani 2011, CitationIravani et al. 2014a, Citation2014b, CitationIravani and Zolfaghari 2013, CitationKorbekandi and Iravani 2013, CitationKorbekandi et al. 2009, Citation2012, Citation2013).
Pulicaria is a plant genus in the family of Asteraceae. It comprises about 100 species distributed from Europe to North Africa and Asia, particularly around the Mediterranean region (CitationKhan et al. 2013). They are commonly used as herbal tea, flavoring agent, and medicinal plant. P. gnaphalodes (Vent.) Boiss. is a persistent plant of about 10–30 cm high, with gold-yellow flowers, which grows on sandy, stony places in the Asia (CitationShariatifar 2012). Kakkosh-Biabani (Pulicaria gnaphalodes Linn) is a plant which grows wild in abandoned areas such as Iran, Iraq, Pakistan, India, Turkey, and Saudi Arabia (CitationMozaffarian 1996). This plant is a worldwide known folk medicine for severe dehydrations, inflammation, etc. (CitationShariatifar 2012). The extracts of P. gnaphalodes (Vent.) Boiss. contains high amounts of phenolic compounds and antioxidant activity, and thus can be considered as a potential source for green synthesis of silver NPs. Herein, the objective was to study the potential of Pulicaria gnaphalodes (Vent.) Boiss. aerial parts for the production of silver NPs and the effect of the extraction solvent on the produced NPs.
Materials and methods
Preparation of the plant extract
Pulicaria gnaphalodes (Vent.) Boiss. aerial parts were freshly collected from Isfahan Agricultural and Natural Resources Research Center (Herbarium number: 1362). After washing, air-drying at room temperature, and powdering them, the powder was screened using a 17-mesh sieve. Methanol and dichloromethane extracts was prepared by percolation. The plant powder (200 g) was extracted with methanol and dichloromethane (500 mL) using a 2-L percolator for 48 h. The extract was concentrated in rotary evaporator to 50 mL and powdered through freeze-drying (Christ ®, Martin Christ; Germany).
Preparation of the reaction mixtures
The reaction mixtures contained (final concentrations) AgNO3 (1 mM) as the substrate, concentrated, and freeze-dried plant extract (equivalent to 100 g plant powder) as the biocatalyst, and phosphate buffer (pH = 7, 100 mM) as the medium in the reaction mixture (50 mL). The aforementioned ingredients were added in appropriate amounts into Duran® bottles (100 ml) and were incubated (70 rpm) at room temperature.
Characterization and analysis
Transmission electron microscopic analysis
Transmission electron microscopy (TEM) was performed on selected samples in order to investigate the process of formation of silver NPs and study the sizes and shapes of them. Samples for TEM were prepared by drop-coating the silver nanoparticle suspensions onto carbon-coated copper grids. Micrographs were obtained using EM 900 ZEISS transmission electron microscope.
Dynamic light scattering (DLS) analysis
Particle size distribution of NPs was analyzed using nano Zeta-Sizer (Malvern Nano®, Nano ZS, ZEN 3600, UK).
Atomic absorption analysis
In order to quantify the substrate (Ag+) and calculate the conversion of it to Ag0 NPs, Ag+ ions were determined using atomic absorption (AA) analysis by Ag lamp at 328 nm. Atomic absorption spectrophotometer (Perkin-Elmer Zeeman 3030, Germany) with pyro-coated tube and platform, pretreatment temperature of 650°C, and atomization temperature of 1600°C was used. The samples (1 mL) were centrifuged (Sigma D-37520, Germany, at 2240 g, for 45 min) before the analysis.
Results and discussion
Bioreduction of Ag0 to Ag+ by methanol extract
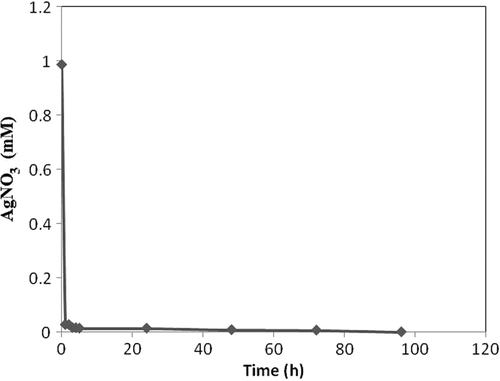
Methanol extract of Pulicaria gnaphalodes (Vent.) Boiss. aerial parts transformed 100% of AgNO3 to Ag NPs after 96 h (). The reaction was almost complete (conversion%, 96) after 5 h, and conversion rate was maximum (0.959 mmole h− 1) between hour 0 and 1.
