Abstract
The oral route for drug delivery is a widely accepted route. For that reason, many researchers are currently working to develop efficient oral drug delivery systems. Use of polymeric nanoparticles (NPs) and lipid carrier systems, including liposomes, solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLC), has limitations such as drug leakage and high water content of dispersions. Thus, lipid polymer hybrid nanoparticles (LPNs) have been explored by the researchers to provide a better effect using properties of both polymers and lipids. The present review is focused on the challenges, possibilities, and future perspectives of LPNs for oral delivery.
Introduction
It is the maximum tendency of all researchers to shield the population from various diseases by developing new strategies or by modifying the existing one to enhance patient compliance. Great expansion has been made in treating the number of diseases by using different drug delivery systems which maintain the concentration, rate, time, and release of therapeutic agents in the body (CitationJain 2008). The main goal of carrier systems is to distribute the drug to a targeted site within a suitable time duration with higher concentration in the diseased site and as low as possible in the normal tissues (CitationUekama et al. 1998). Oral route offers needle-free delivery which is considered to be the more preferable route because of the advantages like easy administration, patient convenience, economical manufacturing processes, non requirement of sterile processing and easy approval from regulatory bodies. Although, being the well accepted route of administration, it is not always possible to deliver every active moiety through the oral route (Citationdes Rieux et al. 2006, CitationPrego et al. 2006).
Challenges for oral drug delivery
Drug has to cross highly fluctuating gastrointestinal (GI) system.
pH of stomach has impact on stability of drug.
Some factors are also responsible like motility, mucus barriers, high metabolic activity and relative impermeability of the epithelium (CitationTrevaskis et al. 2008). For example, oral delivery of the low molecular weight heparins (LMWH) is difficult due to variable gastrointestinal tract (GIT) conditions and physicochemical properties of the drug itself (CitationBaughman et al. 1998, CitationChandy et al. 2002). For example, high molecular weight, high negative charge density, and instability of LMWH in the GIT pose difficulties in its absorption (CitationArbit et al. 2006). These problems can be solved by using a number of strategies like penetration enhancers, development of polymeric carriers, and conjugation of hydrophilic LMWH with hydrophobic lipids (CitationChen et al. 2009). The passage of molecules through intestinal epithelium takes place by passive diffusion (CitationWard et al. 2000). The passive diffusion of hydrophilic molecules generally occurs through paracellular pathway but limited due to presence of junctional complexes as well as insufficient lipophilicity (CitationLutz and Siahaan 1997).
Absorption enhancers are used to promote the assimilation of poorly absorbable drugs like hydrophilic antibiotics and biotechnology derived drugs. These absorption enhancers include surfactants, bile salts, chelating agents, and fatty acids (CitationUchiyama et al. 1999). Sometimes, the damage and irritation have been observed in the intestinal mucosal membrane due to use of penetration enhancers (CitationUchiyama et al. 1996). Some permeation enhancers are listed below in (CitationAungst and Rogers 1988).
Table I. List of permeation enhancers.
Oral drug delivery systems
Conventional drug delivery systems
Plasma drug concentration increases first on administration, and then usually decreases the concentration to an ineffective plasma drug concentration level. The concentration should be in between the toxic level and minimum effective level. Same rise and fall happens again during the next dose. In these types of situations, a higher dose may be the solution of this problem, but this will raise up the toxic effect of the drug as well as treatment cost. In various disorders, a particular amount of the drug should reach the site of action and must remain constant for a longer period of time. However, this is usually not seen in conventional drug delivery systems. In addition, this constant level can be maintained only if the drug release follows zero-order kinetics resulting in decrease in toxicity and ineffectiveness (CitationPankhurst et al. 2003).
Nanotechnology
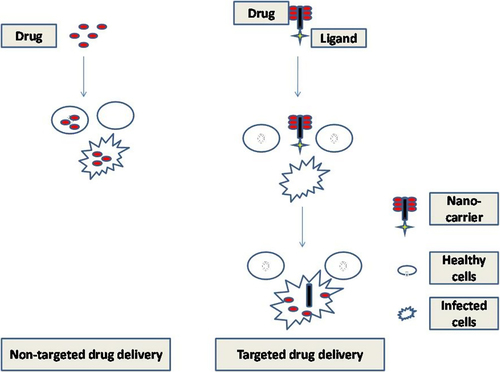
The utility of nanoscience in drug delivery has become very popular these days. The particles which have a size within the nanoscale give better optical, electronic, and structural properties due to their smaller size, surface structure, and high surface area, although, these characteristics are usually not seen in large sized systems (CitationSemete et al. 2010). In order to accomplish efficient drug delivery, the interactions of nanomaterials with target cell-surface receptors, drug release, stability of therapeutic agents, and molecular mechanisms of cell signaling involved in pathology of the disorder must be known initially (CitationSuri et al. 2007). In the novel drug delivery approaches, nanoparticles (NPs) are most explored because of their improved bioavailability (BA), solubility, and the retention time (CitationKumari et al. 2010). Incorporation of therapeutic agents in NPs promotes the efficiency of the drugs due to improved localization. It is reported that NPs could enter the body through various routes and these particles get accumulated more in the defected body's tissues and lesser in healthy (as shown in ) (CitationOberdörster et al. 2005). In oral delivery, uptake of NPs is done via Payer's patches in the gut-associated lymphoid tissue. Payer's patches consist of microfold (M) cells that overspread the lymphoid tissue and are meant for endocytosis and delivery of the drug into intraepithelial spaces and adjacent lymphoid tissue (CitationBrayden and Baird 2004). A theory states that NPs attached to the apical membrane of the M cells undergo rapid internalization and a “shuttling” to the lymphocytes (CitationSanvicens and Marco 2008). NPs are made of various types of raw materials usually detectable on the micro scale, such as gold, lipids, carbon, silica, silicon, and iron (CitationFlorence and Hussain 2001). All the parameters should be identified to design perfect colloidal drug delivery system through oral route for the stability of a biodegradable material in GI fluids (CitationMüller et al. 1996).
Use of polymers in the formation of nanoparticles
The NPs are composed of degradable/nondegradable and hydrophilic/hydrophobic polymers. The natural polymers used in NPs are chitosan, gelatin, sodium alginate, albumin, etc. Chitosan is a biodegradable cationic polymer with mucoadhesive property. Chitosan can make complexes easily in an aqueous medium by encapsulating the therapeutic moieties (CitationBagre et al. 2013). Due to variable purity of natural polymers (proteins or polysaccharides), they cannot be generally used alone. Thus, cross linking is required which may further denature the incorporated therapeutic agent (CitationHans and Lowman 2006). Therefore, synthetic polymers are more useful. For example, polymeric NPs (PNPs) are made up of synthetic polymers like poly (lactide) and poly (glycolide) and their copolymers (CitationKatanec et al. 2004, CitationStolnik et al. 1994) and poly (ϵ-caprolactone) (PCL) are used in the field of nanobiotechnology (CitationKumari et al. 2010). The properties of poly(lactide-co-glycolide) (PLGA) are maintained by the stereochemistry of lactic acid (D, L, or DL), degree of crystallinity, lactic acid/glycolic acid ratio, and the molecular weight (CitationHouchin and Topp 2008).
PEGylation technology also plays a role in:
Increasing the aqueous solubility and stability
Reducing intermolecular aggregation
Decreasing immunogenicity
Prolongation of the systemic circulation time of a compound
Conjugation of polyethylene glycol (PEG) with PLGA is more beneficial (CitationVonarbourg et al. 2006). But there are some limitations with the PNPs like systemic toxicity, cytotoxicity, residue from organic solvents, inadequate encapsulation of water soluble drugs, drug leakage, large scale production, and sterilization problems (CitationSanna et al. 2004). Moreover, the immune system has a variety of mechanisms to identify foreign particles in the body thus the PNPs can easily lead to internalization. This internalization could be prevented by using lipids.
Use of lipids in nanoparticles
A lipid coat over the NPs may increase its BA. It could provide protection to the particulate system from drug retention as well as water permeation. PEG can also be linked as a targeted ligand (CitationSouto and Doktorovova 2009).
Reasons for use of lipids are
Initiation of biliary and pancreatic secretions.
Increased GIT residence time.
Stimulation of lymphatic transport.
Variation in mesenteric and liver blood flow.
Enhanced intestinal wall permeability and reduced metabolism.
Efflux activity which enhances BA (CitationChakraborty et al. 2009).
Lipids used in lipid NPs are of lesser cost as compared to synthetic polymers in PNPs, for example, PLGA (CitationShegokar et al. 2011). Furthermore, lipid-based drug delivery systems are developed to mimic the food (or post-prandial) effect to address the oral BA challenges for less soluble drugs and vitamins. Therefore, the molecules can effectively solubilize in the lipophilic microenvironment developed by the presence of fat and their corresponding digested fatty acid products mixed with endogenous micellar components (CitationMu et al. 2013, CitationPorter et al. 2007). Lipid based formulation may be classified as below in (CitationGabizon 2001).
Table II. Types of lipid based formulations.
Lipid based nanocarriers show great potential in cancer therapy. For example, liposomal doxorubicin (Dox) used in breast and ovarian cancer was the first nanocarrier approved by the FDA (CitationWong et al. 2007). But liposomes show certain storage and drug leakage problems and demand a high price for large-scale production (CitationMontasser et al. 2013). Nowadays, solid lipid nanoparticles (SLNs) may be a good alternative. SLNs are more efficient colloidal drug carriers as compared to PNPs, with the advantage of being prepared with physiological and nontoxic lipids used as common pharmaceutical excipients (CitationMüller et al. 2000). The excipients used in lipoid carrier systems have been mentioned in (CitationKalepu et al. 2013).
Table III. Types of excipients used in lipid based formulations.
