?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Tuberculosis (TB) is one of the major devastating diseases in the world, mainly caused by Mycobacterium tuberculosis. Furthermore, multi-drug resistant TB and extremely drug resistant TB are becoming big problems globally. Bacillus Calmette-Guerin (BCG) is the only available vaccine which provides protection against TB. The BCG vaccine is effective in children but not recommended in adults and elderly patients due to an associated low risk of infection with Mycobacterium tuberculosis and variable effectiveness of the vaccine. The main aim of this research study is to develop such a vaccine which will provide a better and safer profile in children and adults, as well as in elderly patients. In this present study, we prepared pulmonary tubercular vaccine by using an Antigen 85 complex (Ag85)-loaded nanocarrier such as the immunostimulating complex (ISCOM). Immunological outcomes clearly indicated significant improvement in humoral as well as cellular immune responses after pulmonary immunization with ISCOMs containing Quil A in mice.
Introduction
Vaccines are the principal boon of biological sciences to mortals in the previous century, representing the most cost-effective and successful approach to prevent infectious diseases (CitationBaldwin et al. 2013, CitationKaur et al. 2014a, CitationGoyal et al. 2013a). In the last decade, a massive number of novel vaccination techniques have been considered based on extensive increase in the fundamental knowledge of the immune system (CitationGarg et al. 2012b). Tuberculosis (TB) is one of the major infectious diseases, which is mainly caused by the strain Mycobacterium tuberculosis. TB most commonly affects the lungs (pulmonary TB), but some other organs are also affected such as the central nervous system, the lymphatic system, circulatory system, and the genitourinary system (CitationOrme 2013, CitationGarg and Goyal 2014a). According to WHO, an estimated 8.6 million people developed TB and 1.3 million died from the disease (including 3,20,000 deaths among HIV-positive people) in 2012. Furthermore, multi-drug resistant TB (MDR-TB) and extremely drug resistant TB (XDR-TB) are becoming big world problems. By 2012, the global TB mortality rate had been reduced by 45% since 1990 due to advancement in the available drugs and vaccines, and better public awareness. DOTS (directly observed treatment, short-course) is the internationally recommended approach to control TB, diminish TB-related morbidity, thwart TB deaths, and lessen TB transmission. Bacillus Calmette-Guerin (BCG) is only available vaccine for protection against TB. It is found to be effective only in children, but not recommended in adults and elderly patients due to some reasons like low risk of infection with M. tuberculosis and the variable effectiveness of the vaccine against adult pulmonary TB (CitationChol et al. 2013). Thus, the need of the hour is to develop such a vaccine which will provide a better and safer profile in children and adults, as well as in elderly patients. In this present study, we mainly focused on the preparation of pulmonary tubercular vaccine by using Antigen 85 complex (Ag85)-loaded nanocarriers such as immunostimulating complexes (ISCOMs). Pulmonary delivery offers local targeting for the treatment of respiratory diseases and aids the development of both mucosal as well as systemic immunity against the microbes (CitationGagandeep et al. 2014). The pulmonary route is also preferable because drug efflux transporters and metabolizing enzymes are present in the lung at much lower levels than in the gastrointestinal tract (CitationCollins et al. 2012, CitationChaudhary et al. 2014, CitationGarg 2014). Ag85 is a major secretory product of actively growing M. tuberculosis that induces strong cellular and humoral immune response in infected mammals. Pulmonary vaccination of this protein induces a significantly high level of interferon-γ, interleukin-12, and interleukin-4 (CitationTang et al. 2012, CitationKaur et al. 2014b). ISCOMs are mainly composed of adjuvant active saponin, cholesterol and phospholipids which have been found to be more immunogenic (CitationGarg and Goyal 2012, Citation2014b, CitationGarg et al. 2012a). ISCOMs have been shown to induce strong antigen-specific cellular or humoral immune responses to a broad range of antigens of viral, bacterial, and parasitic origin (CitationCruz-Bustos et al. 2012, CitationGarg et al. 2014).
