Abstract
We crosslink hemoglobin (Hb), superoxide dismutase (SOD), catalase (CAT), and carbonic anhydrase (CA) to form a soluble polyHb-SOD-CAT-CA nanobiotechnological complex. The obtained product is a soluble complex with three enhanced red blood cell (RBC) functions and without blood group antigens. In the present study, 2/3 of blood volume was removed to result in 90-min hemorrhagic shock at mean arterial blood pressure (MAP) of 30 mmHg. This was followed by the reinfusion of different resuscitation fluids, then followed for another 60 min. PolyHb-SOD-CAT-CA maintained the MAP at 87.5 ± 5 mmHg as compared with 3 volumes of lactated Ringer's solution, 43.3 ± 2.8 mmHg; blood, 91.3 ± 3.6 mmHg; polyHb-SOD-CAT, 86.0 ± 4.6 mmHg; poly stroma-free hemolysate (polySFHb), 85.0 ± 2.5 mmHg; and polyHb, 82.6 ± 3.5 mmHg. PolyHb-SOD-CAT-CA was superior to the blood and other fluids based on the following criteria. PolyHb-SOD-CAT-CA reduced tissue pCO2 from 98 ± 4.5 mmHg to 68.6 ± 3 mmHg. This was significantly (p < 0.05) more effective than lactated Ringer's solution (98 ± 4.5 mmHg), polyHb (90.1 ± 4.0 mmHg), polyHb-SOD-CAT (90.9 ± 1.4 mmHg), blood (79.1 ± 4.7 mmHg), and polySFHb (77 ± 5 mmHg). PolyHb-SOD-CAT-CA reduced the elevated ST level to 21.7 ± 6.7% and is significantly (< 0.05) better than polyHb (57.7 ± 8.7%), blood (39.1 ± 1.5%), polySFHb (38.3% ± 2.1%), polyHb-SOD-CAT (27.8 ± 5.6%), and lactated Ringer's solution (106 ± 3.1%). The plasma cardiac troponin T (cTnT) level of polyHb-SOD-CAT-CA group was significantly (P < 0.05) lower than that of all the other groups. PolyHb-SOD-CAT-CA reduced plasma lactate level from 18 ± 2.3 mM/L to 6.9 ± 0.3 mM/L. It was significantly more effective (P < 0.05) than lactated Ringer's solution (12.4 ± 0.6 mM/L), polyHb (9.6 ± 0.7 mM/L), blood (8.1 ± 0.2 mM/L), polySFHb (8.4 ± 0.1 mM/L), and polyHb-SOD-CAT (7.6 ± 0.3 mM/L). PolyHb-SOD-CAT-CA can be stored for 320 days at room temperature. Lyophilized poly-Hb-SOD-CAT-CA can be heat pasteurized at 68F for 2 h. This can be important if there is a need to inactivate human immunodeficiency virus, Ebola virus, and other infectious organisms.
Introduction
The blood cannot be pasteurized to inactivate potential infectious agents, and this had been a major problem during the human immunodeficiency virus (HIV) crisis in the year 1989. Other epidemics like Ebola may well come at any time. In addition, accidents, natural or man-made disasters, can result in severe injuries with extensive blood loss requiring urgent blood transfusion (CitationHess et al. 2011, CitationKim and Greenburg 2014). However, much time can be lost because of the need to transport the injured to the hospital for blood group test before transfusion can be given. There is a safety window of delay for blood replacement in severe hemorrhagic shock (CitationHess et al. 2011, CitationKim and Greenburg 2014, CitationChang 1964). Beyond this safety window, there can be problems related to ischemia–reperfusion injuries and irreversible shock. There are also problems related to the transport of donor blood, since it can only be stored for 1 day at room temperature and requires refrigeration at 4°C but even then only for about 42 days. Seven-micron-diameter red blood cells (RBCs) cannot easily perfuse into constricted microcirculation in severe hemorrhagic shock or partially obstructed vessels as in heart attack and stroke.
Polyhemoglobin (polyHb) is a water-soluble oxygen carrier based on the 1964 basic principle of nanobiotechnological assembly of Hb molecules (CitationChang 1964, CitationChang 1971). There was no initial interest in this until the 1989 crisis of HIV-contaminated blood. It took 20 years to develop this and other modified Hb oxygen carriers for clinical trials (CitationGould et al. 1995, CitationChang 1997, CitationKlein 2000, CitationWinslow 2006, CitationChang 2005, CitationChang 2007, CitationJahr et al. 2008, CitationChang 2013, CitationBurhop and Estep 2001, CitationNatanson et al. 2008, CitationMoore et al. 2009, CitationBiro et al. 1995). It was too late for many patients infected with HIV from contaminated donor blood. We need to learn from this lesson and be ready in case there is another unexpected epidemic like Ebola.
Large-scale U.S. clinical trials on bovine polyHb have been carried out in surgical patients (CitationJahr et al. 2008). PolyHb has been approved for routine use in those countries, where these side effects are not as serious as the problem with insufficient supply of donor blood or HIV-contaminated donor blood (CitationKim and Greenburg 2014). A U.S. randomized clinical trial (CitationMoore et al. 2009) in more than 700 prehospital ambulance patients for hemorrhagic shock shows that human polyHb can be given on the spot without the need for crossmatching. It can delay the need for blood transfusion for 12 h, whereas the control group that was given saline required blood transfusion in 30 min. There are nonfatal cardiac side effects in 3% of the polyHb group as compared with 0.6% in the control group.