The solid matrix of the lipid has the ability to control the release of encapsulated moieties. In SLNs, a solid lipid or blend of solid lipids form an o/w emulsion (oil phase dispersed in water phase of emulsion) ranging in particle size between 80 and 1000 nm (CitationMontasser et al. 2013). On the other hand, SLNs have shortcomings like poor drug loading capacity, drug expels out after polymeric transition storage, high water-content of dispersions (70–99.9%) and inability for encapsulation of hydrophilic drugs (CitationJenning et al. 2000a). Solubility of some active ingredients can be increased by formulating NLCs comprising solid lipid phase with a small amount of oil/liquid lipid. The volume of oil to be mixed must be taken into consideration as at large volumes phase separation will take place due to the increase in solubility of oil in solid lipid phase (CitationJenning et al. 2000b, CitationPandey et al. 2005). Incorporation of liquid lipids with solid lipids forms NLCs which exhibit improved drug loading and release behavior (CitationMüller et al. 2002). However, the NLCs are more advantageous than SLNs (CitationMehnert and Mäder 2001) because of:
High drug payload,
Reduced drug leakage during storage of formulations.
Polymer-mediated delivery systems along with lipid NPs are the new advancements in nanotechnology. Polymeric nanometer sized particulate systems such as micelles, nanospheres, nanocapsules, polymerosomes, polyplexes, and hydrogels, etc. are used very much in these days (CitationLiechty and Peppas 2012).
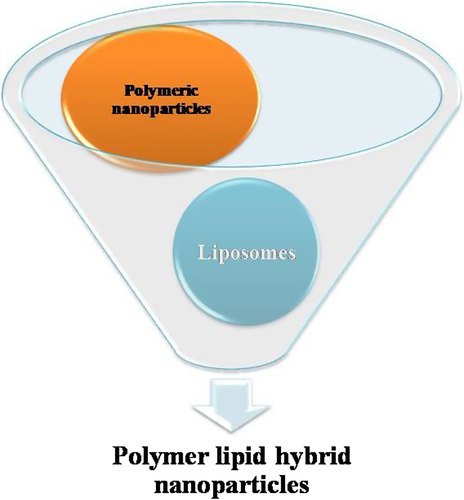
Polymer lipid hybrid nanoparticles
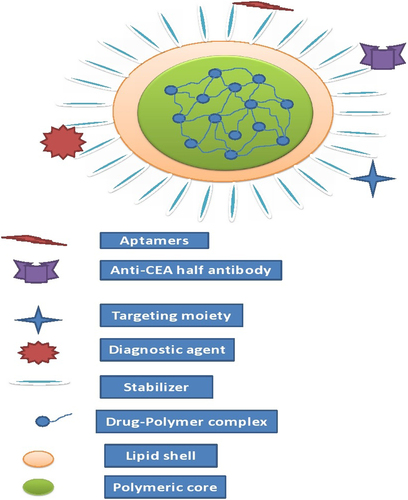
To compensate the shortcomings of both lipid NPs as well as PNPs, a new carrier system emerges out which is known as lipid polymer hybrid nanoparticles (LPNs) (). The hybrid term is used because it possesses the characteristics of both polymer and lipid particles. The polymer controls the drug release and the lipid increases the loading efficiency as well as permeation. LPNs have the potential to enhance physical stability and biocompatibility. Moreover, the lipid can also prevent the first pass metabolism. LPNs have more capacity for in vivo cellular delivery than PNPs and liposomes. The LPNs consist of a biocompatible hydrophobic polymeric core, a monolayer of phospholipids, and an outer layer made of PEG. Although the structure of LPNs is complex, the methods for preparing for LPNs are quite simple, which could play a key role to scale up its production in future (CitationZhang et al. 2008). PLGA NPs inhibit the P-glycoprotein (P-gp) efflux and also shows reversal of multiple drug resistance. The encapsulation of therapeutic moiety into a PLGA NP may be the way to enhance oral BA but it is limited to lipophilic drugs only because it is very hard to incorporate low molecular weight water soluble ionic drugs (CitationSahoo and Labhasetwar 2005). In order to improve the loading of ionic drugs to LPNs, a counter ion polymer can make a stable complex with the ionic drug further enclosed by lipoid membrane on the outer side. Furthermore, it is reported that LPNs are modified form of SLNs that can encapsulate an anionic polymer to load a hydrophilic cationic drug. For example, LPNs of Dox compared to Dox solution result in 8-fold increase in killing cells of P-gp overexpressing human breast cancer cells in clonogenic assay experiments (CitationWong et al. 2006b). In this review we are going to discuss the potential of LPNs for drug delivery.
Types of lipid polymer hybrid nanoparticles
Polymer core-lipid shell nanoparticles
This type of systems usually consists of a polymer as the inside core further surrounded by one or more lipid membranes as shown in . The space between the polymeric core and lipid layer is filled with water or aqueous buffer. In this, the polymer will delay the release of drug and enhance the stability of lipid shell. The hydrophobic drugs can easily incorporate in this system but it is problematic to encapsulate hydrophilic drugs. This can be solved by using a complex of polymers and lipids. Likewise Salidroside (Sal) is a potent antitumor drug with high water-solubility. Polymer core-lipid shell NPs were developed having PLGA-PEG-PLGA triblock copolymers and lipids (lecithin and cholesterol) and Sal were incorporated in the polymer core-lipid shell NPs which gave better entrapment efficiency, small particle size, and increased tumor cell uptake (CitationFang et al. 2014). As discussed earlier drugs have two limitations, one is hydrophilicity and the other is anionic charge. For this, alginate coated chitosan core shell NPs for oral delivery of enoxaparin were prepared using cationic polymer chitosan which act as charge stabilizer (CitationBagre et al. 2013). The ratio of drug to polymer is a very critical parameter because an improper ratio may lead to the formation of lumps which ultimately fails its formation. The reason may be charge interactions between polymer and drug that play important roles in core formation and overall size reduction of LPNs.
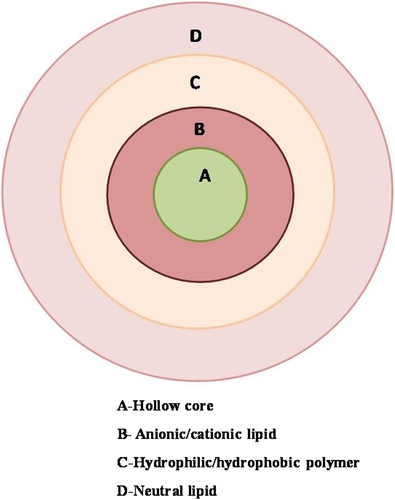
Hollow core/shell lipid–polymer–lipid hybrid nanoparticles
The hybrid NPs combine the unique characteristics of polymeric NPs and PEGylated lipoplexes. It is composed of lipid, polymer and have sizes around 225 ± 8 nm as described in .
A lipid layer of positive charge constituting the inner hollow core.
PLGA layer at the middle which is hydrophobic.
Interface formed in between PLGA and outer PEG layer by neutral lipid layer.
These types of systems are different from simple LPNs because they show properties of both PEGylated lipoplexes as well as PLGA NPs. The hollow core/shell lipid–polymer–lipid hybrid NPs can be prepared by the double emulsification solvent evaporation method (CitationZhang et al. 2008). A PEG-lipid layer is introduced into the system because it will allow the particle to avoid its recognition as a macrophage, improve stability during circulation, and slow polymer degradation and drug release (CitationDavis 2009, CitationZhang et al. 2008). The middle polymer layer will provide sustained release (CitationZhang et al. 2008). Moreover, the inner positively charged hollow core, composed of the cationic lipids, can encapsulate the anionic drug much more efficiently than polymer alone (CitationPautot et al. 2003). Sometimes, to stabilize the system, combination of lipid may be used to construct an outermost layer. One lipid may self assemble itself at water-oil interphase with their hydrophilic head group facing the aqueous droplet and the tail to polymeric phase and the rest forming a complex with PEG. During formation of this sort of complex, the concentrations of both lipids must be below their respective critical micelle concentration (CMC) otherwise risk of liposome and micelle formation could be increased. Nonetheless, the important parameters to be considered for its development are inner cationic lipids, outer PEG chain length, middle polymer composition/molecular weight (CitationSaad et al. 2008). The advantage of this system is that the combination of two active moieties can deliver efficiently. For example, the combination of si-RNA and synergistic small-molecule drugs within the hydrophobic PLGA layer may be helpful for the treatment of several diseases including multidrug-resistant cancers (CitationHu et al. 2011).
Lipid bilayer-coated polymeric particle
Bypass of macrophage uptake and systemic clearance is very important to enhance the residence time of NPs. Thus, LPNs possess two lipid layers which are combination of membrane vesicle and particle. To improve residence time, various approaches have been used of which the most preferred one is PEGylation but its immunological responses have been reported for a limited number of cases. Then RBCs are used to enclose the NPs because they have potential for long time circulation which may easily protect it from macrophage uptake. It has been reported that this combination could retain the functionality of membrane-associated protein (CitationTanaka and Sackmann 2005). RBC was extruded to form RBC-membrane derived vesicles and conjugate with PLGA NPs to form RBC-membrane-camouflaged PNPs (CitationHu et al. 2011). The lipid bilayers-coated polymeric NPs provide the sustained release in a better way than the liposomes and PNPs. Moreover, RBC may exhibit slower release because it possesses more dense lipid barrier against drug release. Besides the advantages it has the drawback that different blood groups have different types of antigens on the surface of the erythrocytes that have to be cross matched during blood transfusion which is really a challenging aspect (CitationLallana et al. 2012).