Materials and method
Materials
Antigen Ag85 was obtained from GensScript, USA. Quil A, Anti-Mouse IgG, mouse monoclonal antibody isotyping reagents, BCA protein estimation kit and MTT reagents were purchased from Sigma Aldrich, USA. Phosphatidylcholine, cholesterol, Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum, penicillin and streptomycin were purchased from HiMedia, Mumbai. Caco-2 cell line was purchased from NCCS, Pune.
Preparation of Antigen-loaded ISCOMs
Antigen Ag85-loaded ISCOMs were prepared by the reverse phase evaporation technique. In this technique, an organic phase was prepared by dissolving phosphatidylcholine (PC) and cholesterol (Chol) in diethyl ether. An aqueous phase was prepared by dissolving saponin (Quil A) and antigen (Ag85) in a phosphate buffer solution (PBS, pH 7.4). The organic phase was then added drop wise to aqueous phase under ultrasonication (Steryl Medi-Equip Systems, India) at amplitude 20 for 1:30 min. After ultrasonication, the solution was subjected to vortexing (Spinix, Tarsons products Pvt. Ltd. India) for 20 min to evaporate the residual organic solvent. All the samples were produced in triplicate for further analysis (CitationKo and Bickel 2012).
Characterization of ISCOMs
Size measurements of ISCOMs
The particle size, polydispersity index (PDI) and zeta- potential of antigen-loaded ISCOMs were determined by using a Zetasizer (DelsaNano C, Beckman coulter counter USA) (CitationSingh et al. 2014).
Morphological characterizations of ISCOMs
Transmission electron microscopy (TEM) was used to examine the morphology of the antigen-loaded ISCOMs prepared in this study. In this study, a drop of particle dispersion was spread onto a 200-mesh copper grid coating and the excess droplets were removed with a filter paper. After 5 min, a drop of 2% phosphotungstic acid was placed onto the copper grid. The grid was dried at room temperature and was observed by TEM, H7500 Hitachi, Japan, at SAIF, Panjab University, Chandigarh (CitationParnami et al. 2013).
Entrapment efficiency (EE) of antigen
Entrapment efficiency was determined by the minicolumn centrifugation technique. In this technique, Sephadex G-50 solution (10% w/v) was prepared in distilled water and kept aside for 24 h for complete swelling. To prepare the minicolumn, a Whatman filter pad was inserted in a 1 ml syringe and the swollen Sephadex was added carefully into it. To avoid air entrapment in the column, excess amount of normal saline was removed by spinning the column at 300 rpm for 3 min using a centrifuge (R-8C, FBLC-5910, India). The ISCOM formulation (100 μl) was slowly added on the prepared column and centrifuged at 500 rpm for 3 min, and then the same procedure was repeated by adding 100 μl of water. The remaining free drug bound to the gel when the ISCOMs were passed through the gels which were collected from the first and second stage of centrifugation. The eluted ISCOMs were diluted with PBS (pH 7.4) and treated with 0.1%v/v Triton X-100 solution to disrupt the vesicles and centrifuged for 20 min at 14,000 rpm. The percent encapsulation was calculated by quantifying the free antigen available in the supernatant by using BCA protein estimation kit (Sigma, USA). A blank formulation in the same way was used to correct the absorbance (CitationKirby et al. 2008, CitationKaur et al. 2014c).
In vitro release studies
In vitro release studies were performed using the dialysis bag technique. The dialysis membrane (molecular weight 110 kDa) was soaked in PBS (pH 7.4) for 24 h prior to use. Phosphate buffered saline, pH 7.4, was used as the dissolution medium for in vitro antigen release from the ISCOMs matrix. Three ml formulation was put into a dialysis bag and then placed into a beaker containing 50 ml PBS. The beaker was placed in shaking incubator (LSB-1005RE, Daihan Labtech Co. Ltd., Korea) (37°C) at the speed of ∼75 rpm. At predetermined time intervals, 2 ml of sample was withdrawn from the incubation medium and analyzed for protein content using the BCA protein estimation kit and standard protocol (CitationModgill et al. 2014, CitationMorie et al. 2014, CitationGoyal et al. 2013b).
Toxicological analysis
Cytotoxicity studies
The Caco-2 cell line was cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) heat inactivated fetal bovine serum. Cells were maintained in a humidified 5% CO2 incubator at 37°C.