Some other types of modified Hb have more severe side effects (CitationNatanson et al. 2008). In conditions with endothelial dysfunction as in diabetes, there could be vasoconstriction (CitationYu et al. 2010). In severe ischemia as in sustained severe hemorrhagic shock, myocardial ischemia or cerebral ischemia, there is the potential for ischemia–reperfusion injuries (CitationBiro et al. 1995, CitationAlayash 2004, CitationD’Agnillo and Chang 1998, CitationPowanda and Chang 2002, CitationMa and Hsia 2013).
It is important to resolve the adverse effect for more widespread use. Especially, this is because now there is again the potential of infectious agents that can lead to epidemics and contaminated donor blood in the same way as the disastrous HIV crisis. In addition, large-scale wars, terrorisms, and major accidents that require on the spot use of blood substitutes can also happen any time. We are wiser now and know that we cannot wait for the needs to come before carrying out serious research to resolve the side effects.
PolyHb is only an oxygen carrier but in addition to the transport of oxygen, RBCs have other two important functions, removal of oxygen radicals and transport of carbon dioxide (CO2). We have shown earlier that polyHb needs antioxidant enzymes for the removal of oxygen radicals especially in ischemia–reperfusion (CitationD’Agnillo and Chang 1998, CitationPowanda and Chang 2002). This results in the removal of oxygen radicals and prevention of ischemia–reperfusion injuries (CitationPowanda and Chang 2002, CitationChang et al. 2004, CitationNadithe and Bae 2011). Superoxide dismutase (SOD) can also remove superoxide that would otherwise remove nitrate oxide to form peroxynitrate. This may help in preventing the removal of nitrate oxide that can lead to vasoconstriction in conditions with endothelial dysfunction (CitationYu et al. 2010). An extension of this is oxygen carrier with synthetic antioxidant enzymes (CitationMa and Hsia 2013).
Do we need the third function of RBC—CO2 transport (CitationGeers and Gros 2000)—for the treatment of sustained severe hemorrhagic shock? The use of a novel intracellular pCO2 microelectrode shows that in hemorrhagic shock, increase in intracellular pCO2 is related to increase in mortality (CitationSims et al. 2001). In addition, increase in tissue pCO2 is related to the severity of myocardial ischemia (CitationTronstad et al. 2010). Thus, we have added the CO2 transport function of RBC by using carbonic anhydrase (CA) to form a soluble biotechnological complex of polyHb-SOD-catalase (CAT)-CA (CitationBian et al. 2011). With adjustments of the enzyme concentrations, this complex can have these RBC functions at enhanced levels.
Our earlier in vivo studies show that polyHb-SOD-CAT-CA could reduce the elevated tissue pCO2 level in a rat model of hemorrhagic shock within the 60-min safety window (CitationBian et al. 2013). We now present our detailed research data in a 90-min severe hemorrhagic shock rat model with 2/3 blood volume loss, which is beyond the 60-min safety window for rats.
Methods
Materials and methods
The following are the places of purchase and the materials used in this experiment: Sigma: potassium phosphate, toluene, sodium phosphate, 99% lysine monohydrochloride, 25% glutaraldehyde, Sephacryl S-300HR, 37% hydrochloric acid, CAT, SOD, CA, 30% Brij 35 solution, Drabkin's solution, and 3% (w/w) hydrogen peroxide; Fisher: 1.5 × 98 cm chromatography column and Millipore 100 kDa centrifugal filter; B. Braun Medical Inc.: lactated Ringer's solution; McGill University (MacDonald campus): fresh bovine blood; and Biopure Cooperation: ultrapure bovine Hb.
Preparation of SFHb
Stroma-free hemolysate (SFHb) is the content of RBCs with their membrane stroma removed. For preparing this, we used the method described in detail elsewhere (CitationChang 2007). Fresh bovine blood with heparin was centrifuged at 4000 g for 60 min at 4°C to remove the plasma supernatant and upper layer of cell pellet. The RBCs were washed four times with sterile, ice-cold saline and then suspended in twice the volume of potassium phosphate (12.5 mM; pH, 7.4) for 30 min. Then 2 volumes of ice-cold reagent-grade toluene was used to remove stromal lipids. The sample was centrifuged at 16,000 g for 2 h at 4°C to remove cellular debris. The concentration of Hb was tested using Drabkin's solution. Our analysis shows that SFHb contains enzymes (SOD, CAT, and CA) that are normally present in RBCs ().
Table I. Composition of test liquids.