Bioreduction of Ag0 to Ag+ by dichloromethane extract
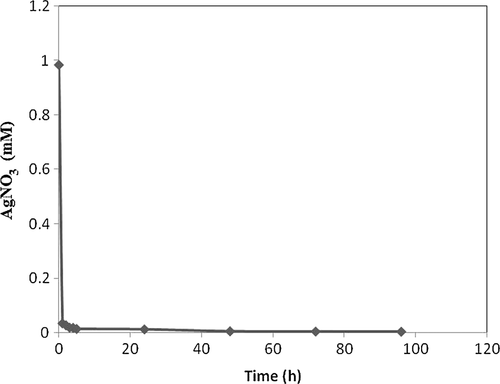
Dichloromethane extract of P. gnaphalodes (Vent.) Boiss. aerial parts transformed 99.7% of AgNO3 to Ag NPs after 96 h (). The reaction was also complete (conversion%, 98.6%) after 5 h, and conversion rate was maximum (0.951 mmoleh− 1) between hour 0 and 1.
DLS analysis
Methanol extract
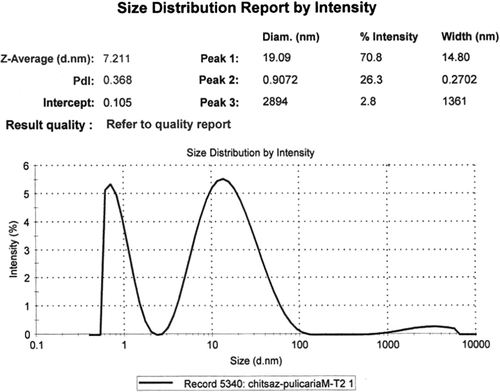
Three peaks were seen in DLS analysis of the reaction mixture after 2 h; therefore, the NPs were polydispersed (). Single NPs with diameter of 19.1 nm were the most frequent ones (70.8%), and the mean size was 7.2 nm (Z-Average). The NPs with smallest diameter were 0.91 nm (26.3%). The polydispersity index (PDI) was 0.368 (near to optimal value).The average diameter was increased by increasing the reaction time (72 h), and the NPs were aggregated further ().
Dichloromethane extract
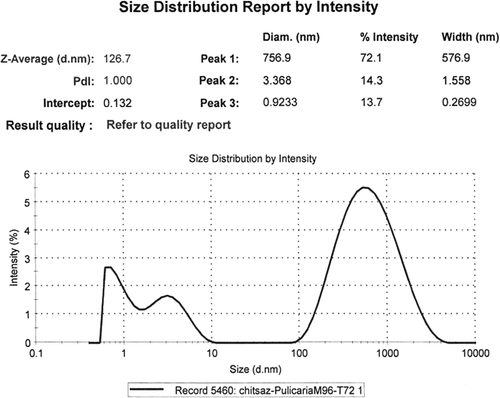
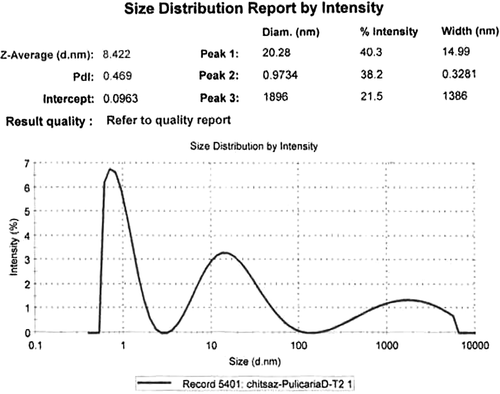
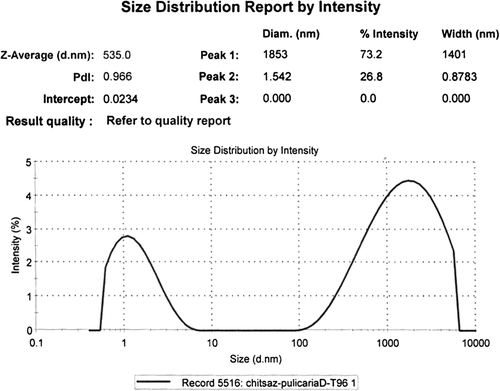
Three peaks were seen on DLS analysis of the reaction mixture after 2-h biotransformation; therefore, the NPs were polydispersed (). Single NPs with diameter of 20.3 nm were the most frequent ones (40.3%), and the mean size was 8.4 nm (Z-Average). The second most frequent (38.2%) particles were with diameter of 0.97 nm (smallest NPs). By increasing the reaction time (96 h, ), the average size of the NPs was increased to 535 nm and the percentage of the aggregated NPs (73.2%) was increased further.
