Abstract
Polyethyleneimine (PEI) coated-silica nanoparticles were prepared by the Stöber method. The formation and the structure of the nanoparticles were characterized by ATR-FT-IR spectroscopy and transmission electron microscopy (TEM). TEM images of the silica and PEI-coated nanoparticles revealed that they were well dispersed and that there was no agglomeration. The acetylcholineesterase enzyme was immobilized onto these nanoparticles. The effects of pH and temperature on the storage stability of the free and immobilized enzyme were investigated. The optimum pHs for free and immobilized enzymes were determined as 7.0 and 8.0, respectively. The optimum temperatures for free and immobilized enzymes were found to be 30.0 and 35.0°C, respectively. The maximum reaction rate (Vmax) and the Michaelis-Menten constant (Km) were investigated for the free and immobilized enzyme. The storage stability of acetylcholinesterase was increased when immobilized onto the novel PEI-coated silica nanoparticles. The reuse numbers of immobilized enzyme were also studied. These hybrid nanoparticles are desirable as carriers for biomedical applications.
Introduction
Silica nanoparticles exhibit special functions such as bioactivity, crosslinking ability and amphiphilic properties by grafting of functional polymers onto the nanoparticle surface (CitationYang et al. 2008). Hybrid nanomaterials comprised of an inorganic core and a polymer shell have great attraction due to their excellent properties. Polymers are ideal building blocks for nanofabrication and engineering of surfaces. They can graft onto the nanoparticle core and enhance its flexibility, stability and dispersibility in various solvents. Therefore, these nano-sized particles can find a wide range of applications in optics, electronics, engineering and biosciences (CitationZhu et al. 2004, CitationMcCormick and Lowe 2004, CitationLi et al. 2005, CitationShan et al. 2005, CitationLi et al. 2006, CitationYokoyama et al. 2006, CitationGuo et al. 2006).
Polyethyleneimine (PEI) is a water-soluble polyamine, which has a large quantity of nitrogen atoms. Generally, commercial PEI chains are branched and have primary, secondary and tertiary amine groups in a ratio of 1:2:1. A large part of the amine groups on the molecular chains of PEI are in the protonated state in an aqueous solution of pH less than10, because PEI is a cationic polyelectrolyte (CitationAmara and Kerdjoudj 2003).
In this study, a novel nanoparticle support was prepared for enzyme immobilization and acetylcholinesterase (AChE) was used as a model enzyme. Firstly, PEI was grafted onto the surfaces of silica nanoparticles by Van der Waals interaction and hydrogen bonding between the silica nanoparticles and PEI. Then, AChE was immobilized onto nanoparticles by electrostatic interaction. There is a strong physical adsorption between the PEI chains and the negatively charged enzyme. The purpose of this study was to develop new composite materials for enzyme immobilization.
Experimental materials and methods
Materials
Poly(ethyleneimine) (PEI) (Mw:25,000 g/mol), Ammonium hydroxide solution (28 wt.%, NH3 in H2O) from Aldrich. Acetylcholinesterase (from Electrophorus electricus) (AChE) (EC 3.1.1.7), acetylthiocholine iodide, 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB) from Fluka. Ethanol (≥ 99.5%). Free AChE solution was prepared in 20 mL gelatin solution (1.0%) and diluted with distilled water (1:200). Acetylthiocholine iodide and 5 5’-dithiobis(2-nitrobenzoic acid) (DTNB) solutions were prepared in PBS.
Preparation of silica nanoparticles
Silica nanoparticles were synthesized according to the Stöber method (CitationStöber et al. 1968). Briefly, 5.7 mL of concentrated ammonia solution was added to a flask containing different volumes (1.5–5 mL) of tetraethyl orthosilicate and 114 mL of ethanol, with magnetic stirring. The stirring was continued for 5 h. Silica nanoparticles were centrifuged at 14,000 rpm, washed thoroughly with water to remove the remaining reactants, and dried under vacuum at 30°C.