Mixed lipid polymer nanoparticles
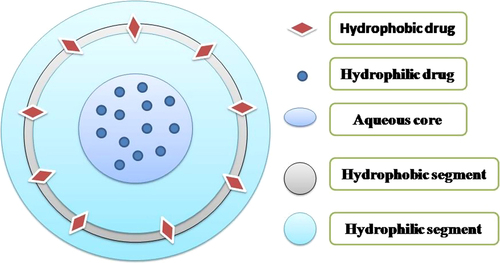
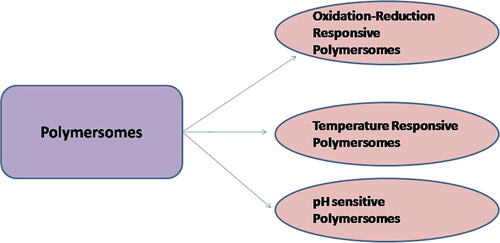
As the name indicates these are the mixtures of amphiphilic copolymer and lipids. The liposomes have a composition identical to the cell because they contain phospholipids that have the ability to form vesicles (CitationLin et al. 2004). However, phospholipids have limited flexibility for chemical modification by PEGylation as PEG-lipids at higher PEG densities could form micelles due to large sized PEG head groups. In order to solve this issue, the need to add a polymers was raised. Furthermore, the polymer solved the problems regarding stability, rapid uptake by reticuloendothelial systems (RES), retention of encapsulated agent, and degradation. The factors which may be responsible for the stability of the liposomal system are polymer's molecular weight, polydispersity, and relative hydrophilic to hydrophobic block ratio (CitationMeng et al. 2003, CitationNielsen et al. 2004). Polymersomes are hollow, lamellar, and spherical structures. This system is more advantageous over liposomes and PNPs alone. Liposomes (CitationLee et al. 2007) and amphiphilic block polymers (CitationO’Halloran et al. 2013) both have the ability to self-assemble into vesicular systems as shown in . Liposomes have disadvantages like poor modular chemical functionality and osmotic shock leading to weak stability. The toughness and permeability make polymersomes more preferable as compared to liposomes (CitationZhang et al. 2010). Moreover, the cell biomimetic character of polymer vesicles is less compared to liposomes as block copolymers are usually synthetically made, however, phospholipids are generally natural components of the cell membrane. To solve these problems, the primary advantage of such hybrid structures is the fine tuning of the membrane physical properties. The capacity of self-assembled structures depends upon the amphiphilic nature of macromolecules and in the case of polymers, the presence of two blocks having a different solubility in the aqueous environment that is hydrophilic and hydrophobic in nature. The ratio of these blocks controls the arrangement of these assemblies (CitationAhmed and Discher 2004, CitationVriezema et al. 2005). As described in , polymersomes can incorporate synergistic drugs using a large water loving reservoir for hydrophilic molecules and a thick hydrophobic wall for hydrophobic molecules. At specific sites, hydrolytic degradation of the block copolymer takes place after changing molecular shape due to greater amount of the hydrophilic phase. Afterwards, macromolecular surfactant is formed due to damage of the hydrophobic block resulting in release of incorporated moiety in cytoplasm (CitationAhmed et al. 2006). Moreover, the number of types of release mechanisms can be designed through response to external stimuli such as pH (CitationChiang et al. 2010), temperature (CitationLi and Guan 2011, CitationNapoli et al. 2004, CitationQin et al. 2006), and oxidation reduction conditions (CitationConnor et al. 1984) as shown in .
Polymer caged nanoparticles
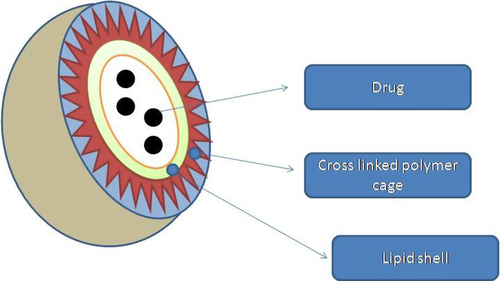
These are liposomes with surface modification by polymers to get better effects. For example, liposomes were prepared and a cholesterol-functionalized poly(acrylic acid) was linked to its surface which will provide surface-active carboxylate groups which will cross-link to telechelic 2,2-(ethylenedioxy) bis(ethylamine) linkers as shown in . These polymer-caged liposomes are more stable and possess pH-sensitive behavior (CitationLee et al. 2007). By this, not only may surface properties change but drug release is also adjustable. There may be two solutions to this, one is PEGylation and another a is pH-responsive cross-linked polymer shell. PEGylation decreases opsonization, and a pH-responsive cross-linked polymer shell will enhance the stability and reduce the drug leakage of liposomal NPs and also will improve drug release rate at low pH, which will be beneficial in tumor therapy (CitationO’Halloran et al. 2013). The cross-linked polymer cage offers protection to the drug payload and also serves as a pH-responsive trigger that enhances drug release in the acidic environments commonly seen in solid tumors and endosomes. Varying the degree of cross-linking in the polymer cage allows the surface potential for in vivo circulation lifetime of the nanocarriers to be tuned. During these days, pH-sensitive lipid components (CitationGerasimov et al. 1999), such as phosphatidylethanolamine (CitationBergstrand et al. 2003) and acid-labile PEG, (CitationLee et al. 2007, Citation2009) have been used to enhance drug release, which have a size of 100 nm and are called polymer-caged nanobin (PCN). It is possible due to chemical modification for the attachment of targeting ligand or imaging agents (CitationTannock and Rotin 1989, CitationVaupel et al. 1989). Likewise, the polymer cage provides steric stability around the lipid shell and decreases the expulsion of drug. The surface of the polymer cage enables drug release at low-pH target sites such as tumor interstitium (CitationCasey et al. 2009) and cellular endosomal vesicles (CitationFlorence 2004). Presumably, the free carboxylate groups in the cross-linked acrylamide polymer cage are protonated in acidic environments, which results in pockets of increased local hydrophobicity on the surface of the PCN leading to the collapse of the vesicle and the release of the drugs (CitationTannock and Rotin 1989).
Uptake of LPN's from oral route
Absorption is a very important process that occurs from the mouth to the stomach, small intestine, and at last colon. Drugs also undergo absorption through GIT membrane by one or more transport mechanisms as same as micro/macromolecules (CitationFlorence 1997). Absorption of nanoparticulate systems take place by one or more mechanisms. Absorption of any NP system is based on the different mechanistic approaches of absorption and also their properties, which ultimately have impact on its absorption.
Absorption mechanism
NPs are colloidal drug carriers that are useful for oral drug delivery. Usually, non-engineered NPs of 50–1000 nm (CitationBargoni et al. 1998) and microspheres < 10 μm have shown the sufficient particulate uptake into lymphatics. However, microparticles showed only 2–3% of absorption through Payer's patches and were retained in the gut of rats and mice for prolonged time periods (CitationFlorence et al. 1995, CitationKreuter 1991). Furthermore, NPs were taken up in particulate form by the intestine and transferred to various organs of lymphatic systems in the body. Two possible mechanisms of NP uptake are:
| (1) | intracellular uptake via the M cells of Payer's patches in the gut | ||||
| (2) | intercellular/paracellular uptake (CitationSanjula et al. 2009). | ||||
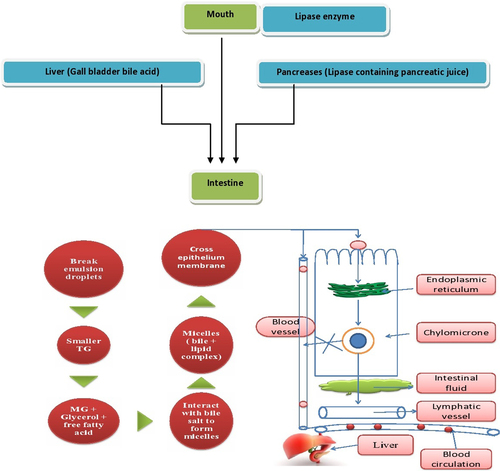
Moreover, in case of lipid based systems containing self-emulsifying excipients, apart from M cell and paracellular uptake, absorption occurs through lipase mediated chylomicrons formation into lymphatic system (similar to absorption of long chain fatty acids via facilitated chylomicrons formation) that further increases the absorption. Digestion of lipids starts in the mouth by means of lipase enzymes. When lipids enter the intestine, it is immediately exposed to lipase containing pancreatic juice as well as bile acid secreted out from the gall bladder of the liver which breaks the lipid vesicle to small droplets, i.e., smaller triglycerides (TGs) which are further converted to monoglycerides (MGs), glycerol, and free fatty acids. Moreover, the MGs interact with bile salt produced by micelles which cross the epithelial barrier and then again convert to TGs with the help of the endoplasmic reticulum and become chylomicrons. These chylomicrons do not have a very small size, so that they can enter blood circulation directly to the liver. Thus, the first transport to lymphatic vessel is followed by blood circulation via the thoracic duct to the jugular vein. Thus lipid can bypass liver metabolism (CitationSung et al. 2009). The whole scheme is demonstrated in . Through M cell uptake, therapeutic moieties can be successfully transported to the systemic circulation via intestinal lymphatics via the thoracic lymph duct. At the capillary level, the intercellular junctions between the endothelial cells of lymphatic capillaries are more open compared to blood capillaries which results in molecular sieving of NPs of a large size directly into the lymphatics, avoiding direction to the blood capillaries. The M cell uptake of NPs was found to be size-dependent (i.e., smaller the size, higher the uptake), but independent of the animal model (CitationSanjula et al. 2009). Thus, nanoparticulate systems can effectively improve BA and mean residence time (MRT) thereby improving therapeutic efficacy. Lymphatic delivery is supportive not only for absorption of poorly soluble drugs but also for targeting drug carriers to the lymphatics. Moreover, lymphatic delivery of NPs escapes the hepatic first-pass effect and enhances plasma concentration of the drug. This transcellular process coupled with stimulation of chylomicrons formation by enterocytes further enhanced the absorption process. In chylomicrons formation, dissolution and assimilation of lipophilic molecules into nonpolar core is achieved and thereby it promotes the absorption of lipophilic drugs. Since SLNs are composed of a lipid core, apart from M cell uptake, lipase mediated chylomicrons formation is another mechanism of absorption that differs from polymeric NPs (CitationFlorence et al. 1995).