TEM analysis
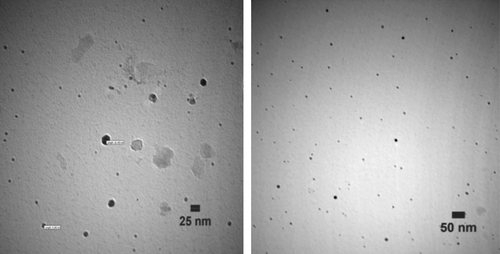
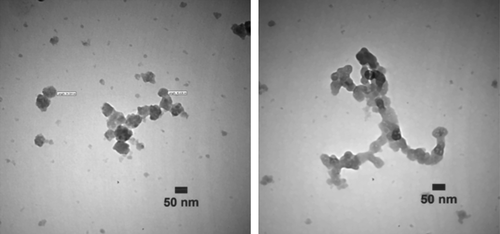
TEM images of the NPs produced with 100% methanol extract of P. gnaphalodes (Vent.) Boiss. after 24 h of biotransformation were shown in . The silver NPs produced were spherical. Furthermore, TEM images of the produced NPs by dichloromethane extract of P. gnaphalodes (Vent.) Boiss. after 24 h of biotransformation were shown in . The produced NPs were porous polyhedral, and in some places aggregated in assemblies.
Effect of the extraction solvent
Biotransformation time courses, bioconversion percentages, and bioreduction rates of Ag+ to Ag0 NPs by both the extracts ( and ) were almost the same. By comparing the NPs shapes and size distributions, produced by methanol (, , and ) and dichloromethane (, , and ) extracts of Pulicaria gnaphalodes (Vent.) Boiss., it can be seen that methanol extract produced spherical, smaller, and less aggregated spherical silver NPs.
Previously, CitationKhan et al. (2013) used the aqueous extract of aerial parts of P. glutinosa, which was collected from local fields in Saudi Arabia. They soaked small pieces of the plant in deionized water and refluxed them for 3 h. The solution was filtered and dried at 50°C under reduced pressure in a rotary evaporator to give a powder of dark brownish color. They stirred the reaction mixture at 90°C for 2 h and produced spherical nanosilver particles with the size range of 40–60 nm. Comparing our work with the study of CitationKhan et al. (2013), we used the species, P. gnaphalodes, grown in Iran, while they used P. glutinosa from Saudi Arabia but both of us used the aerial parts from the same genus. In our case, the bioreduction reaction was almost finished in 5 h, at room temperature, but Khan et al., using aqueous extract, spent 20 h at this condition to complete the reaction; then, they used 90°C for the reaction mixture. As we mentioned earlier, methanol extract of P. gnaphalodes produced dense spherical NPs, but dichloromethane extract synthesized porous polyhedral NPs. Using aqueous extract of P. glutinosa, Khan et al. produced spherical NPs, as well, which confirms the effect of extraction solvent on the produced NPs. After 5-h bioreduction, our methanol extract produced nanosilver particles with the mean size of 7.2 nm, and dichloromethane extract synthesized NPs with the mean size of 8.4 nm. However, using the aqueous extract and 2 h reaction, Khan et al. produced nanosilver particles with the size range of 40–60 nm. It was reported that less aggregated, small, and spherical silver NPs are more effective for most applications (CitationKhan et al. 2013).