Preparation of PEI-coated silica nanoparticles
The above prepared silica nanoparticles were then added into a PEI solution (0.5 g PEI dissolved in 25 mL of ethanol), with mechanical stirring at room temperature for 24 h. The PEI-coated silica nanoparticles were centrifuged at 14,000 rpm, washed completely with water to remove the remaining reactants, and dried under vacuum at 30°C.
AChE immobilization onto PEI-coated silica nanoparticles
Briefly, an aqueous solution of AChE (2 mg/mL) was prepared by dissolving the lyophilized enzyme in PBS (10 mM, pH 7.0) at room temperature. After that, 0.5 g PEI- coated silica nanoparticles were added to the enzyme solution and stirred at 25°C for 24 h in PBS (pH = 7.0). Unreacted enzyme on the nanoparticle surface was removed by washing the modified silica nanoparticles with distilled water. The immobilized enzyme was stored at + 4°C in PBS until needed.
Results and discussion
Preparation and characterization of PEI coated silica nanoparticles
Stöber et al. reported a simple synthesis of monodisperse spherical silica nanoparticles (CitationStöber et al. 1968). The synthesis proceeds with the hydrolysis and condensation of tetraethyl orthosilicate (TEOS) in a mixture of alcohol, water and ammonia (catalyst). The resulting nanoparticles are stabilized by electrostatic repulsion due to the ions in the ammonia solution. Some researchers have reported the mechanism of particle nucleation and growth (CitationVan Blaaderen and Vrij 1993).
According to Gao et al., PEI is only physically adsorbed on the silica nanoparticles through Van der Waals interaction and hydrogen bonding, since the amine group will not react with SiO2. The reaction processes to prepare the support material PEI-coated silica nanoparticles can be expressed as in their study (CitationGao et al. 2006).
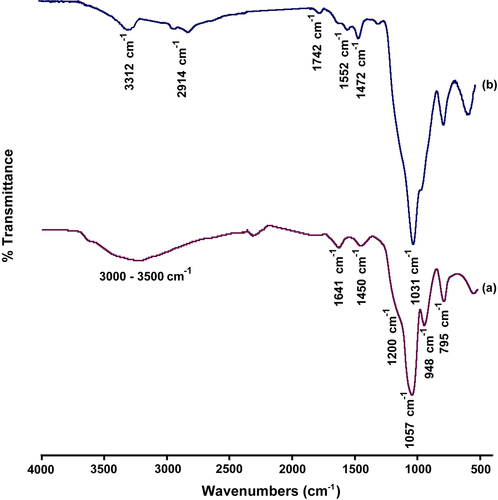
The chemical structures of bare silica and PEI-coated silica nanoparticles were characterized by FT-IR spectroscopy and the spectrums are shown in . For bare silica nanoparticles, the wide band at 3000–3500 cm− 1 are attributed to the silanol groups. Intensive bands at 1200 and 1057 cm− 1 are attributed to the asymmetric stretching and bending of siloxane groups (Si–O–Si) in (CitationLi et al. 2005). The FT-IR spectrum of PEI of silica-coated nanopar ticles is shown in . The characteristic absorption band of the N–H bond appeared at 3312 cm− 1 and the characteristic absorptions of bend vibration of C–N bond appeared at 1472 and 1552 cm− 1. All of these absorption bands showed that PEI macromolecules have been attached onto silica nanoparticles.