Cell viability: MTT assay
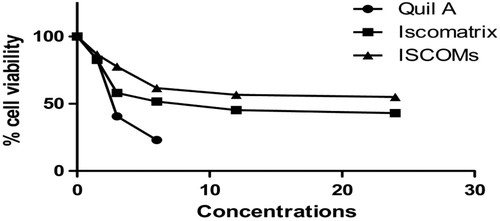
The 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide dye reduction assay was performed to determine the cytotoxic effect of the ISCOMs loaded with Quil A at various concentrations. The assay depends on the reduction of MTT by mitochondrial dehydrogenase, an enzyme present in the mitochondria of viable cells, to a blue formazan product. The cells were plated onto 96-well flat bottom culture plates at a concentration of 6 × 103 per 100 μl per well on the basis of growth characteristics of each cell line. All cultures were incubated for 24 h at 37°C in a humidified incubator. After 24 h of incubation (37°C, 5% CO2 in a humid atmosphere), 2 μl of MTT (5 mg/ml in DMSO) was added to each well, and the plate was incubated for a further 4 h at 37°C. The resulting formazan was dissolved in 200 μl dimethyl sulfoxide and absorbance of the solution was read at 570/630 nm using an ELISA plate reader (Bio-Rad, Model 680, Japan). All determinations were carried out in triplicate with varying concentrations of different formulations. Concentrations of ISCOMs showing 50% reduction in cell viability (i.e. IC50 values) were then calculated (CitationSylvester 2011).
Hemolytic activity
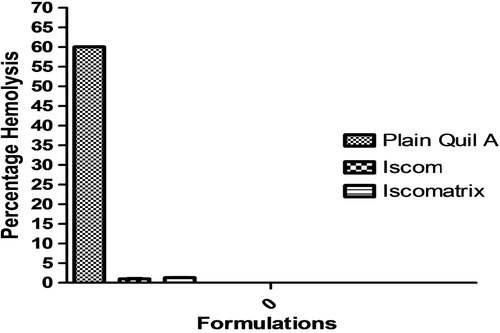
Hemolytic studies were conducted on fresh human blood. To perform the hemolytic assay, the anticoagulant EDTA was added at a concentration of 1.8 (mg/ml) to 5 ml of whole blood sampled from a healthy volunteer. Blood was centrifuged at 3000 rpm for 20 min in a REMI R4C centrifuge (Mumbai, India). The buffy coat was removed and the packed cells were washed thrice with normal saline solution. The saline was added to the cells to obtain 50% hematocrit (HCT). Hemolysis experiments were in accordance with a method used by Renata et al. (2002) with a slight modification. Briefly, to prepare the negative control, a volume of 100 μl cell suspension was added to 3 ml normal saline solution. The sample showed no hemolysis, i.e. red blood cells (RBC) had not lysed in normal tonic conditions. Likewise, 100 μl of cells were added to a second test tube and the volume was made up to 3 ml with double-distilled water. In this case, the sample showed 100% hemolysis and was used as positive control, as RBC lysed in a hypotonic medium. Plain Quil A, Iscomatrix containing Quil A, and ISCOMs containing Quil A loaded with Ag85 were separately added to a 100 μl cell suspension. All the samples were incubated at 37°C for 1 h in a water bath. The reaction was interrupted by addition of 50 μl of 2.5% glutaraldehyde, since it stops the hemolysis by abolishing hypertonic cryoheniolysis. The blood samples were then centrifuged at 3000 rpm for 15 min and the absorbance of supernatant was measured at 412 nm using a UV/VIS spectrophotometer (Schimadzu, Japan). The experiment was performed in triplicate. The percentage hemolysis was calculated applying the following equation:-
In vivo studies
The in vivo study protocol was approved by the Institutional Animals Ethical Committee and CPCSEA. The studies were carried out according to the guidelines of the Council for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India (ISF/CPCSEA/IAEC/2013-90). The description of immunization protocol is mentioned in . To study the antibody response in serum, three groups of female Balb/c mice were immunized by the pulmonary route with ISCOMs and Iscomatrix. Control groups were immunized with a fixed dose of antigen in PBS. A booster dose was administered after 21 days. Blood sampling was done from each group on days 14, 28 and 42 . The collected blood samples were allowed to coagulate, so as to separate the serum, which was stored at − 40°C until tested by ELISA for the estimation of IgG, IgG1 and IgG2a, and IgM by using an ELISA-based isotyping kit (Sigma, USA). Lung secretions were collected on day 42 by sacrificing mice and isolating their lungs and were used to determine IgA titer. All data were expressed as mean ± standard deviation.