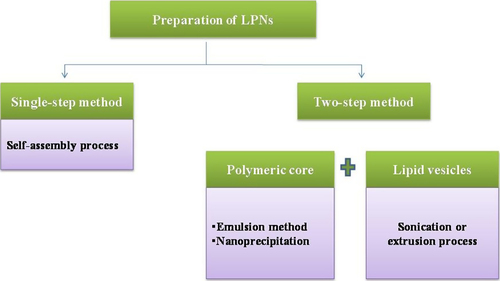
Method of preparation
Conventional methods for preparation of LPNs is more difficult as compared to preparation of liposomes or PNPs particles. Earlier, PNPs were mixed with liposomes to form lipid polymer complexes which need two steps for synthesis (as shown in ). First, the preparation of PNPs followed by the encapsulation of PNPs into the liposomes. This is very big task and also may hamper its physicochemical structure. To make it easier, a single step has been reported consisting of the combination of nanoprecipitation and self-assembly (CitationZhang et al. 2008).
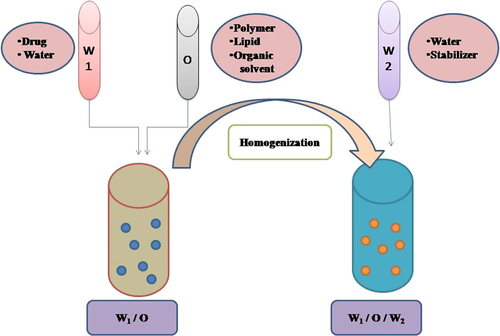
Double emulsification
It is a two-step process which involves the addition of one emulsion to the other (as shown in ). This method is useful for the encapsulation of all types of drugs like hydrophilic, lipophilic as well as amphiphilic. The addition of a primary emulsion into an aqueous external phase should be slow because it is a very critical step. A low shearing device should be used at room temperature, otherwise the chances of elimination of the internal phase to external continuous phase will be more (CitationGrossiord and Seiler 1999). It has been reported that antibiotics were incorporated in these hybrid NPs used for lung biofilm infection via modified emulsification-solvent evaporation method which possess lipid as surfactant; PLGA and PC were used as polymer and lipid, respectively. A modified emulsification-solvent evaporation method have been used to prepare hybrid NPs of three fluoroquinolone antibiotics levofloxacin, ciprofloxacin, and ofloxacin having different ionicity, lipophilicity, and aqueous solubility. The prepared hybrid NPs were examined for their drug encapsulation efficiency, drug loading, stability, and in vitro drug release profile. Charge between drug and lipid also has great impact which may interrupt the formation of NPs. Subsequently, counterionic surfactant, for example, stearyl amine may be used to stabilize the charge related issues. Lipid layer is responsible for controlled drug release during in vitro studies mainly depending on lipophilicity (CitationCheow and Hadinoto 2011). The major drawback of this method is lesser capacity to incorporate hydrophilic moiety for their partitioning into the aqueous phase of the emulsion (CitationBodmeier and McGinity 1988).
Two-step desolvation method
The emulsification method has a negative aspect in that the organic solvent is required to remove both the oily residues of preparation and the stabilizers. This could be overcome by using an alternative approach called the desolvation method in which NPs in aqueous phase are prepared by a coacervation process and then stabilized by a cross-linking agent. Desolvation factor like natural salts or alcohol added to protein solution plays a vital role because it leads to a change in the third protein structure. This change leads to clump formation of protein and unfortunately NPs formed by these clumps aggregate followed by cross linking (CitationJahanshahi and Babaei 2008). For oral delivery, amphotericin B (AmB) loaded lipid polymer hybrid NPs (AmB-LPNs) contained lecithin (anionic lipid) and gelatin (Type A, cationic below its isoelectric point 7.0–9.0) have been prepared by using this technique. The particle size, polydispersity index and entrapment efficiency of the hybrid NPs were 253 ± 8 nm, 0.274 ± 0.008, and 50.61 ± 2.20%, respectively. The NPs were spherically shaped lecithin core with a gelatin coat which has been confirmed by fluorescent resonance energy transfer (FRET) analysis. The sustained release was proved by Higuchi kinetics. The intestinal permeation (by Caco-2 cell lines) was more in case of hybrid NPs and oral BA was found to be increased by 4.69 fold as compared to free drug, fungizone (micellar solution of AmB) and fungisome (liposomal formulation of AmB) with less toxicity (CitationJain et al. 2012).
Bath sonication
Bath sonication is reported as a hasty method to prepare LPNs with appropriate particle size. Unlike the other time taking processes, it takes less time, approximately 5 min. By regulating the concentrations of all constituting components, including the size and polydispersity of NPs, the system does not show aggregation in PBS buffer and serum up to 5 days. Production rate of hybrid NPs is also enhanced by 20-fold. Thus, this method could be better than others for large scale production with easy optimization (CitationFang et al. 2010).
Nanoprecipitation
In this, an organic phase is used which is miscible with an external aqueous phase and will leach out to external phase. The complete miscibility of both phases will cause precipitation of the polymer quickly. Consequently, no separation and extraction of solvent is needed to precipitate the polymer. The method is useful only for slightly water soluble drugs (CitationVyas and Khar 2004). The nanoprecipitation method is very beneficial for preparation of NPs with a range between 150 and 170 nm. Actually, there is a very wide range of size variation so the filtration step is required. Hence some modifications have to be carried out to decrease the size to less than 150 nm and a variation in that range. For this reason, moderate stirring is replaced by sonication to get the desired reduced particle size to eliminate the filtration step (CitationChidambaram and Krishnasamy 2014). It has been reported that vincristine sulfate (VCR), novel self-assembled dextran sulphate-PLGA hybrid NPs (DPNs) were prepared by a self-assembly technique and nanoprecipitation method with some modification. By using the negative polymer (dextran sulphate sodium), 93.6% encapsulation of VCR was recorded. 80.4% sustained release was achieved within 96 h. Further, apparent BA of VCR-DPNs is quite higher than VCR-sol alone (CitationLing et al. 2010).
Factor affecting formation of LPNs
Optimal lipid amount in the LPNs preparation
In addition to minimizing drug leakage from the NPs, the self-assembled lipid as well as surfactant stabilizer play an important role in stability of the o/w (oil in water type emulsion) or W1/O/W2 emulsions (this is double emulsion having internal water phase dispersed in oil then again dispersed in external water phase). Thus a fixed ratio of lipids is required to prepare LPNs. By varying the mass of lipid with percentage of polymer mass the final formulation may be optimized on the basis of size, PDI, etc., parameters. However, it was found that increasing Wlipid/Wpolymer above 15% sharply decreases the NPs size, though no further decrease in size is observed even when ratio is increased up to 90%. When the amount of lipid added into the formulation is not appropriate, the reduction in emulsion stability causes the oil droplets to coalesce resulting in the formation of large particles. The coalescence of oil droplets contributes to polymer clumping that lowers the yield. Significantly, compared to the amount of PVA required in the nonhybrid NPs in 2% (w/v) aqueous phase, the hybrid NPs don't increase material wastage, as not only a smaller amount of surfactant is used, but also the majority of lipid is incorporated into the hybrid NPs, whereas only a minute amount of PVA (≈2%, w/w NPs) remains adsorbed on the non-hybrid NPs (CitationRowe 1989).
Selection of lipid
The mechanism of drug loading and release highly depends upon the thermodynamics for binding a drug to a polymer. This may not satisfy the design of LPNs. The compatibility of drug with excipients may be identified by means of thermal and non-thermal methods such as DSC, PXRD to predict the optimal compositions of different dosage forms including SLN (CitationNovoa et al. 2005, CitationSchenderlein et al. 2004). In addition, solubilization of a drug-polymer complex in molten lipid is a very critical step (CitationGardon 1966); to examine it, screening of lipid is required experimentally which may be a very costly and time consuming process. But, by using theoretical solubility parameters it may provide an early, quick screening tool for selection of lipid candidates. It is based on the hypothesis that the best miscibility of a drug and constituting agent is expected when both materials have the same total solubility parameters and polarities (including additive effect of hydrogen bonding) (CitationJelesarov and Bosshard 1999). Miscibility of the drug polymer complex in various lipids and to optimize the ionic molar ratio of polymer to drug may be checked by partition experiments (CitationValenta and Auner 2004).
Factorial design
Choice of best lipid for polymer-drug complex is not a single issue to develop LPNs. On the other hand, particular ratio of all excipients plays very important role. In order to choose best ratio all the ratios have to vary which may be possible by the factorial design. Best combination may be considered by evaluating formulation with certain characterization parameters like particle size, zeta potential (ZP), entrapment efficiency etc. Thus, this approach may be beneficial to screen best in vivo as well as in vitro results. The impact of variables (lipid, polymer, surfactant) have been reported earlier.
Effect of variables on particle size. The concentration of polymer is a major factor responsible for the enlargement of LPNs in size. This could be explained on the basis of inherent property of the polymer to produce globules of larger diameters (CitationChang et al. 2012). Moreover, it has been reported that generally during double emulsification PVA is used. By keeping the amount of lipid constant, the concentration of PVA was varied and it was concluded that the particle size was found to decrease with increase in PVA concentration. In emulsification, due to high shearing droplet size usually gets reduced and also droplets have a tendency to form aggregate in order to reduce their surface energy. But presence of surfactant molecule stabilizes the emulsion by providing a thick protective layer around the droplet to solve aggregation problem. Similarly, at low concentration of PVA, with amount of lipid increased, size generally increases due to lack of ability of PVA solution to stabilize emulsion at low concentration (CitationSingh et al. 2010).
Effect of variables on zeta potential. ZP reflects the extent of the electric charge on particle surface which offers an electrical barrier, and acts as a ‘repulsive factor’ in the process of emulsion stabilization (CitationSugiura et al. 2001). High surface energy has a big role in the stability of the formulation as like-charges at the interface prevent coalescence of particles (CitationMiller et al. 2000). Stability of NPs gets decreased for low ZP. LPNs may possess negative charge on their surfaces due to adsorption of -OH ions of lipid at the interface. Thus, concentration of polymer is an important factor to stabilize LPNs dispersions. The polymer provides electro-steric stabilization by induced shielding of surface charges (CitationXie et al. 2008).