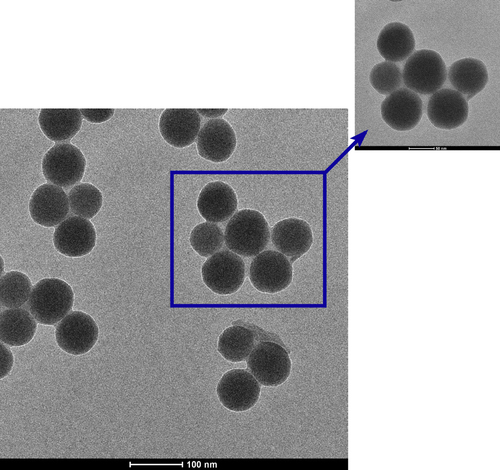
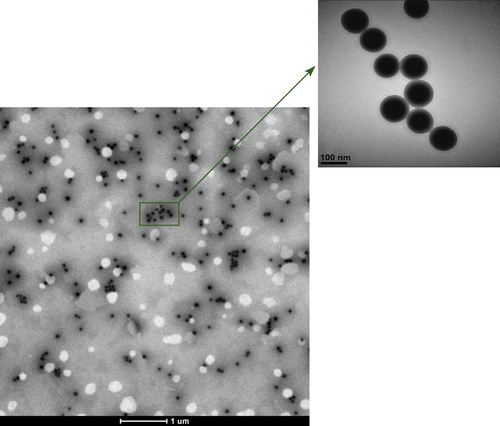
The diameters of bare silica and PEI-coated silica nanoparticles were characterized by TEM. The TEM images, as shown in , showed that the silica nanoparticles fabricated by the Stöber method were spherical, with a mean diameter of about 60 ± 10 nm. The morphology consisted of spherical nanosized particles, monodisperse with no aggregation (CitationMcConnell et al. 2009).
shows the morphology of the PEI-coated silica nanoparticles. As shown in the TEM image, the resulting hybrid silica nanoparticles have a clear spherical and core–shell structure (CitationMu et al. 2009), the dark-colored core for silica and light-colored shell for polymers. As it can be seen, with branched PEI modification, silica nanoparticles were wrapped in the PEI molecule.
Enzyme immobilization onto PEI-coated silica nanoparticles
According to Gao et al., PEI-coated silica nanoparticles can provide strong physical adsorption against enzyme molecules by electrostatic interaction at pH 7.0. Due to this structure, the surface of the PEI-coated silica nanoparticles is positively charged. The isoelectric point of AChE was between pH 5.6–6.0, therefore the AChE enzyme was negatively charged under the optimum pH conditions of pH 7.0. Hence, the nanoparticles can provide strong physical adsorption against enzyme molecules by electrostatic interaction. Moreover, the efficiency of AChE immobilization as well as the activity of bound enzyme were found to be 91.6%. This result, that is the percentage of bound AChE (91.6%), showed that the binding process was successful. The unbound enzyme was determined by assaying the protein content in the supernatant (CitationGao et al. 2006).
Effects of pH and temperature on the immobilized AChE activity
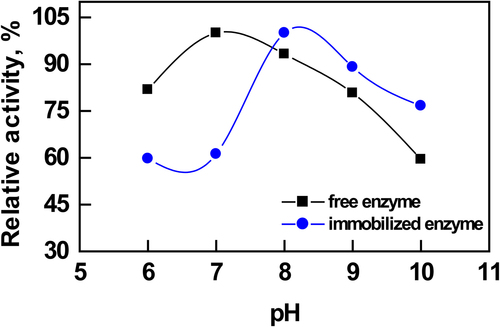
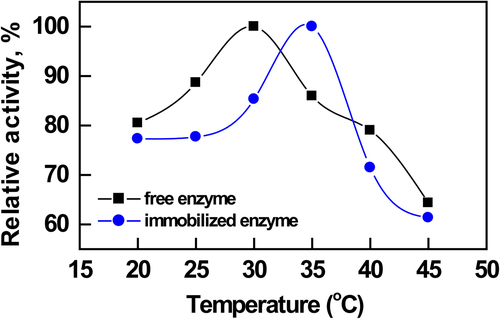
The pH stabilities of the free and immobilized enzymes on modified nanoparticles were determined by incubating in different buffers (pH 6.0–10.0) for 20 min at 30.0°C (). The activities of free and immobilized enzymes were assayed under optimal conditions. It is well known that the immobilization of enzymes leads to a change in their optimum pH and this change depends on the ionic charge, and on the nature of the support used for immobilization. In our previous study, the optimum pH value for free AChE enzyme was found to be 7.0 (CitationŞahin et al. 2007). The enzyme immobilized onto the PEI-coated silica nanoparticles also showed an optimum pH of 8.0. The basic chain structure of PEI is [–CH2–CH2–NH–]n, so there are a large number of nitrogen atoms of the amine groups on the macromolecular chains of PEI. In aqueous solution, these nitrogen atoms of the amine groups combine easily with H+ to form [–CH2–CH2–NH2+–]n. Thus, a majority of amine groups on PEI chains were protonated and the macromolecular chains of PEI were positively charged.