Preparation of polyHb-SOD-CAT-CA, polySFHb, polyHb, and polyHb-SOD-CAT
PolyHb-SOD-CAT-CA
PolyHb-SOD-CAT-CA was prepared by the method described in detail elsewhere (CitationBian et al. 2011). SOD, CAT, and CA were added to SFHb with a final concentration as shown in . These were crosslinked by glutaraldehyde to construct polyHb-SOD-CAT-CA. First, 1.3M lysine was added at a molar ratio of 7:1 lysine/Hb before the crosslinking reaction. The reaction vessel was flushed with nitrogen gas and kept at 4°C at all times to prevent the formation of metHb. Twenty milliliters of the mixture was placed on a shaker at 4°C for 1 h at 140 rpm. Without stopping the shaker, 0.7 ml of 5% glutaraldehyde was slowly added to it at a molar ratio of 16:1 glutaraldehyde/Hb at a rate of 0.15–0.20 mL every 5–10 min. The reaction mixture was allowed to crosslink in the shaker for 24 h at 4°C. The crosslinking was stopped by adding 2.0M lysine at a molar ratio of 200:1 lysine/Hb.
PolySFHb
PolySFHb was prepared using the same method, but using the stroma-free content of RBCs (SFHb) with the normal blood cell concentration of SOD, CAT, and CA without the addition of more enzymes.
PolyHb
PolyHb was prepared by crosslinking ultrapure Hb that contained no RBC enzymes following the above-mentioned procedure but without adding enzymes. This represented the polyHb used in clinical trial.
PolyHb-SOD-CAT
PolyHb-SOD-CAT was prepared following the first procedure mentioned above, except that ultrapure Hb was used with the additional CAT and SOD to make up the final concentration as shown in . This represented the sample with O2 transport and antioxidant functions but without CO2 transport function.
Purification and concentration
Purification and concentration were carried out as follows: polyHb-SOD-CAT-CA, polySFHb, polyHb-SOD-CAT, and polyHb were each filtered using a sterile 0.45 uM filter and dialyzed against lactated Ringer's solution overnight at 4°C using a dialysis membrane. A Sephacryl S-300 column in a 4°C cabinet was used to remove only the fraction of free tetrameric Hb with molecular weight greater than 300 Ka. The samples were placed in a dialysis membrane on a bed of Sephacryl/Gel absorbent at 4°C and concentrated to a final Hb concentration of 10 g/dL.
The measurement of Hb concentration and SOD, CAT, and CA enzyme activities
Hb concentration
The Hb concentration was determined by colorimetry.
The samples were first reacted with Drabkin's solution (Sigma-Aldrich).
Then the concentration of the resulting cyanmetHb solution was measured by spectrophotometry at 540 nm.
SOD
SOD activity was determined by HT Superoxide Dismutase Assay Kit (R&D Systems), which followed the inhibition of nitroblue tetrazolium reduction using xanthine/xanthine oxidase as a superoxide generator.
CAT
CAT measurement was based on the rate of disappearance of H2O2 as described elsewhere (CitationChang 2007).
CA
For CA measurement, one Wilbur–Anderson (W-A) unit of CA activity was defined as the amount of enzyme that caused the pH of a 0.02 M Tris buffer to drop from 8.3 to 6.3. The reaction was initiated by the addition of substrate, and the time (T) needed for the pH of the reaction mixture to drop from 8.3 to 6.3 was recorded. A Fisher Accumet Basic pH Meter (Fisher Scientific, Pittsburgh, PA) with MI-407 (P) Needle pH Electrode (Microelectrodes Inc., Bedford, NH) was used to measure the change in pH caused by the hydration reaction of CO2 catalyzed by CA. The control for the assay consisted of the same mixture without the test sample. The measurements in seconds were converted into W-A units according to the following formula:
The units were then plotted versus the Hb/CA concentration (CitationBian et al. 2011).
Effects on tissue pCO2, mean arterial pressure, and electrocardiogram in a hemorrhagic shock rat model
The Animal Ethics Committee of McGill University has approved this experimental protocol.
Sprague-Dawley rats, weighing 300 ± 30 g, were randomly divided into 6 groups and each group contained 4 rats. Each of them was anesthetized with pentobarbital of 30 mg/g body weight. The animals were freely breathing and no ventilation support or oxygen was given during the protocol. This was to simulate hemorrhagic shock, where no ventilation support is available. Heparinized catheters were inserted into the right femoral vein and right femoral artery. Mean arterial pressure (MAP) was reduced by slowly withdrawing up to 2/3 volume of blood from the right femoral artery and maintained at 30 mmHg for 90 min. After this, each rat received one of the following resuscitation fluids intravenously through the femoral vein in 15 min: (The detailed composition of each fluid is shown in ).
| (1) | Sham control group: This group received the same general anesthesia, cannulation, and continuous monitoring for blood pressure, tissue pCO2, and electrocardiogram. No blood was removed and no fluid was infused. | ||||
| (2) | Whole blood shed by rats. | ||||
| (3) | Lactated Ringer's solution, three volumes of shed blood. | ||||
| (4) | PolyHb-SOD-CAT-CA in lactated Ringer's solution equivalent to the volume of shed blood. | ||||
| (5) | PolySFHb in lactated Ringer's solution equivalent to the volume of shed blood. | ||||
| (6) | PolyHb in lactated Ringer's solution equivalent to the volume of shed blood. | ||||
| (7) | PolyHb-SOD-CAT in lactated Ringer's solution equivalent to the volume of shed blood. | ||||
MAP and electrocardiogram (ECG) were recorded using a Biopac Lab system (BIOPAC Systems Inc., Goleta, CA). Tissue pCO2 was measured by placing a pCO2 microelectrode (Lazar Research Labs, USA) on the surface of vastus medialis muscle at the incision site used for cannulation. MAP, tissue pCO2, and ECG were followed throughout the experiment and continued for 60 min after the completion of the above reinfusions. After this, the rats were given IP Nembutal to terminate these acute studies. The blood was collected at the end of the resuscitation and centrifuged at 5000 g for 10 min at 4°C to collect the plasma. The plasma was stored at − 80°C before the assay. Cardiac troponin T (cTnT) was analyzed using a cTnT assay kit. Lactate assay kit was purchase from Biosource.