Table I. Description of immunization protocol.
Measurement of specific IgG immunoglobulins
Antibody responses in immunized animals were monitored using a microplate ELISA. Microtiter plates were coated with 100 μl/well of 1 μg/ml Ag85 in PBS (pH 7.4) and incubated overnight at 4°C. The plates were washed three times with PBS-Tween 20 (0.5%, v/v) (PBST) and blocked with PBS-BSA (3% w/v) for 2 h at 37°C, followed by washing with PBS-T. The serum was serially diluted with PBS and 100 μl of each sample was added to each well of coated ELISA plates. The plates were incubated for 1 h at room temperature and washed three times with PBST. Next, 100 μl of peroxidase labeled Goat Anti-Mouse IgG (1:1000 dilution, Sigma, USA) was added to each well. The plates were covered and after incubation for 1 h at room temperature, washing was repeated. One hundred microliters of tetramethylbenzidine (TMB) solution was added to each well followed by addition of 50 μl of 1N H2SO4. The plate was read at 450 nm using a plate reader (Bio-Rad, USA). End point titers were expressed as the log of the reciprocal of the last dilution, which gave an optical density (OD) at 450 nm above the OD of negative controls.
Measurement of subsets of immunoglobulins (IgG)
For this study, a similar procedure was followed. In this case, the wells were coated with specific isotype reagents (100 μl/well) of mouse monoclonal antibody isotyping kit (Sigma, USA). For determination of isotypes, 1:5000 dilutions of Goat Anti-Mouse IgG1, Goat Anti-Mouse IgG2a, Goat Anti-Mouse IgM and Goat Anti-Mouse IgA were used. The coated plates were then incubated overnight at 4°C. The plates were washed three times with PBS-T followed by addition of 100 μl/well of blocking solution (3% w/v BSA) to prevent non-specific adsorption of antibodies. Appropriately diluted sample fluid (100 μl/well) was then added after the washing step. Subsequently, HRP-conjugated Goat Anti-Mouse IgG (1:500 dilutions, Sigma, USA) was added followed by addition of 100 μl of substrate (TMB) and 50 μl of 1 N H2SO4 to develop color and stop the reaction. Absorbance was read at 450 nm using a microtiter plate reader (Bio-Rad, USA) (CitationGranfors 1979).
Results and discussion
Lipid nanocarriers have been reported as useful tools in pulmonary immunization. ISCOMs containing Quil A formulations were successfully developed for pulmonary administration to enhance the antitubercular activity. Quil A used as surfactant decreased the viscosity by surrounding the lipid nanocarrier and prevented the particles from aggregation with each other.
Morphology of ISCOMs
The particle size and zeta potential of finally optimized ISCOMs formulation based on Quill A (Ag85-A) were found to be 87.1 nm and − 37 mv respectively. The values of particle size as well as zeta potential for both the formulations are indicative of the stable formulations. They were found to be safe for administration through pulmonary route due to their acceptable size. The morphologies of ISCOMs containing Quil A were observed by TEM and are shown in .
Entrapment efficiency
Optimized ISCOMs containing Quil A formulations showed a high entrapment efficiency of 79.12% due to their lipophilic nature. However drug payload depends on the solubility of antigen in the aqueous phase. Minimum drug payload was observed at the isoelectric pH. Higher drug payload was observed at pH higher than isoelectric pH. However, at a pH more than 8.6, drug payload was decreased.
In vitro release profile
The in vitro release study of the optimized formulation showed a biphasic pattern that is burst release in the initial stage followed by sustained release. Approximately 24% of antigen from Quil A-based ISCOMs was released within the first 2 h followed by the release of 57% within 48 h of study. Burst release characteristic indicated that some drug molecules were adsorbed on the particle surface. Upon immediate contact of ISCOMs with the release medium, a fraction of drugs diffused rapidly into the surrounding liquid, accounting for the rapid initial part of the release profiles. In the later stage, the release of antigen became slow and sustained due to the diffusion of drug from the core of the lipid matrix.