Mechanistic aspects
The extract of P. gnaphalodes contains high amounts of phenolic compounds and antioxidant activity, and it can be used as a potential source of antioxidant compounds for food and pharmaceutical industry (CitationKamkar et al. 2013, CitationShariatifar 2012). Five compounds including one phenol acid (salicylic acid), two clerodane diterpenoids (salvifolin and salvicin) and two flavonoids (pulicarin and giperoside) were isolated from alcoholic extract of the aerial parts of P. gnaphalodes (Vent.) Boiss. (CitationEshbakova 2011). Members of the genus Pulicaria were investigated extensively all over the world, and the chemical literature survey shows the presence of flavonoids, sesquiterpenoids, diterpenoids, and sesquiterpenoid lactones (CitationShaiq Ali et al. 1999). Phytochemicals such as phenolic and flavonoid compounds are the largest group of antioxidant compounds, and they scavenge peroxide, lipid peroxyl, and hydroperoxide, and chelate metals (CitationSakai et al. 1996). Antioxidant action of phenolic compounds is due to their high tendency to chelate metals. Phenolic compounds possess hydroxyl and carboxyl groups, which may inactivate iron ions by chelating and additionally suppressing the superoxide-driven Fenton reaction, which is believed to be the most important source of reactive oxygen species (CitationIravani and Zolfaghari 2011, Citation2013, Citation2014).
Bioreduction of silver ions to yield NPs using plant extracts such as Geranium leaf broth (CitationShankar et al. 2003), Neem leaf broth (CitationShankar et al. 2004), and Aloe vera plant extracts (CitationChandran et al. 2006) has been reported. It seems that the basis of all metal NPs synthesis methods is the bioreduction of metal ions by reducing agents. Accordingly, it might be interpreted that the aforementioned phytochemicals and phenolic compounds have the potential role in phytoreduction of silver ions and formation of silver NPs.
Conclusion
Both the extracts of P. gnaphalodes (Vent.) Boiss. successfully produced small and polydispersed NPs with low aggregates in early hours of the biotransformation. The optimum conditions for the reaction mixture were as follows (final concentrations): AgNO3 (1 mM) as the substrate, concentrated and freeze-dried plant extract (equivalent to 100 g of plant powder) as the biocatalyst, and phosphate buffer (pH = 7, 100 mM) as the medium in the reaction mixture (50 mL). The aforementioned ingredients were added in appropriate amounts into Duran® bottles (100 ml) and were incubated (70 rpm) at room temperature. The conversion was fast and completed in 5 h. Results from TEM and DLS analysis demonstrated that by increasing the reaction time, the NPs were aggregated further. Methanol extract produced spherical and more single NPs, whereas dichloromethane produced porous polyhedral and more aggregated NPs. Therefore, it might be concluded that the solvent of extraction may influence the shapes and sizes of the produced silver NPs. Methanol extract of this plant seems to be quiet attractive for industrial scale production of silver NPs.
Acknowledgments
The authors appreciate the support of Isfahan University of Medical Sciences.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
This study was a part of the research project No. 392034 and was financially supported by Faculty of Pharmacy, Isfahan University of Medical Sciences.
References
- Chahardooli M, Khodadadi E, Khodadadi E. 2014. Green synthesis of silver nanoparticles using oak leaf and fruit extracts (Quercus) and its antibacterial activity against plant pathogenic bacteria. Int J Biosci. 4:97–103.
- Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M. 2006. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog. 22:577–583.
- Eshbakova KA. 2011. Chemical constituents of Pulicaria gnaphalodes Boiss. Med Plants 3:161–163.
- Gade A, Gaikwad S, Duran N, Rai M. 2014. Green synthesis of silver nanoparticles by Phoma glomerata. Micron. 59:52–59.
- Korbekandi H, Iravani S. 2012. Silver nanoparticles In: Hashim AA, Ed. The Delivery of Nanoparticles. Rijeka: InTech: 1–36. Available from: http://www.intechopen.com/books/thedelivery-of-nanoparticles/silver-nanoparticles.
- Iravani S. 2011. Green synthesis of metal nanoparticles using plants. Green Chem. 13:2638–2650.
- Iravani S, Korbekandi H, Mirmohammadi SV, Mekanik H. 2014a. Plants in nanoparticle synthesis. Rev Adv Sci Eng. 3:261–274.
- Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B. 2014b. Synthesis of silver nanoparticles: chemical, physical, and biological methods. Res Pharm Sci. 9:385–406.
- Iravani S, Zolfaghari B. 2011. Pharmaceutical and nutraceutical effects of Pinus pinaster bark extract. Res Pharm Sci. 6:1–11.