Statistics
Primary endpoint is 60 min after the completion of the reinfusions of the resuscitation fluids. Data were expressed as mean ± standard deviation (SD). Analysis of variance and Student's t-test were used to assess the difference between treatment groups. Statistical significance was defined as p value of less than 0.05 to reject a null hypothesis.
Results
Effects on mean arterial blood pressure
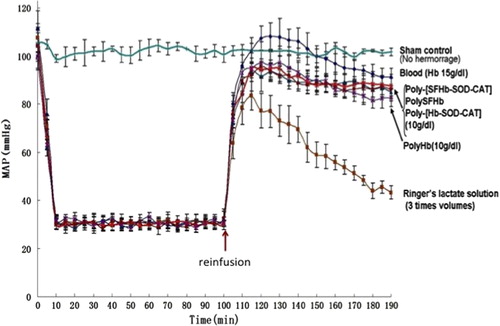
In the groups with 2/3 volume of the blood removed, the MAP fell to 30 mmHg. In each group after 90 min at MAP of 30 mmHg, reinfusions of resuscitation fluids with different compositions () had the effects that are shown in . The results of these individual groups were pooled together for comparison (). Here, a sham control group was also included. The sham control group showed no significant changes in MAP (). When transfused with 3 volumes of lactated Ringer's solution, the MAP increased transiently from 30 mmHg to 43.3 ± 2.8 mmHg after 1 h ( and ). Reinfusion of the lost blood (with Hb of 15 g/dl) maintained the MAP at 91.3 ± 3.6 mmHg. When transfused with polyHb-SOD-CAT-CA (with Hb at 10 g/dL), the MAP was maintained at 87.5 ± 5 mmHg when followed for 1 h after the infusion. PolySFHb, polyHb-SOD-CAT, and polyHb maintained the MAP at 85.0 ± 2.5 mmHg, 86.0 ± 4.6 mmHg, and 82.6 ± 3.5 mmHg, respectively. However, there were other important criteria besides maintaining the MAP, which included changes in ST elevation, tissue pCO2, plasma lactate, and the integrity of the intestine and other organs.
Figure 1. Mean arterial blood pressure. 2/3 volume of blood was removed to result in 90 min of hemorrhagic shock at MAP of 30 mmHg. After this, different fluids were reinfused. Reinfusion with 3 volumes of lactated Ringer's solution increased MAP transiently and fell to 43.3 ± 2.8 mmHg. Blood (Hb 15 g/dl) maintained the MAP at 91.3 ± 3.6 mmHg, while polyHb-SOD-CAT-CA (Hb 10 g/dL) maintained it at 87.5 ± 5 mmHg, polyHb-SOD-CAT at 86.0 ± 4.6 mmHg, polySFHb at 85.0 ± 2.5 mmHg, and polyHb at 82.6 ± 3.5 mmHg.
Effects on elevated tissue pCO2
The mean and standard deviation of each group were calculated and compared with the other groups. In the test groups with 2/3 volume of the blood removed, the baseline tissue pCO2 of 54.7 ± 2.5 mmHg increased steadily to 98 ± 4.5 mmHg by 90 min (). The effects on tissue pCO2 during the 60-min period after the reinfusions of different resuscitation fluids are shown in and . The sham control group showed no significant changes in tissue pCO2 (). The results of the comparison with other groups are as follows:
| (1) | Reinfusion of lactated Ringer's solution did not significantly (P > 0.05) reduce the tissue pCO2 level that remains at 98 ± 4.5 mmHg. | ||||
| (2) | Rats that received their shed whole blood showed significant (P < 0.05) reduction in the tissue pCO2 levels, 79.1 ± 4.7 mmHg. | ||||
| (3) | Reinfusion of polyHb (crosslinked ultrapure Hb with no RBC enzymes) did not significantly (P > 0.05) reduce the elevated tissue pCO2 levels that remained at 90.1 ± 4.0 mmHg. | ||||
| (4) | Reinfusion of polyHb-SOD-CAT (crosslinked ultrapure Hb plus the addition of antioxidant enzymes—SOD and CAT but not CA) did not significantly (P > 0.05) reduce the elevated tissue pCO2 levels that remain at 90.9 ± 1.4 mmHg. | ||||