Toxicological analysis
Cell toxicity studies
Subjecting the formulations to the Caco-2 cell line showed that mean cell viability was found to be 75% at 1.5 μg/ml concentration whereas at 24 μg/ml, the percentage of mean cell viability was found to be 30%. The graph representation between percentage mean cell viability and concentration has been depicted in . The cell toxicity study clearly indicates that free Quil A shows significantly higher cytotoxicity over the ISCOMs and Iscomatrix. We might conclude from the results that there was no free Quil A present in the optimized formulation. Presence of Quil A helps to achieve higher membrane rigidity, which in turns minimizes the release of Quil A from the lipid matrix, which helps in low cytotoxicity in ISCOMs and Iscomatrix.
Hemolytic studies
To check the acceptability of the prepared formulation for pulmonary immunization, assessment of hemolysis was performed by incubation with isolated RBC. Positive control (Double Distilled water) showed 100% hemolysis of RBCs. The percentage of hemolysis in Plain Quil A solution was found to be 60%. The hemolysis of Quil A may be due to the lipophilic group present in the saponin interacting with the RBC. The hemolytic activity of Quil A reduced drastically after the formation of vesicles which may be due to interaction of lipid and the saponin. However, in the case of Iscomatrix containing Quil A and ISCOMs containing Quil A, the percentage of hemolysis was found to be 1% and 1.3% respectively. These results indicated that the tested formulations existed within acceptable range for pulmonary delivery. shows the comparative hemolytic profiles of all the three formulations.
In vivo studies
In vivo results of the ISCOMs showed excellent immune response, which were significantly higher than the control. The adjuvant property of the saponin is associated with the presence of structurally heterogeneous groups. Saponin influences the titer, duration, isotypes, and avidity of antibody and affects the properties of cell-mediated immunity.
Antibody titer estimation
Measurement of specific serum IgG immunoglobulin
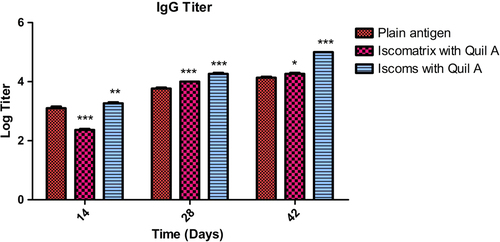
The serum IgG profile was determined for all experimental groups after 14, 28, and 42 days following immunization to evaluate the systemic immune response which was produced by the developed formulations. It was found that after 14 days following primary immunization, the IgG titer produced by Iscomatrix and ISCOMs was more in comparison to the antigen. After a booster dose on the 21 st day, Iscomatrix statistically showed a significantly higher rise in IgG titer compared to the antigen, due to better interaction of the lipophilic group of the antigen. ISCOM-based Quil A showed more penetration of the antigen, resulting an increased pattern of IgG titer after days 28 and 42. depicts the relationship between the IgG titers produced after administration of the formulations. Higher specific IgG titre in ISCOMs indicated that the antigen retained its structural integrity. This could be due to the hydrogen, hydrophobic and electrostatic interaction between the saponin and antigen. Further, the size distribution and morphology of ISCOMs play an important role in the presentation of antigen to the antigen-presenting cell. The hydrophobic and hydrophilic characters of ISCOMs are particularly useful to improve the antigen avidity for the antigen-presenting cell through MHC receptors. Experimental results were further supported by the earlier literature in which the adjuvant properties of ISCOMs were well established. The size distribution of ISCOMs is particularly important to minimize mucociliary clearance, where free amino groups of saponin help interaction with negatively charged phospholipids of the mucosal cells. The above process helps to retain the carrier systems at the target site in Iscomatrix. There is a non-specific interaction between Quil A and antigen, leading to poor interaction between the antigenic epitopes and the T-cell receptor. Further, the weak interaction between antigen and saponin leads to faster clearance of antigen from the target site, resulting in poor immunological response.