- Iravani S, Zolfaghari B. 2013. Green synthesis of silver nanoparticles using Pinus eldarica bark extract. Biomed Res Int. 2013:639725.
- Iravani S, Zolfaghari B. 2014. Phytochemical analysis of Pinus eldarica bark. Res Pharm Sci. 9:243–250.
- Kamkar A, Ardekani MRS, Shariatifar N, Misagi A, Mozaffari Nejad AS, Jamshidi AH. 2013. Antioxidative effect of Iranian Pulicaria gnaphalodes L. extracts in soybean oil. S Afr J Bot. 85:39–43.
- Khan M, Khan M, Adil SF, Tahir MN, Tremel W, Alkhathlan HZ, et al. 2013. Green synthesis of silver nanoparticles mediated by Pulicaria glutinosa extract. Int J Nanomedicine. 8:1507–1516.
- Korbekandi H, Ashari Z, Iravani S, Abbasi S. 2013. Optimization of biological synthesis of silver nanoparticles using Fusarium oxysporum. Iran J Pharm Res. 12:289–298.
- Korbekandi H, Iravani S. 2013. Biological synthesis of nanoparticles using algae. In: Rai M, Posten C, Eds. Green Biosynthesis of Nanoparticles: Mechanisms and Applications. Wallingford, UK: CABI, pp. 53–60.
- Korbekandi H, Iravani S, Abbasi S. 2009. Production of nanoparticles using organisms. Crit Rev Biotechnol. 29:279–306.
- Korbekandi H, Iravani S, Abbasi S. 2012. Optimization of biological synthesis of silver nanoparticles using Lactobacillus casei subsp. casei. J Chem Technol Biotechnol. 87:932–937.
- Mariselvam R, Ranjitsingh AJA, Usha Raja Nanthini A, Kalirajan K, Padmalatha C, Selvakumar PM. 2014. Green synthesis of silver nanoparticles from the extract of the inflorescence of Cocos nucifera (Family: Arecaceae) for enhanced antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc. 129:537–541.
- Mozaffarian V. 1996. A Dictionary of Iranian Plant Names Farhang Moaser: Tehran, Iran.
- Sakai N, Inada K, Okamoto M, Shizuri Y, Fukuyama Y. 1996. Portuloside A, a monoterpene glucoside, from Portulaca oleracea. Phytochemistry. 42:1625–1628.
- Shaiq Ali M, Jahangir M, Saleem M, Ahmad VU. 1999. Chemical constituents of Pulicaria gnaphalodes. Nat Prod Sci. 5:134–137.
- Shankar SS, Absar A, Murali S. 2003. Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol Prog. 19: 1627–1631.
- Shankar SS, Rai A, Ahmad A, Sastry M. 2004. Rapid synthesis of Au, Ag and bimetallic Au shell nanoparticles using Neem. J Colloid Interf Sci. 275:496–502.
- Shariatifar N. 2012. Quantitative and qualitative study of phenolic compounds and antioxidant activity of plant Pulicaria gnaphalodes. Horizon Med Sci. 17:35–41.
- Sun Q, Caia X, Li J, Zheng M, Chen Z, Yu CP. 2014. Green synthesis of silver nanoparticles using tea leaf extract and evaluation of their stability and antibacterial activity. Colloids Surf A Physicochem Eng Aspects. 444:226–231.
- Suresh G, Gunasekar PH, Kokila D, Prabhu D, Dinesh D, Ravichandran N, et al. 2014. Green synthesis of silver nanoparticles using Delphinium denudatum root extract exhibits antibacterial and mosquito larvicidal activities. Spectrochim Acta A Mol Biomol Spectrosc. 127:61–66.
- Umoren SA, Obot IB, Gasem ZM. 2014. Green synthesis and characterization of silver nanoparticles using red apple (Malus domestica) fruit extract at room temperature. J Mater Environ Sci. 5:907–914.
- Velmurugan P, Anbalagan K, Manosathyadevan M, Lee KJ, Cho M, Lee SM, et al. 2014. Green synthesis of silver and gold nanoparticles using Zingiber officinale root extract and antibacterial activity of silver nanoparticles against food pathogens. Bioprocess Biosyste Eng. doi:https://doi.org/10.1007/s00449-014-1169-6.