| (5) | Reinfusion of polySFHb (crosslinking Hb and the content of RBC with the enzymes SOD, CAT, and CA at the concentration normally present in the RBC) was as efficient as blood in reducing the elevated tissue pCO2 levels to 77 ± 5 mmHg. | ||||
| (6) | PolyHb-SOD-CAT-CA was formed as in (5) above, but more SOD, CAT, and CA enzymes were added to enhance the normal RBC enzyme activities. Reinfusion reduced the elevated tissue pCO2 to 68.6 ± 3 mmHg toward the normal levels after 60 min. This was significantly more effective than blood (p < 0.05) that only reduced the tissue pCO2 to 79.1 ± 4.7 mmHg. | ||||
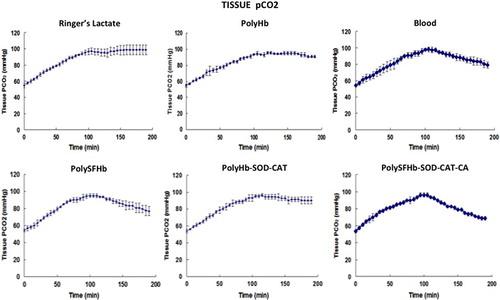
Figure 4. Comparison of pCO2. The above measurements of each group were pooled together for comparison. Sham control measurement, where no blood loss occurred, was added as control. The results showed that reinfusion with polyHb-SOD-CAT-CA reduced this to 68.6 ± 3 mmHg in 1 h. This was significantly (p < 0.05) more effective than lactated Ringer's solution (98 ± 4.5 mmHg), polyHb (90.1 ± 4.0 mmHg), polyHb-SOD-CAT (90.9 ± 1.4 mmHg), blood (79.1 ± 4.7 mmHg), and polySFHb (77 ± 5 mmHg).
Effects on ST elevation
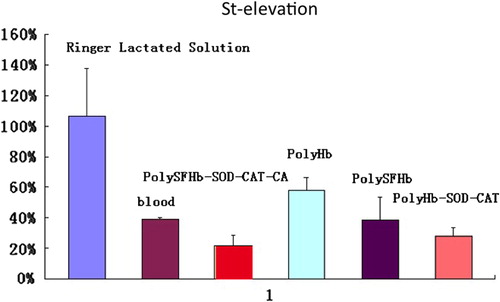
ST elevation in the ECG could be caused by ischemia, ischemia– reperfusion injury, or abnormal tissue metabolism. The results of reinfusion of different fluids are shown in . Infusion of lactated Ringer's solution did not reduce the ST as it remained at 106 ± 3.1% of the 90-min hemorrhagic shock ST level (). For polyHb, an oxygen carrier without any enzyme activities, the ST only recovered to 57.7 ± 8.7% of the elevated level. Blood reduced the ST to 39.1 ± 1.5%, while polySFHb to 38.3% ± 2.1%. PolyHb-SOD-CAT with its enhanced antioxidant enzymes reduced the ST to 27.8 ± 5.6%. PolyHb-SOD-CAT-CA reinfusion reduced the ST to 21.7 ± 6.7% of the hemorrhagic shock ST level, which is significantly better than the others (P < 0.05). This is likely because polyHb-SOD-CAT-CA has all three RBC functions at an enhanced level, that is, oxygen and CO2 transport plus antioxidant functions.
Figure 5. ST elevation. PolyHb-SOD-CAT-CA reduced the ST to 21.7 ± 6.7%. This was significantly (p < 0.05) better than polyHb (57.7 ± 8.7%), blood (39.1 ± 1.5%), polySFHb (38.3% ± 2.1%), and polyHb-SOD-CAT (27.8 ± 5.6%). Lactated Ringer's solution exhibited no significant (p > 0.05) effects (106 ± 3.1%).
Effects on plasma lactate levels
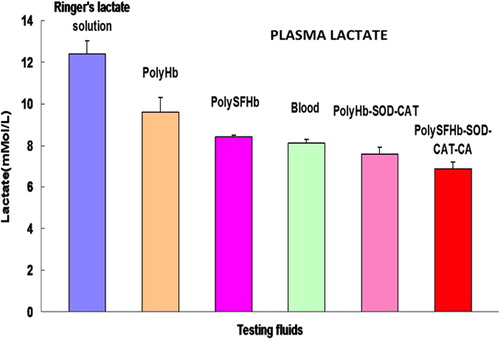
In the case of plasma lactate level, after 90 min of sustained severe hemorrhagic shock it increased to 18 ± 2.3 mM/L. PolyHb-SOD-CAT-CA reduced this to 6.9 ± 0.3 mM/L in 60 min (). It is significantly more effective (P < 0.05) than lactated Ringer's solution (12.4 ± 0.6 mM/L), polyHb (9.6 ± 0.7 mM/L), blood (8.1 ± 0.2 mM/L), polySFHb (8.4 ± 0.1 mM/L), and polyHb-SOD-CAT (7.6 ± 0.3 mM/L).
Figure 6. Plasma lactate. PolyHb-SOD-CAT-CA reduced the plasma lactate level from 18 ± 2.3 mM/L to 6.9 ± 0.3 mM/L. It was significantly (p < 0.05) more effective than lactated Ringer's solution (12.4 ± 0.6 mM/L), polyHb (9.6 ± 0.7 mM/L), blood (8.1 ± 0.2 mM/L), polySFHb (8.4 ± 0.1 mM/L), and polyHb-SOD-CAT (7.6 ± 0.3 mM/L).