Effect of variables on entrapment efficiency. The concentration of polymer and lipid has great influence on the encapsulation. Less concentrations of polymer and lipid than surfactant reduce the entrapment efficiency (EE). Thus, the high entrapment could be the combined result of polar nature of lipid and the influence of polymer and surfactant on the viscosity build up and subsequent formation of a diffusion barrier. Moreover, The EE directly increases with increased PVA concentration. PVA is a polymer which has influence on the nanoparticulate suspension formulation. It efficiently coats the particles and imparts viscosity to the external phase (CitationCoombes et al. 1998, CitationGasco 1993).
Effect of variables on drug release. LPNs show burst release initially may be because of release of unentrapped drug deposited at polymer shell and the partial hydrophilic nature of lipid matrix. The diffusion and desorption processes might play a significant role in this regard. On the other hand, polymer induced diffusion barrier may control drug release from the carrier matrix (CitationWahlgren et al. 2009). Incorporation of surfactant (HLB-10) has shown to decrease the mean size of LPN. This could have resulted in an improved dissolution rate and enhanced drug release. Thus, the partial hydrophilic nature of lipid, and the presence of amphiphilic surfactant were significant to modulate drug release from LPN (CitationDeok Kong et al. 2013).
Nowadays, the release of drug from LPNs is also possible via external stimulation. Camptothecin (CPT) lipid polymer hybrid particles have been prepared further by activating by an applied RF field (at 100 kHz). The extent of such remotely triggered heating of magnetic NPs depends directly on the following:
| ~ | Number of Fe3O4 NPs | ||||
| ~ | Duration of the applied RF magnetic field | ||||
| ~ | Low glass transition temperature of PLGA | ||||
A temperature rise in the PLGA matrix can loosen the polymer mesh and cause the loaded CPT to more easily diffuse out in 5 h (CitationCarmona-Ribeiro and Midmore 1992).
Surface adsorption. Physical adsorption of charged bilayers onto oppositely charged polymeric particles was done via electrostatic attractions (CitationCarmona-Ribeiro and Herrington 1993). Langmuir type adsorption isotherms were used for the three different lipids studied in which polymer/water interface was consistent with bilayer deposition. Electro kinetic properties of the covered particles were very similar to those of vesicles; the mean-z-average diameter of particles in the latex/vesicle mixtures increased by 10 nm, consistently with the increase in diameter expected from the deposition of one layer on the particles. Besides, for neutral phospholipids such as phosphatidylcholines (PC) and dipalmitoylphosphatidylcholine (DPPC), lipid adsorption was evaluated from adsorption isotherms and determination of mean-z-average diameter of particles in the latex/vesicle mixtures for three different latex dispersions: polystyrene with amidine, sulfate, or carboxylate as functional groups (CitationFahmy et al. 2005).
Lipid segregation to particle surfaces observed during dispersion and subsequent evaporation of the organic solvent, the lipid constituent acts as a surfactant that stabilizes the oil-water interface, consequently forming a coating over the solid PLGA micro particles or NPs. This “lipid surfactant” strategy provides great flexibility because of the ease with which the surface chemistry can be modified. The lipid also shows two-dimensional fluidity along the particle surface, as detected by fluorescence recovery after photo bleaching (FRAP), which shows diffusion of fluorescent lipids into a region within seconds of bleaching with a high-powered laser (CitationSilva et al. 2014).
Application of LPNs
In optical imaging of virus/host cell interaction
Hepatitis C in chronic stage leads to liver cirrhosis and hepatocellular carcinoma. Hepatitis C virus (HCV) is usually seen as three types in the serum of patient:
Enveloped in a lipid bilayers (HCV pseudo particles)
Non-enveloped and
Associated to lipoproteins (CitationBartenschlager et al. 2011).
Lipid-polymer conjugates are able to interact with artificial lipid bilayers and fluorescent lipid-polymer probes that can label living cells such as HeLa and T lymphocytes. The synthesis of novel lipid-polymer probes aims to label HCV viral particles and to follow their interaction and entrance in the host cells (CitationBathfield et al. 2008). Lipid polymer probes are made up of polymeric chains having a phospholipid moiety at one end and fluorophores along with polymer chain which give more brightness than molecular probes (CitationCepraga 2012). This is done in following steps:
The formation of fluorescent lipid-polymer probes; by studying their structure (size of the polymer chain, nature of the phospholipids, number of chromophores per chain) to label the viral particles.
To observe interactions of these lipid-polymer probes with model lipid bilayers such as liposomes and lipoparticles to select the appropriate lipid-polymer probes in terms of lipid layer insertion and membrane labeling (CitationTroutier et al. 2005).
Nanofibrous hybrid technology
Hybrid nanofibers for oral delivery via fast-dissolving drug delivery membranes (FDMs) to enclose poorly water soluble drugs have been prepared with ibuprofen and polyvinylpyrrolidone (PVP) K30 which act as filament forming polymer and drug carrier through electro-spinning. By some tests it comes out that ibuprofen was distributed uniformly in the fiber as nanosolid dispersion and drug was in amorphous form unlike pure drug as well as combination of PVP and ibuprofen. Electro-spun ultrafine fibers might be used as solid dispersions to enhance the dissolution of poorly water-soluble moieties or as oral fast disintegrating drug delivery systems (CitationYu et al. 2009). It has been reported that use of pure titanium NPs in PCL nanofibers may improve the precipitation of bone where the doped nanofibers are drenched in a simulated body fluid. Large porosity of eletrospun nanofibrous mats by addition of titanium NPs in it formed PCL nanofibrous mat may be used for the hard-tissue engineering applications (CitationBarakat et al. 2011).
Polymer lipid hybrid nanoparticulate blanket
Binding of polymer covalently to lipid on the surface of lipid nanovesicles could be a good approach for oral delivery of various drugs. In this case, polymer’ act as blanket may be beneficial to protect drugs from variable gastric conditions and lipid nanovesicles may enhance encapsulation efficiency. It has been reported that CMC-tethered nanovesicles (LN-CPTX) in the size range of 200 − 300 nm enhance the GI resistance and mucoadhesion properties as compared with unmodified lipid nanovesicles (LN-PTX). CMC-conjugated nanovesicles were administered to rat increased the plasma concentration profile of paclitaxel by 1.5-fold its BA and 5.5-folds elimination half-life as compared to taxol. Furthermore, CMC plays dual role: one is protection from GIT conditions and other one is showing stealth nature in order to reduce their uptake by reticuloendothelial system (RES) in liver and spleen. By this, no need for PEGylation is there with better tumor growth inhibition. In addition, LN-C-PTX exhibited therapeutic efficacy comparable to taxol and abraxane, and reduced toxicity and improved survival have been observed. Paclitaxel is a hydrophobic moiety that can be incorporated in lipid nanovesicles. CMC have positive charge and lipid have negative charge so there is very much chance for their electrostatic interactions but still covalent coupling is preferred due to greater stability (CitationJoshi et al. 2013) unlike electrostatic interactions. Thus no negative charge of lipid considers only -NH2 free group have been taken in account. Thus, stable CMC will show perfect adhesion to intestinal mucosa as required as the first thing for oral delivery (CitationKovacsovics-Bankowski et al. 1993).
Lipid-based biomimetic for drug and vaccine delivery
A particulate system could be targeted to antigen presenting cells (APC). Therefore, a particulate system can deliver an antigen to an APC more predicatively as compared to a soluble antigen (CitationOsaka et al. 2009, CitationVidard et al. 1996). Moreover, the internalization into human breast cancer cells are more in the case of positively charged NPs as compared to negatively charged into the human umbilical vein endothelial cells, which was almost the same (CitationLincopan et al. 2007). Nowadays, use of polymer along with a lipid is very popular. For example, bilayers of cationic lipid dioctadecyldimethylammonium bromide (DODAB) over NPs of polystyrene sulfate (PSS) have been reported (CitationJohnsson and Edwards 2003, CitationLincopan et al. 2009); consequently discoid micelles are formed on shifting of dispersed lamellar phase (liposomes) to micellar phase. Moreover, this took place at very low concentration of PEG-lipid and by increasing its concentration fine size discs can be obtained (CitationVieira and Carmona-Ribeiro 2008). Encapsulation of amphotericin B is carried out at high drug to lipid molar ratio. The insoluble drug particle is shielded with a cationic bilayer, then covered by a layer of carboxymethylcellulose and an outer layer of polydiallyldimethylamonium polymer (PDDA) to form a positively charged species which is active against Candida albicans (CitationBershteyn et al. 2008).
The physicochemical perspective in which molecules are offered at the surfaces of microbes has fabulous implications for the immune reaction to vaccination. The arrangement and mobility of molecules may manage interactions of their receptors, impacting immune cell activation, pathogen uptake, and antigen processing. The chemical environment of antigens also affects the specificity of the humoral immune response, because antibodies recognize antigen in its three-dimensional shape. Unfortunately, physical properties of antigen, such as diameter, impact immune response on both a cellular and tissue level. Thus, new achievement has been reported named as synthetic pathogens comprising a biodegradable polymeric core further covered by a lipid shell in order to resemble with a bilayer-enveloped pathogen. This is simply O/W/O type emulsion (a type of double emulsion having internal oil phase dispersed in water) based hybrid NPs of size 100 nm identical to lipid-enveloped viral pathogen or bacteria (CitationKol et al. 2007). Furthermore, biodegradable nature of polymer may be helpful to resolve all clinical issues. Example of such a system is the 45-monomer PEG chains containing lipid shell enclosed PLGA core have been considered as perfect as models in studies of immune response to pathogens. As vaccine carriers, these particles are identical to the structure and chemistry of pathogen surfaces. But still there are certain things which are difficult to explain. For example, mechanics of particulate antigen may play a role in antigen transport and processing. Viruses could alter their physical properties radically during the cycle of viral replication in the case of HIV by a factor of 14 (CitationKaneko et al. 1998).