The immobilized and free enzyme activities were measured at different temperatures (). The optimum temperature was determined to be 30.0°C for free enzyme (CitationŞahin et al. 2007). The optimum temperature for immobilized AChE was determined to be 35.0°C. This was probably due to the conformational limitations of the mobility of the immobilized enzyme with the increase of temperature, as a result of the physical adsorption with the carrier, which requires higher activation energy. Moreover, the temperature of the bulk solution was lower than the actual temperature matrix microenvironment, therefore high activity for the immobilized enzyme could hold a wider range of temperatures. The activity of enzymes immobilized in a more soluble form is known to be more resistant to heat.
Kinetic parameters
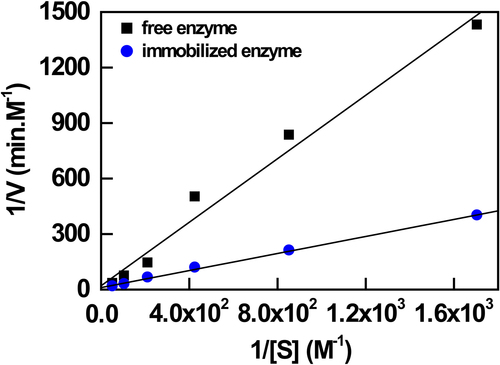
Kinetic parameters of the free and immobilized AChE, Km and Vmax, were assayed at substrate concentrations ranging from 0.5 to 18.75 mM. From the Lineweaver–Burk plot of 1/V versus 1/S Michaelis constant (Km), the maximum reaction velocity (Vmax) of the free and immobilized enzymes were calculated and results are given in . The Km value for free enzyme was 6.35 mM, whereas the Vmax value was 50 mM min−1. The Km values of immobilized enzyme were found to be 21mM, and the Vmax value for immobilized enzyme was found to be 1.42 mM min− 1, respectively. The increase in the Km value after the immobilization of AChE can be due to the changes in the conformation of the enzyme molecules, which blocks the enzyme-substrate interaction, or due to the hindered access of the substrate to the active sites of the immobilized enzyme (CitationŞahin et al. 2007).
Storage stability
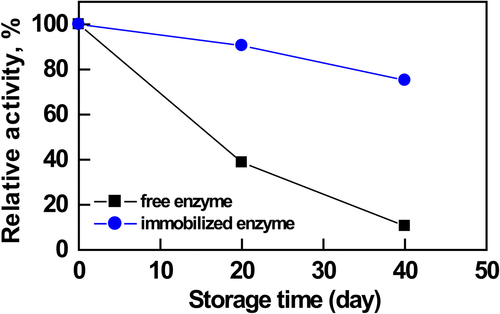
In order to investigate the industrial capability of an immobilized enzyme, the loss of enzyme activity, known as storage stability, is an important parameter to be taken into account. The immobilized and free AChEs (2 mg/mL) were stored in PBS (10 mM, pH 7.0) at 4°C and activities were measured periodically over a duration of 40 days (). After 40 days of storage, the catalytic activity of the free enzyme was retained at about 4% of its original activity. After 40 days of storage, the immobilized enzyme retained about 80% of the initial activity. This result indicated that the immobilized enzyme effectively decreases denaturation, as well as obviously increases the endurance and stability of immobilized AChE. The multipoint ionic interactions generated between the enzyme and PEI-coated silica nanoparticles should also convey a higher conformational stability to the immobilized enzyme. Thus, the nanoparticles and the immobilization method provided higher shelf life compared to that of the free enzyme.