Figure 4. IgG titer after 14, 28 and 42 days following pulmonary immunization. Statistical analysis was carried out by two-way analysis of variance followed by post-hoc Bonferroni post-tests comparing all vs. control. Data compared with plain antigen as control. ‘*’ denotes P < 0.05, considered as significant, ‘**’ denotes P < 0.01 and ‘***’ denotes P < 0.001.
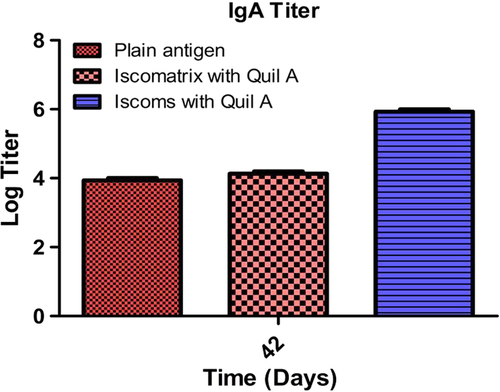
Mucosal IgA response
Tuberculosis is primarily associated with the mucosal site, so mucosal immunity is a critical parameter to access the immunological performance of the developed vaccine. depicts IgA levels produced in the lung secretions after 42 days. It was observed from the data that the ISCOMs produced maximum mucosal immune response due to the immuno modulator activity. All Quil A-based formulations showed an increased IgA titer. ISCOMs showed a slight increase in the titer in comparison to the plain antigen but the response was fairly low. Developed ISCOMs and Iscomatrix show significantly higher IgA responses over free antigens. The higher IgA titer in ISCOMs could be attributed to preferential localization of antigen at the target site. Further, the association of Quil A and antigen in close proximity helps in proper presentations of antigenic epitopes to the antigen-presenting cell. Increased avidity of the antigen with the APC stimulate the singling pathway for the activation of T helper cells, with subsequent increase in the B cell population, resulting in higher IgA turnover. Further, the adjuvant activity of Quil A is associated with the secretion of TGF-β, causing transformation of B cells for the production of IgA. However, non-specific interaction of Quil A and antigen in the Iscomatrix leads to rapid clearance of antigen from the target site. Non-specific interaction further leads to improper presentation of antigenic epitopes to antigen-presenting cells, probably, a major concern for poor IgA titer in the Iscomatrix. The least IgA titer associated to pure antigen is attributed to lack of adjuvant activity and fast clearance of antigen from the target site.
IgM response
depicts IgM response in serum immune responses for all the formulations after days 14, 28 and 42 following primary immunization. The data reveal that the IgM responses are higher after 14 days than after 28 and 42 days following primary immunization, which suggests that IgM is dominant in primary immune responses. Iscomatrix and ISCOMs formulations produced statistically significant response after the 14th day. These results confirm an efficient adjuvant character of the formulations on the IgM response and absolute physical association between antigen and adjuvant for the potentiation of IgM response. A comparable low IgM titer in all formulations followed by secondary immunization is associated with TGF-β. As discussed in the above section, TGF-β serves as a major singling pathway for the transformation of B cell for the production of IgA from IgM. Immunological responses are followed by secondary immunization, clearly shown in a positive shift in IgA titer with a subsequent reduction in the value of IgM titer. We infer a decrease in population of B-cell iso types responsible for IgM production.
Figure 6. IgM titer after 14, 28 and 42 days following pulmonary immunization. Statistical analysis was carried out by two-way analysis of variance followed by post-hoc Bonferroni post-tests comparing all data vs. control. Data compared with plain antigen as control. ‘*’ denotes P < 0.05, considered as significant, ‘**’ denotes P < 0.01 and ‘***’ denotes P < 0.001.