Histology
Detailed results of the histological studies will be finalized and reported elsewhere. The most striking changes were in the intestine. Lactated Ringer's solution group showed damaged villi and glands. The polyHb group showed damaged villi and mild damage of glands. The blood reinfusion group showed damaged villi but intact glands. There were no signs of injury in the polySFHb, polyHb-SOD-CAT, and polyHb-SOD-CAT-CA groups. The histological results suggest that the presence of antioxidant enzymes, SOD and CAT, in the polySFHb, polyHb-SOD-CAT, and polyHb-SOD-CAT-CA could protect the intestine from ischemia–reperfusion injuries in severe 90-min hemorrhagic shock beyond the 60-min safety window. A summary of other findings can be found in .
Table II. Histology of organs 60 min after reinfusion.
Stability at different temperatures including pasteurization
PolyHb can be heat sterilized and stored for 1 year even at room temperature (CitationKim and Greenburg 2014, CitationGould et al. 1995). However, polyHb-SOD-CAT-CA contains both Hb and enzymes, and enzymes are particularly sensitive to storage and heat. We thus carried out studies to analyze its heat sterilization stability and storage stability. The detailed methods and results will be finalized and reported elsewhere. A summary of the result is as follows ():
RBCs cannot be heat sterilized. On the other hand, after heat sterilization at 68°C for 2 h, lyophilized polyHb-SOD-CAT-CA retained good enzyme activities of SOD, 100 ± 2.5%; CAT, 63.8 ± 4%; and CA, 97 ± 4%. More CAT can be added during the crosslinking process to maintain the same enzyme ratio after heat sterilization. It is important to note that heat sterilization at 68°C for 2 h is possible only for the lyophilized form of polyHb-SOD-CAT-CA and not for the solution. After sterilization, the lyophilized form can be stored as such and can be easily reconstituted by dissolving in suitable solutions. Allowable storage time for RBC is about 1 day at room temperature and 42 days at 4C. The lyophilized form of polyHb-SOD-CAT-CA can retain sufficient enzyme activities when stored at room temperature for 320 days ().
Table III. Effects of storage time and temperature on stability.
Discussions
In ischemia–reperfusion study of hemorrhagic shock, stroke or myocardial infarction in animals, researchers use an ischemic period ranging from 30 min to 1 h with variable results. Our earlier studies on ischemia–reperfusion injuries in a rat model of combined hemorrhagic shock–brain ischemia show that a minimum of 60 min is needed (CitationPowanda and Chang 2002). However, our more recent results show that 60 min is the safety window for the treatment of hemorrhagic shock in rats (CitationBian et al. 2013). Thus, in order to avoid the pitfall of a very short ischemic period, and also to carry out a more stringent test, we are using 90 min for the present study.
We show in the present study that in severe hemorrhagic shock lasting for 90 min beyond the 60-min safety window, the novel nanobiotherapeutic polyHb-SOD-CAT-CA is superior to blood and other resuscitation fluids. It is more effective than RBC in reducing tissue pCO2. This is important since increased tissue pCO2 has been related to increased mortality in hemorrhagic shock (CitationSims et al. 2001) and severity of cardiac ischemia (CitationTronstad et al. 2010). It is also more effective than blood and the other resuscitation fluids for the recovery of ST and reducing the elevated plasma lactate level. It is also more effective in protecting the intestine in the reperfusion and resuscitation process. Being a solution, it can also better perfuse into the constricted microcirculation than RBC in hemorrhagic shock or partially obstructed vessels in heart attack and stroke. The enhanced antioxidant enzymes, CAT and SOD, can remove damaging oxygen radicals. SOD also removes superoxide and prevents it from reacting and removing nitric oxide to cause vasoconstriction. The lyophilized form can be heat sterilized to remove infectious agents like HIV or future infectious agents like ebola to avoid contaminated donor blood crisis in the future. It is not specific to blood groups and the lyophilized powder can be stored at room temperature for 320 days (). The lyophilized powder can be quickly reconstituted and dissolved for use. Since it does not need refrigeration or blood group testing, medics or ambulance can easily carry it and use it on the spot. This would avoid the life-threatening delays in emergency situations like mass disasters, war, and remote regions. However, polyHb-CAT-SOD-CA is just a potential nanobiotherapeutic with enhanced functions for specific applications. It is not meant to replace donor RBC with its much longer circulation time, which is essential for other long-term elective nonemergency uses.
PolyHb-SOD-CAT-CA requires the use of enzymes that can be expensive if purchased in purified form from commercial sources. Thus, we have successfully extracted enzymes from RBC and used them in the preparation of polyHb-SOD-CAT (CitationGu and Chang 2009). We have more recently successfully extracted all three enzymes, SOD, CAT, and CA, for the pre-paration of polyHb-SOD-CAT-CA (Chen, Glynn and Chang, personal communication). This way, Hb and RBC enzymes can be extracted from outdated donor blood, placental cord blood (CitationLi et al. 2006), and from other sources, thus further utilizing RBC that would otherwise be discarded.