Magnetically stimulated polymer lipid hybrid nanoparticles
Stimuli-responsive NPs (SRNPs) possess the capacity for enhancing the therapeutic efficacy and minimizing the side-effects of chemotherapeutics by releasing the encapsulated drug at the target site in a controlled manner. Currently, drug release from a carrier system via external activation is quite famous and a more challenging issue. A LPNs system is proved to be effective comprising magnetic beads for stimuli-responsive drug release using a remote radio frequency (RF) magnetic field. The external activators are of several types like acidity, temperature, magnetic fields, and light irradiation (CitationKong et al. 2010, CitationLuong et al. 2010). In addition, there are hollow silica nanocapsules capable of on–off switchable drug release via application of a remote radio frequency (RF) magnetic field; a one-step nanoprecipitation process was used to prepare 80 nm LPNs co-encapsulating 10-nm magnetic beads and a model anticancer drug, camptothecin (CPT). The lipid coating provided a diffusional gradient that slowed down the drug release in the absence of an external stimulus. On magnetic field exposure, the polymer core gets loosened by ferric oxide ultimately increasing the release. The lipid PLGA hybrid NPs gave controlled drug release by application of a remote RF field with long-term stability (CitationHall et al. 2007).
LPNs in pharmaceutical research
Nowadays the LPNs strategy has become very popular in all fields of research. The number of examples are given in .
Table 4. Studies performed to explore the potential of Lipid polymer hybrid nanoparticles in drug delivery.
Limitations of LPNs
The advancement of novel approaches such as hybrid lipid-polymeric NPs for drug delivery is necessary for successful drug delivery, but the ability to precisely control and predict properties of these systems is critical for their accomplishment in clinical translation. Furthermore, it may be obligatory to screen and select NPs with optimal properties for a certain application, which demands reproducible synthesis of NPs with a distinct size, charge, and ligand density (CitationGu et al. 2008).
Conclusion
During the last decades, interest to develop nanocarrier based drug delivery systems for oral delivery of therapeutics has been increasing constantly. Nanocarriers have many advantages including high absorption and stability of the therapeutic molecules in the GI environment. Among various nanocarriers developed for oral delivery, LPNs are more advantageous from all aspects. It has a very complex structure but still easy to prepare by simple methods. They are used to a great extent in all spheres of research, like in cancer therapy as well as in delivery of the gene, nucleic acids, small interfering RNA, etc. They are also used to encapsulate both hydrophilic as well as hydrophobic drugs for oral drug delivery. The LPNs have the ability for sustained and controllable release, with excellent in vivo properties. In future, these carriers have great avenues for the delivery of therapeutics via an oral route.
Declaration of interest
The authors report no declarations of interest. The author alone are responsible for the content and writing of the paper.
References
- Ahmed F, Discher DE. 2004. Self-porating polymersomes of PEG–PLA and PEG–PCL: hydrolysis-triggered controlled release vesicles. J Control Release. 96:37–53.
- Ahmed F, Pakunlu RI, Srinivas G, Brannan A, Bates F, Klein ML, et al. 2006. Shrinkage of a rapidly growing tumor by drug-loaded polymersomes: pH-triggered release through copolymer degradation. Mol Pharm. 3:340–350.
- Arbit E, Goldberg M, Gomez-Orellana I, Majuru S. 2006. Oral heparin: status review. Thromb J. 4:6.
- Aungst BJ, Rogers NJ. 1988. Site dependence of absorption-promoting actions of laureth-9, Na salicylate, Na2EDTA, and aprotinin on rectal, nasal, and buccal insulin delivery. Pharm Res. 5:305–308.
- Bagre AP, Jain K, Jain NK. 2013. Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: In vitro and in vivo assessment. Int J Pharm. 456:31–40.
- Barakat NA, Sheikh FA, Al-Deyab SS, Chronakis IS, Yong Kim H. 2011. Biologically active polycaprolactone/titanium hybrid electrospun nanofibers for hard tissue engineering. Sci Adv Mater. 3:730–734.
- Bargoni A, Cavalli R, Caputo O, Fundarò A, Gasco MR, Zara GP. 1998. Solid lipid nanoparticles in lymph and plasma after duodenal administration to rats. Pharm Res. 15:745–750.
- Bartenschlager R, Penin F, Lohmann V, André P. 2011. Assembly of infectious hepatitis C virus particles. Trends Microbiol. 19:95–103.
- Bathfield M, Daviot D, D’Agosto F, Spitz R, Ladavière C, Charreyre M-T, Delair T. 2008. Synthesis of lipid-α-end-functionalized chains by RAFT polymerization. Stabilization of lipid/polymer particle assemblies. Macromolecules. 41:8346–8353.
- Baughman RA, Kapoor SC, Agarwal RK, Kisicki J, Catella-Lawson F, FitzGerald GA. 1998. Oral delivery of anticoagulant doses of heparin a randomized, double-blind, controlled study in humans. Circulation. 98:1610–1615.
- Bergstrand N, Arfvidsson MC, Kim J-M, Thompson DH, Edwards K. 2003. Interactions between pH-sensitive liposomes and model membranes. Biophys Chem. 104:361–379.
- Bershteyn A, Chaparro J, Yau R, Kim M, Reinherz E, Ferreira-Moita L, Irvine DJ. 2008. Polymer-supported lipid shells, onions, and flowers. Soft Matter. 4:1787–1791.
- Bhavsar MD, Tiwari SB, Amiji MM. 2006. Formulation optimization for the nanoparticles-in-microsphere hybrid oral delivery system using factorial design. J Control Release. 110:422–430.
- Bodmeier R, McGinity J. 1988. Solvent selection in the preparation of poly (DL-lactide) microspheres prepared by the solvent evaporation method. Int J Pharm. 43:179–186.
- Brayden DJ, Baird AW. 2004. Apical membrane receptors on intestinal M cells: potential targets for vaccine delivery. Adv Drug Deliv Rev. 56:721–726.
- Carmona-Ribeiro A, Herrington T. 1993. Phospholipid adsorption onto polystyrene microspheres. J Colloid Interface Sci. 156:19–23.
- Carmona-Ribeiro A, Midmore B. 1992. Synthetic bilayer adsorption onto polystyrene microspheres. Langmuir. 8:801–806.
- Casey JR, Grinstein S, Orlowski J. 2009. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 11:50–61.
- Cepraga C. 2012. Conjugués polymère-chromophores biphotoniques greffés sur des nanoparticules d'or comme sondes fluorescentes pour la bioimagerie et la photothérapie dynamique. INSA de Lyon.
- Chakraborty S, Shukla D, Mishra B, Singh S. 2009. Lipid–an emerging platform for oral delivery of drugs with poor bioavailability. Eur J Pharm Biopharm. 73:1–15.
- Chandy T, Rao GH, Wilson RF, Das GS. 2002. Delivery of LMW heparin via surface coated chitosan/peg-alginate microspheres prevents thrombosis. Drug Deliv. 9:87–96.
- Chang DP, Jankunec M, Barauskas J, Tiberg F, Nylander T. 2012. Adsorption of lipid liquid crystalline nanoparticles on cationic, hydrophilic, and hydrophobic surfaces. ACS Appl Mater Interfaces. 4:2643–2651.
- Chen M-C, Wong H-S, Lin K-J, Chen H-L, Wey S-P, Sonaje K, et al. 2009. The characteristics, biodistribution and bioavailability of a chitosan-based nanoparticulate system for the oral delivery of heparin. Biomaterials. 30:6629–6637.
- Cheow WS, Hadinoto K. 2011. Factors affecting drug encapsulation and stability of lipid–polymer hybrid nanoparticles. Colloids Surf B Biointerfaces. 85:214–220.
- Chiang W-H, Hsu Y-H, Tang F-F, Chern C-S, Chiu H-C. 2010. Temperature/pH-induced morphological regulations of shell cross-linked graft copolymer assemblies. Polymer. 51:6248–6257.
- Chidambaram M, Krishnasamy K. 2014. .Modifications to the conventional nanoprecipitation technique: an approach to fabricate narrow sized polymeric nanoparticles.Adv Pharm Bull. 4:205–208.
- Connor J, Yatvin MB, Huang L. 1984. pH-sensitive liposomes: acid-induced liposome fusion. Proc Natl Acad Sci. 81:1715–1718.
- Coombes A, Yeh M-K, Lavelle E, Davis S. 1998. The control of protein release from poly (DL-lactide co-glycolide) microparticles by variation of the external aqueous phase surfactant in the water-in oil-in water method. J Control Release. 52:311–320.
- Davis ME. 2009. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm. 6:659–668.
- Deok Kong S, Sartor M, Jack Hu C-M, Zhang W, Zhang L, Jin S. 2013. Magnetic field activated lipid–polymer hybrid nanoparticles for stimuli-responsive drug release. Acta Biomater. 9:5447–5452.
- des Rieux A, Fievez V, Garinot M, Schneider Y-J, Préat V. 2006. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release. 116:1–27.
- Fahmy TM, Samstein RM, Harness CC, Mark Saltzman W. 2005. Surface modification of biodegradable polyesters with fatty acid conjugates for improved drug targeting. Biomaterials. 26:5727–5736.
- Fang D-L, Chen Y, Xu B, Ren K, He Z-Y, He L-L, et al. 2014. Development of lipid-shell and polymer core nanoparticles with water-soluble salidroside for anti-cancer therapy. Int J Mol Sci. 15:3373–3388.
- Fang RH, Aryal S, Hu C-MJ, Zhang L. 2010. Quick synthesis of lipid− polymer hybrid nanoparticles with low polydispersity using a single-step sonication method. Langmuir. 26:16958–16962.
- Florence AT. 1997. The oral absorption of micro-and nanoparticulates: neither exceptional nor unusual. Pharm Res. 14:259–266.
- Florence AT. 2004. Issues in oral nanoparticle drug carrier uptake and targeting. J Drug Target. 12:65–70.
- Florence AT, Hillery AM, Hussain N, Jani PU. 1995. Nanoparticles as carriers for oral peptide absorption: studies on particle uptake and fate. J Control Release. 36:39–46.