Reuse numbers
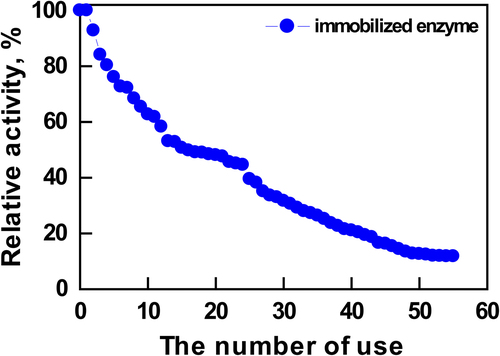
The reusability of immobilized enzymes is important for their practical application. The maximum activity in the range of 100% was obtained at the beginning of reusability experiments. As shown in , AChE immobilized by using nanoparticles was used repeatedly 55 times within 3 days. After 10 reuses, the enzyme immobilized onto the PEI-modified support retained 90% residual activity. After 30 reuses, the residual activity of the immobilized enzyme was still about 72%. This result showed that immobilized enzyme activities were decreased due to diffusional effects, while the reuse number was increased.
Conclusions
A facile method was developed for the preparation of PEI-coated silica nanoparticles from the primary silica nanoparticles in the Stöber process. AChE was immobilized on PEI-coated silica nanoparticles and the optimal immobilization conditions were achieved. Although the Km value of free enzyme was higher than the Km value of immobilized enzyme. However, the immobilized enzyme showed excellent stability to pH and temperature variations when compared to free enzyme. Its optimal catalytic pH and temperature were determined to be 8.0 and 35°C, respectively. These supports have certain advantages over other materials, such as easy preparation procedure, low cost, high immobilization capacity and good mechanical stability, for various biotechnological and biomedical applications, like being a part of an enzyme electrode or an enzyme reactor.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Amara M, Kerdjoudj H. 2003. Talanta. 60:991–1001.
- Gao B, Wang X, Shen Y. 2006. Biochem Eng J. 28:140–147.
- Guo TY, Liu P, Zhu JW, Song MD, Zhang BH. 2006. Biomacromolecules. 7:1196–1202.
- Li D, Jones GL, Dunlap JR, Hua FJ, Zhao B. 2006. Langmuir. 22:3344–3351.
- Li D, Sheng X, Zhao B. 2005. J Am Chem Soc. 127:6248–6256.
- McConnell MD, Bassani AW, Yang S, Composto RJ. 2009. Langmuir. 25:11014–11020.
- McCormick CL, Lowe AB. 2004. Acc Chem Res. 37:312–325.
- Mu Y, Qiu T, Li X. 2009. Mat Lett. 63:1614–1617.
- Şahin F, Demirel G, Tümtürk H. 2007. Bioprocess Biosyst Eng. 30: 141–145.
- Shan J, Nuopponen M, Jiang H, Viitala T, Kauppinen E, Kontturi K, Tenhu H. 2005. Macromolecules. 38:2918–2926.
- Stöber W, Fink A, Bohn E. 1968. J Colloid Inter Sci. 26:62–69.
- Van Blaaderen A, Vrij A. 1993. J Colloid Interface Sci. 156:1–18.
- Yang Y, Yan X, Cui Y, He Q, Li D, Wang A, et al. 2008. J Mater Chem. 18:5731–5737.
- Yokoyama R, Suzuki S, Shirai K, Yamauchi T, Tsubokawa N, Tsuchimochi M. 2006. Eur Polym J. 42:3221–3229.
- Zhu MQ, Wang LQ, Exarhos GJ, Li ADQ. 2004. J Am Chem Soc. 126:2656–2657.