Our earlier study shows that polySFHb (contains enzymes at the same concentration as those in the RBCs) does not involve in immunological reactions and is not activated by anycomplement (CitationZhu et al. 2010). If the higher amounts of enzymes in the new polyHb-CAT-SOD-CA resulted in immunological reaction, then we can use human enzymes extracted from outdated human donor RBC or human placenta cord RBC (CitationLi et al. 2006), or recombinant human enzymes.
Massive fluid and RBC replacement in severe injuries can lead to dilution of platelets and clotting factors resulting in bleeding (CitationHess et al. 2011, CitationKim and Greenburg 2014, CitationMoore et al. 2009). Our earlier study in rats also shows that the clotting time is markedly increased when more than 80% of blood is exchanged with polyHb (Wong and Chang 2009). We have designed a soluble polyHb–fibrinogen complex that does not cause any increase in clotting time even when 98% of the blood has been exchanged with polyHb-fibrinogen (CitationWong and Chang 2007). If needed, we can apply this to the polyHb-SOD-CAT-CA to obtain a nanobiotherapeutic that has not only enhanced RBC functions, but also a platelet-like function.
One theory regarding vasoconstriction in endothelial dysfunction is the removal of nitrate oxide by modified Hb (CitationYu et al. 2010). Superoxide may contribute to this problem since it removes nitric oxide to form peroxynitrate. The SOD component in polyHb-CAT-SOD-CA is effective in removing superoxide. This would prevent the removal of nitrate oxide to avoid vasoconstriction. In the unlikely event that polyHb-CAT-SOD-CA still causes vasoconstriction in endothelial dysfunction, we can use one of the available Hbs that has been modified to prevent vasoconstriction (CitationLui and Kluger 2011). Other ways to prevent adverse vasoconstriction of modified Hb are also available (CitationYu et al. 2010, CitationJia and Alayash 2013).
Stem cell culture for producing blood cells (CitationMazurier et al. 2011) has much promise for platelets and leucocytes that require only small volume. However, scaling up the liters of RBC needed for clinical use is still a major problem. When scale-up becomes practical, universal donor—group O—RBC can be produced. This will be very important for many important nonurgent uses of RBC. However, we shall still need polyHb-CAT-SOD-CA for emergency uses like sustained severe hemorrhagic shock for a number of reasons ().
Table IV. Comparison of polyHb-SOD-CAT-CA with RBC.
Declaration of interest
The authors have an agreement with McGill University for a patent application on this invention to allow for future development. They do not have conflicts of interest related to any connections with commercial companies in this.
The support for this research was provided by operating grants from the Canadian Institutes of Health Research (CIHR) and the Canadian Blood Service/CIHR joint award to Professor TMS Chang.
References
- Alayash AI. 2004. Oxygen therapeutics: can we tame Hb. Nat Rev Drug Discov. 3:152–159.
- Bian Y, Rong Z, Chang TMS. 2011. PolyHb-superoxide dismutase-catalase-carbonic anhydrase: a novel biotechnology-based blood substitute that transports both oxygen and carbon dioxide and also acts as an antioxidant. Artif Cells Blood Substit Biotechnol. 39:127–136.
- Bian YZ, Wei G, Chang TMS. 2013. Lowering of elevated tissue PCO2 in a hemorrhagic shock rat model after reinfusion of a novel nanobiotechnological PolyHb-superoxide dismutase-catalase- carbonic anhydrase that is an oxygen and carbon dioxide carrier with enhanced antioxidant properties. Artif Cells Nanomed Biotechnol. 41:60–68.
- Biro GP, Ou C, Ryan-MacFarlane C, Anderson PJ. 1995. Oxyradical generation after resuscitation of hemorrhagic shock with blood or stromafree Hb. Artif Cells Blood Substit Immobiliz Biotechnol. 23:631–645.
- Burhop KE, Estep TE. 2001. Hb induced myocardial lesions. Artif Cells Blood Substit Immobil Biotechnol. 29:101–106.
- Chang EJ, Lee TH, Mun KC, Kim HC, Suh SI, Bae JH, et al. 2004. Effects of polyHb-antioxidant enzyme complex on ischemia-reperfusion in kidney. Transplant Proc. 36:1952–1954.
- Chang TMS (Ed.). 2013. Selected Topics in Nanomedicine. Singapore, London: World Science Publisher/Imperial College Press, 590 pages.
- Chang TMS. 1964. Semipermeable microcapsules. Science. 146: 524–525.
- Chang TMS. 1971. Stabilisation of enzymes by microencapsulation with a concentrated protein solution or by microencapsulation followed by cross-linking with glutaraldehyde. Biochem Biophys Res Commun. 44:1531–1536.
- Chang TMS. 1997. Red Blood Cell Substitutes: Principles, Methods, Products and Clinical Trials, Vol. I (Monograph). Karger/Landes Systems, Basel, Switzerland (available for free online viewing at www.medicine.mcgill.ca/artcell).
- Chang TMS. 2005. Therapeutic applications of polymeric artificial cells. Nat Rev Drug Discov. 4:221–235.