- Florence AT, Hussain N. 2001. Transcytosis of nanoparticle and dendrimer delivery systems: evolving vistas. Adv Drug Deliv Rev. 50:S69–S89.
- Gabizon AA. 2001. Pegylated liposomal doxorubicin: metamorphosis of an old drug into a new form of chemotherapy. Cancer Invest. 19:424–436.
- Gardon JL. 1966. The influence of polarity upon the solubility parameter concept. J Paint Technol. 38:43–57.
- Gasco MR. 1993. Method for producing solid lipid microspheres having a narrow size distribution. Google Patents, US 5250236A.
- Gerasimov OV, Boomer JA, Qualls MM, Thompson DH. 1999. Cytosolic drug delivery using pH-and light-sensitive liposomes. Adv Drug Deliv Rev. 38:317–338.
- Grossiord JL, Seiler M. 1999. Multiple Emulsions: Structure Properties and Applications Paris: Editions de Sante.
- Gu F, Zhang L, Teply BA, Mann N, Wang A, Radovic-Moreno AF, et al. 2008. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc Natl Acad Sci. 105:2586–2591.
- Hall JB, Dobrovolskaia MA, Patri AK, McNeil SE. 2007. Characterization of nanoparticles for therapeutics. Nanomedicine. 2:789–803.
- Hans ML, Lowman AM. 2006. 23 Nanoparticles for Drug Delivery. Philadelphia, Pennsylvania: Drexel University.
- Houchin M, Topp E. 2008. Chemical degradation of peptides and proteins in PLGA: a review of reactions and mechanisms. J Pharm Sci. 97:2395–2404.
- Hu C-MJ, Kaushal S, Cao HST, Aryal S, Sartor M, Esener S, et al. 2010. Half-antibody functionalized lipid− polymer hybrid nanoparticles for targeted drug delivery to carcinoembryonic antigen presenting pancreatic cancer cells. Mol Pharm. 7:914–920.
- Hu C-MJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. 2011. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci. 108:10980–10985.
- Jahanshahi M, Babaei Z. 2008. Protein nanoparticle: a unique system as drug delivery vehicles. Afr J Biotechnol. 7.
- Jain K. 2008. Nanomedicine: application of nanobiotechnology in medical practice. Med Princ Pract. 17:89–101.
- Jain S, Valvi PU, Swarnakar NK, Thanki K. 2012. Gelatin coated hybrid lipid nanoparticles for oral delivery of amphotericin B. Mol Pharm. 9:2542–2553.
- Jelesarov I, Bosshard HR. 1999. Isothermal titration calorimetry and differential scanning calorimetry as complementary tools to investigate the energetics of biomolecular recognition. J Mol Recognit. 12:3–18.
- Jenning V, Gysler A, Schäfer-Korting M, Gohla SH. 2000a. Vitamin A loaded solid lipid nanoparticles for topical use: occlusive properties and drug targeting to the upper skin. Eur J Pharm Biopharm. 49:211–218.
- Jenning V, Thünemann AF, Gohla SH. 2000b. Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int J Pharm. 199:167–177.
- Johnsson M, Edwards K. 2003. Liposomes, disks, and spherical micelles: aggregate structure in mixtures of gel phase phosphatidylcholines and poly (ethylene glycol)-phospholipids. Biophys J. 85:3839–3847.
- Joshi N, Saha R, Shanmugam T, Balakrishnan B, More P, Banerjee R. 2013. Carboxymethyl-chitosan-tethered lipid vesicles: hybrid nanoblanket for oral delivery of paclitaxel. Biomacromolecules. 14:2272–2282.
- Kalepu S, Manthina M, Padavala V. 2013. Oral lipid-based drug delivery systems–an overview. Acta Pharm Sin B. 3:361–372.
- Kaneko Y, Nakamura S, Sakai K, Kikuchi A, Aoyagi T, Sakurai Y, Okano T. 1998. Deswelling mechanism for comb-type grafted poly (N-isopropylacrylamide) hydrogels with rapid temperature responses. Polym Gels Netwo. 6:333–345.
- Katanec D, Pavelić B, Ivasović Z. 2004. Efficiency of polylactide/ polyglycolide copolymers bone replacements in bone defects healing measured by densitometry. Coll Antropol. 28:331–336.
- Kol N, Shi Y, Tsvitov M, Barlam D, Shneck RZ, Kay MS, Rousso I. 2007. A stiffness switch in human immunodeficiency virus. Biophys J. 92:1777–1783.
- Kong SD, Zhang W, Lee JH, Brammer K, Lal R, Karin M, Jin S. 2010. Magnetically vectored nanocapsules for tumor penetration and remotely switchable on-demand drug release. Nano Lett. 10:5088–5092.
- Kovacsovics-Bankowski M, Clark K, Benacerraf B, Rock KL. 1993. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci. 90:4942–4946.
- Kreuter J. 1991. Peroral administration of nanoparticles. Adv Drug Deliv Rev. 7:71–86.
- Kumari A, Yadav SK, Yadav SC. 2010. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 75:1–18.
- Kumbhar DD, Pokharkar VB. 2013. Physicochemical investigations on an engineered lipid–polymer hybrid nanoparticle containing a model hydrophilic active, zidovudine. Colloids Surf A Physicochem Eng Aspects. 436:714–725.
- Lallana E, Sousa-Herves A, Fernandez-Trillo F, Riguera R, Fernandez-Megia E. 2012. Click chemistry for drug delivery nanosystems. Pharm Res. 29:1–34.
- Lee S-M, Chen H, Dettmer CM, O’Halloran TV, Nguyen ST. 2007. Polymer-caged lipsomes: a pH-responsive delivery system with high stability. J Am Chem Soc. 129:15096–15097.
- Lee S-M, Chen H, O’Halloran TV, Nguyen ST. 2009. “Clickable” polymer-caged nanobins as a modular drug delivery platform. J Am Chem Soc. 131:9311–9320.
- Li J, He Y-Z., Li W, Shen Y-Z., Li Y-R, Wang Y-F. 2010. A novel polymer-lipid hybrid nanoparticle for efficient nonviral gene delivery. Acta Pharmacol Sin. 31:509–514.
- Li Z, Guan J. 2011. Thermosensitive hydrogels for drug delivery. Expert Opin Drug Deliv. 8:991–1007.
- Liechty WB, Peppas NA. 2012. Expert opinion: responsive polymer nanoparticles in cancer therapy. Eur J Pharm Biopharm. 80: 241–246.
- Lin JJ, Silas JA, Bermudez H, Milam VT, Bates FS, Hammer DA. 2004. The effect of polymer chain length and surface density on the adhesiveness of functionalized polymersomes. Langmuir. 20:5493–5500.
- Lincopan N, Espíndola N, Vaz A, Carmona-Ribeiro A. 2007. Cationic supported lipid bilayers for antigen presentation. Int J Pharm. 340:216–222.
- Lincopan N, Santana MR, Faquim-Mauro E, da Costa MH, Carmona-Ribeiro AM. 2009. Silica-based cationic bilayers as immunoadjuvants. BMC Biotechnol. 9:5.
- Ling G, Zhang P, Zhang W, Sun J, Meng X, Qin Y, et al. 2010. Development of novel self-assembled DS-PLGA hybrid nanoparticles for improving oral bioavailability of vincristine sulfate by P-gp inhibition. J Control Release. 148:241–248.
- Luong A, Issarapanichkit T, Kong SD, Fong R, Yang J. 2010. pH-sensitive, N-ethoxybenzylimidazole (NEBI) bifunctional crosslinkers enable triggered release of therapeutics from drug delivery carriers. Org Biomol Chem. 8:5105–5109.
- Lutz KL, Siahaan TJ. 1997. Molecular structure of the apical junction complex and its contribution to the paracellular barrier. J Pharm Sci. 86:977–984.
- Mehnert W, Mäder K. 2001. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 47:165–196.
- Meng F, Hiemstra C, Engbers GH, Feijen J. 2003. Biodegradable polymersomes. Macromolecules. 36:3004–3006.
- Miller R, Fainerman V, Makievski A, Krägel J, Grigoriev D, Kazakov V, Sinyachenko O. 2000. Dynamics of protein and mixed protein/ surfactant adsorption layers at the water/fluid interface. Adv Colloid Interface Sci. 86:39–82.
- Montasser I, Shahgaldian P, Perret F, Coleman AW. 2013. Solid lipid nanoparticle-based calix [n] arenes and calix-resorcinarenes as building blocks: synthesis, formulation and characterization. Int J Mol Sci. 14:21899–21942.
- Mu H, Holm R, Müllertz A. 2013. Lipid-based formulations for oral administration of poorly water-soluble drugs. Int J Pharm. 453: 215–224.
- Müller RH, Mäder K, Gohla S. 2000. Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur J Pharm Biopharm. 50:161–177.
- Müller R, Radtke M, Wissing S. 2002. Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm. 242:121–128.
- Müller RH, Rühl D, Runge SA. 1996. Biodegradation of solid lipid nanoparticles as a function of lipase incubation time. Int J Pharm. 144:115–121.
- Napoli A, Valentini M, Tirelli N, Müller M, Hubbell JA. 2004. Oxidation-responsive polymeric vesicles. Nat Mater. 3:183–189.
- Nielsen SO, Lopez CF, Srinivas G, Klein ML. 2004. Coarse grain models and the computer simulation of soft materials. J Phys Condens Matter. 16:R481.
- Novoa GAG, Heinämäki J, Mirza S, Antikainen O, Iraizoz Colarte A, Suzarte Paz A, Yliruusi J. 2005. Physical solid-state properties and dissolution of sustained-release matrices of polyvinylacetate. Eur J Pharm Biopharm. 59:343–350.
- O’Halloran TV, Ahn R, Hankins P, Swindell E, Mazar AP. 2013. The many spaces of uPAR: delivery of theranostic agents and nanobins to multiple tumor compartments through a single target. Theranostics. 3:496.