- Chang TMS. 2007. Monograph on “ARTIFICIAL CELLS: Biotechnology, Nanotechnology, Blood Substitutes, Regenerative Medicine, bioencapsulation, cell/stem cell therapy”. Singapore, London: World Scientific Publisher/Imperial College Press, 454 pages. (available for free online viewing/download at www.medicine.mcgill.ca/artcell).
- D’Agnillo F, Chang TMS. 1998. PolyHb-superoxide dismutasecatalase as a blood substitute with antioxidant properties. Nat Biotechnol. 16:667–671.
- Geers C, Gros G. 2000. Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol Rev. 80:681–715.
- Gould, SA, Sehgal LR, Sehgal HL, GS Moss. 1995. The development of Hb solutions as red cell substitutes:Hb solutions. Transfu Sci. 16:5–17.
- Gu G, Chang TMS. 2009. Extraction of erythrocyte enzymes for the preparation of PolyHb-catalase-superoxide dismutase. Artif Cells Blood Substit Biotechnol. 37:69–77.
- Hess JR, Johansson PI, Holcomb JB, et al. 2011. Massive transfusion and transfusion therapy in trauma In: Mintz PD, Ed. Transfusion Therapy: Clinical Principles and Practice. 3rd ed. Bethesda, Maryland: AABB Press, pp. 305–322.
- Jahr JS, Mackenzie C, Pearce LB, Pitman A, Greenburg AG. 2008. HBOC-201 as an alternative to blood transfusion: efficacy and safety evaluation in a multicenter phase III trial in elective orthopaedic surgery. J Trauma. 64:1484–1497.
- Jia YP, Alayash AI. 2013. Molecular Basis of Haptoglobin and Hb Complex Formation and Protection against Oxidative Stress and Damage in “Selected Topics in Nanomedicine” Editor TMS Chang, World Science Publisher/Imperial College Press, Singapore London 149–168.
- Kim HW, Greenburg AG (Eds.). 2014. Hemoglobin-Based Oxygen Carriers as Red Cell Substitutes and Oxygen Therapeutics. New York, London: Springer, 738 pages
- Klein HG. 2000. The prospects for red-cell substitutes. N Engl J Med. 342:1666–1668.
- Li T, Yu R, Zhang HH, Liang WG, Yang XM, Yang CM. 2006. A method for purification and viral inactivation of human placenta Hb. Artif Cells Blood Substit Biotechnol. 34:175–188.
- Lui FE, Kluger R. 2011. Reviving artificial blood: meeting the challenge of NO scavenging by hemoglobin. Chem Biochem. 11:1816–1824.
- Ma L, Hsia CJC. 2013. Polynitroxylated Hb as a multifunctional therapeutic for critical care and transfusion medicine. In: Chang TMS (Ed.). Selected Topics in Nanomedicine. Singapore, London: World Science Publisher/Imperial College Press, pp. 169–194.
- Mazurier C, Douay L, Lapillonne H. 2011. RBC from induced pluripotent stem cells: hurdles and developments. Curr Opin Hematol. 18:249–253.
- Moore E, Moore FA, Fabian TC, Bernard AC, Fulda GJ, Hoyt DB, et al. 2009. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA multicenter trial. J Am Coll Surg. 208:1–13.
- Nadithe V, Bae YH. 2011. Hb Conjugates with antioxidant enzymes(Hb-superoxide dismutase-catalase) via poly(ethylene glycol) crosslinker for protection of pancreatic beta RINm5F cells in hypoxia. Tissue Eng. 17:2453–2462.
- Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. 2008. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 299:2304–2312.
- Powanda D, Chang TMS. 2002. Cross-linked polyHb-superoxide dismutase-catalase supplies oxygen without causing blood brain barrier disruption or brain edema in a rat model of transient global brain ischemia-reperfusion. Artif Cells Blood Substit Immobiliz Biotechnol. 30:25–42.
- Sims C, Seigne P, Menconi M, Monarca J, Barlow C, Pettit J, Puyana JC. 2001. Skeletal muscle acidosis correlates with the severity of blood volume loss during shock and resuscitation. J Trauma. 51:1137–1146.
- Tronstad C, Pischke SE, Holhjem L, Tonnessen TI, Martinsen OG, Grimnes S. 2010. Early detection of cardiac ischemia using a conductometric PCO 2 sensor: real-time drift correction and parameterization. Physiol Meas. 31:1241–1255.
- Winslow RM (Ed.). 2006. Blood Substitutes. Amsterdam: Academic Press.
- Wong N, Chang TMS. 2007. PolyHb-fibrinogen: a novel blood substitutes with platelet-like activity for extreme hemodilution. Artif Cells Blood Substit Biotechnol. 35:481–489.
- Yu B, Shahid M, Egorina EM, Sovershaev MA, Raher MJ, Lei C, et al. 2010. Endothelial dysfunction enhances vasoconstriction due to scavenging of nitric oxide by a hemoglobin-based oxygen carrier Anesthesiology. 112:586–594.
- Zhu HL, Du QQ, Chen C, Chang TMS. 2010. The immunological properties of stroma-free polyhemolysate containing catalase and superoxide dismutase activities prepared by polymerized bovine stroma-free hemolysate. Artif Cells Blood Substit Biotechnol. 38:57–63.