- Oberdörster G, Oberdörster E, Oberdörster J. 2005. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 113:823.
- Osaka T, Nakanishi T, Shanmugam S, Takahama S, Zhang H. 2009. Effect of surface charge of magnetite nanoparticles on their internalization into breast cancer and umbilical vein endothelial cells. Colloids Surf B Biointerfaces. 71:325–330.
- Pandey R, Sharma S, Khuller G. 2005. Oral solid lipid nanoparticle-based antitubercular chemotherapy. Tuberculosis. 85:415–420.
- Pankhurst QA, Connolly J, Jones S, Dobson J. 2003. Applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys. 36:R167.
- Pautot S, Frisken BJ, Weitz D. 2003. Engineering asymmetric vesicles. Proc Natl Acad Sci. 100:10718–10721.
- Porter CJ, Trevaskis NL, Charman WN. 2007. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 6:231–248.
- Prego C, Torres D, Fernandez-Megia E, Novoa-Carballal R, Quiñoá E, Alonso M. 2006. Chitosan–PEG nanocapsules as new carriers for oral peptide delivery: effect of chitosan pegylation degree. J Control Release. 111:299–308.
- Qin S, Geng Y, Discher DE, Yang S. 2006. Temperature-controlled assembly and release from polymer vesicles of poly (ethylene oxide)-block-poly (N-isopropylacrylamide). Adv Mater. 18:2905–2909.
- Rowe R. 1989. Polar/non-polar interactions in the granulation of organic substrates with polymer binding agents. Int J Pharm. 56:117–124.
- Saad M, Garbuzenko OB, Minko T. 2008. Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine. 3:761–776.
- Sahoo SK, Labhasetwar V. 2005. Enhanced antiproliferative activity of transferrin-conjugated paclitaxel-loaded nanoparticles is mediated via sustained intracellular drug retention. Mol Pharm. 2: 373–383.
- Sanjula B, Shah FM, Javed A, Alka A. 2009. Effect of poloxamer 188 on lymphatic uptake of carvedilol-loaded solid lipid nanoparticles for bioavailability enhancement. J Drug Target. 17:249–256.
- Sanna V, Kirschvink N, Gustin P, Gavini E, Roland I, Delattre L, Evrard B. 2004. Preparation and in vivo toxicity study of solid lipid microparticles as carrier for pulmonary administration. AAPS PharmSciTech. 5:17–23.
- Sanvicens N, Marco MP. 2008. Multifunctional nanoparticles– properties and prospects for their use in human medicine. Trends Biotechnol. 26:425–433.
- Schenderlein S, Lück M, Müller B. 2004. Partial solubility parameters of poly (D, L-lactide-co-glycolide). Int J Pharm. 286:19–26.
- Semete B, Booysen L, Lemmer Y, Kalombo L, Katata L, Verschoor J, Swai HS. 2010. In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomedicine. 6:662–671.
- Shegokar R, Singh K, Müller R. 2011. Production & stability of stavudine solid lipid nanoparticles—From lab to industrial scale.Int J Pharm. 416:461–470.
- Shi J, Xiao Z, Votruba AR, Vilos C, Farokhzad OC. 2011. Differentially charged hollow core/shell lipid–polymer–lipid hybrid nanoparticles for small interfering RNA delivery. Angew Chem Int Ed Engl. 123:7165–7169.
- Silva CO, Sarmento B, Reis CP. 2014. Oral Delivery of Biopharmaceuticals. Mucosal Delivery of Biopharmaceuticals. New York: Springer.
- Singh S, Dobhal AK, Jain A, Pandit JK, Chakraborty S. 2010. Formulation and evaluation of solid lipid nanoparticles of a water soluble drug: zidovudine. Chem Pharm Bull. 58:650–655.
- Souto EB, Doktorovova S. 2009. Solid lipid nanoparticle formulations: pharmacokinetic and biopharmaceutical aspects in drug delivery. Methods Enzymol. 464:105–129.
- Stolnik S, Dunn SE, Garnett MC, Davies MC, Coombes AG, Taylor D, et al. 1994. Surface modification of poly (lactide-co-glycolide) nanospheres by biodegradable poly (lactide)-poly (ethylene glycol) copolymers. Pharm Res. 11:1800–1808.
- Sugiura S, Nakajima M, Iwamoto S, Seki M. 2001. Interfacial tension driven monodispersed droplet formation from microfabricated channel array. Langmuir. 17:5562–5566.
- Sung JC, Padilla DJ, Garcia-Contreras L, VerBerkmoes JL, Durbin D, Peloquin CA, et al. 2009. Formulation and pharmacokinetics of self-assembled rifampicin nanoparticle systems for pulmonary delivery. Pharm Res. 26:1847–1855.
- Suri SS, Fenniri H, Singh B. 2007. Nanotechnology-based drug delivery systems. J Occup Med Toxicol. 2:16.
- Tanaka M, Sackmann E. 2005. Polymer-supported membranes as models of the cell surface. Nature. 437:656–663.
- Tannock IF, Rotin D. 1989. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 49:4373–4384.
- Trevaskis NL, Charman WN, Porter CJ. 2008. Lipid-based delivery systems and intestinal lymphatic drug transport: a mechanistic update. Adv Drug Deliv Rev. 60:702–716.
- Troutier A-L, Véron L, Delair T, Pichot C, Ladavière C. 2005. New insights into self-organization of a model lipid mixture and quantification of its adsorption on spherical polymer particles. Langmuir. 21:9901–9910.
- Uchiyama T, Sugiyama T, Quan YS, Kotani A, Okada N, Fujita T, et al. 1999. Enhanced permeability of insulin across the rat intestinal membrane by various absorption enhancers: their intestinal mucosal toxicity and absorption-enhancing mechanism of n-Lauryl-βD-maltopyranoside. J Pharm Pharmacol. 51: 1241–1250.
- Uchiyama T, Yamamoto A, Hatano H, Fujita T, Muranishi S. 1996. Effectiveness and toxicity screening of various absorption enhancers in the large intestine: intestinal absorption of phenol red and protein and phospholipid release from the intestinal membrane. Biol Pharm Bull. 19:1618.
- Uekama K, Hirayama F, Irie T. 1998. Cyclodextrin drug carrier systems. Chem Rev. 98:2045–2076.
- Valenta C, Auner BG. 2004. The use of polymers for dermal and transdermal delivery. Eur J Pharm Biopharm. 58:279–289.
- Vaupel P, Kallinowski F, Okunieff P. 1989. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 49:6449–6465.
- Vidard L, Kovacsovics-Bankowski M, Kraeft S-K, Chen LB, Benacerraf B, Rock KL. 1996. Analysis of MHC class II presentation of particulate antigens of B lymphocytes. J Immunol. 156:2809–2818.
- Vieira DB, Carmona-Ribeiro AM. 2008. Cationic nanoparticles for delivery of amphotericin B: preparation, characterization and activity in vitro. J Nanobiotechnol. 6:1–13.
- Vonarbourg A, Passirani C, Saulnier P, Benoit J-P. 2006. Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials. 27:4356–4373.
- Vriezema DM, Comellas Aragonès M, Elemans JA, Cornelissen JJ, Rowan AE, Nolte RJ. 2005. Self-assembled nanoreactors. Chemical Rev. 105:1445–1490.
- Vyas SP, Khar RK. 2004. Targeted and Controlled Drug Delivery: Novel Carrier Systems. New York: CBS publishers & distributors.
- Wahlgren M, Christensen KL, Jørgensen EV, Svensson A, Ulvenlund S. 2009. Oral-based controlled release formulations using poly (acrylic acid) microgels. Drug Dev Ind Pharm. 35:922–929.
- Wang J, Ornek-Ballanco C, Xu J, Yang W, Yu X. 2012a. Preparation and characterization of vinculin-targeted polymer–lipid nanoparticle as intracellular delivery vehicle. Int J Nanomedicine. 8:39–46.
- Wang Y, Kho K, Cheow WS, Hadinoto K. 2012b. A comparison between spray drying and spray freeze drying for dry powder inhaler formulation of drug-loaded lipid–polymer hybrid nanoparticles. Int J Pharm. 424:98–106.
- Ward PD, Tippin TK, Thakker DR. 2000. Enhancing paracellular permeability by modulating epithelial tight junctions. Pharm Sci Technol Today. 3:346–358.
- Wong HL, Bendayan R, Rauth AM, Li Y, Wu XY. 2007. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv Drug Deliv Rev. 59:491–504.
- Wong HL, Bendayan R, Rauth AM, Xue HY, Babakhanian K, Wu XY. 2006a. A mechanistic study of enhanced doxorubicin uptake and retention in multidrug resistant breast cancer cells using a polymer-lipid hybrid nanoparticle system. JPharmacol Exp Ther. 317:1372–1381.
- Wong HL, Rauth AM, Bendayan R, Manias JL, Ramaswamy M, Liu Z, et al. 2006b. A new polymer–lipid hybrid nanoparticle system increases cytotoxicity of doxorubicin against multidrug-resistant human breast cancer cells. Pharm Res. 23:1574–1585.
- Xie S, Wang S, Zhao B, Han C, Wang M, Zhou W. 2008. Effect of PLGA as a polymeric emulsifier on preparation of hydrophilic protein-loaded solid lipid nanoparticles. Colloids Surf B Biointerfaces. 67:199–204.
- Yu D-G, Shen X-X, Branford-White C, White K, Zhu L-M, Bligh SA. 2009. Oral fast-dissolving drug delivery membranes prepared from electrospun polyvinylpyrrolidone ultrafine fibers. Nanotechnology. 20:055104.
- Zhang L, Chan JM, Gu FX, Rhee J-W, Wang AZ, Radovic-Moreno AF, et al. 2008. Self-assembled lipid− polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano. 2:1696–1702.
- Zhang Y, Dou X, Jin T. 2010. Synthesis and self-assembly behavior of amphiphilic diblock copolymer dextran-block-poly (ϵ-caprolactone) (DEX-b-PC) in aqueous media. Express Polym Lett. 4.