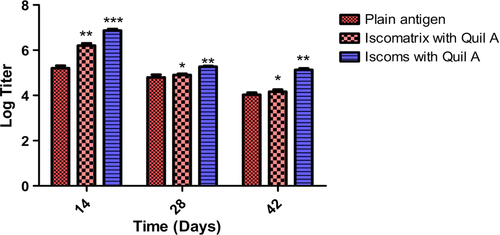
IgG isotyping (IgG1 and IgG2a)
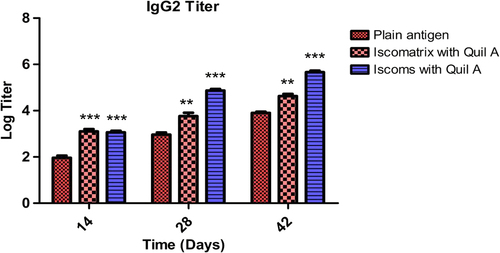
To generate a complete immune response, the system should be able to generate the humoral and cellular immunity. In the study, the IgG1 and IgG2a isotypes were determined. It was found that after 42 days, Iscomatrix and ISCOM formulations produced a high level of IgG1 which indicates good humoral as well as cellular response (). All formulations showed an increased IgG2a titer in comparison to plain antigen after 42 days (). Thus, data reveal that Iscomatrix and ISCOM formulations showed adjuvanticity which generates both humoral and cellular immune response in mice. The results of IgG isotyping clearly demonstrated that Quil A in ISCOMs and Iscomatrix is capable of inducing both humoral and cellular immunological responses. Further, the higher IgG1 and IgG2 associated with ISCOMs could be attributed to localized antigen at the target site and better presentation of antigen to the antigen-presenting cell.
Figure 7. IgG1 titer after 14, 28 and 42 days following pulmonary immunization. Statistical analysis was carried out by two-way analysis of variance followed by post-hoc Bonferroni post-tests comparing all data vs. control. Data compared with plain antigen as control. ‘*’ denotes P < 0.05, considered as significant, ‘**’ denotes P < 0.01 and ‘***’ denotes P < 0.001.
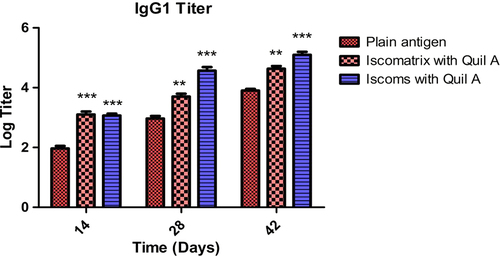
Figure 8. IgG2a titer after 14, 28 and 42 days following pulmonary immunization. Statistical analysis was carried out by two-way analysis of variance followed by post-hoc Bonferroni post-tests comparing all data vs. control. Data compared with plain antigen as control. ‘*’ denotes P < 0.05, considered as significant, ‘**’ denotes P < 0.01 and ‘***’ denotes P < 0.001.
Conclusions
Immunological outcomes clearly indicated significant improvement in immune response after pulmonary immunization with ISCOMs containing Quil A in mice. The antigen-based formulation directly transports antigen to the antigen-presenting cells (macrophages or dendritic cells), which in turn present and process the antigen of interest in a better way and secrete various cytokines to augment humoral as well as cellular immune responses. The present study suggests enhanced humoral as well as cellular response but it needs an extensive work to prove the scientific and clinical implication of this system for tubercular vaccination.
Acknowledgement
The author Dr. Amit K. Goyal is thankful to the Department of Biotechnology (DBT), New Delhi, INDIA for providing financial assistance to carry out research.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Baldwin SL, Ching LK, Pine SO, Moutaftsi M, Lucas E, Vallur A, et al. 2013. Protection against tuberculosis with homologous or heterologous protein/vector vaccine approaches is not dependent on CD8 + T cells. J Immunol. 191:2514–2525.
- Chaudhary S, Garg T, Murthy RS, Rath G, Goyal AK. 2014. Recent approaches of lipid-based delivery system for lymphatic targeting via oral route. J Drug Target. 1–12.
- Chol C, Guy C, Jacquet A, Castot-Villepelet A, Kreft-Jais C, Cambazard F, et al. 2013. Complications of BCG vaccine SSI® recent story and risk management plan: the French experience. Pharmacoepidemiol Drug Saf. 22:359–364.
- Collins SL, Chan-Li Y, Hallowell RW, Powell JD, Horton MR. 2012. Pulmonary vaccination as a novel treatment for lung fibrosis. PLoS One. 7:e31299.
- Cruz-Bustos T, Gonzalez-Gonzalez G, Morales-Sanfrutos J, Megia-Fernandez A, Santoyo-Gonzalez F, Osuna A. 2012. Functionalization of immunostimulating complexes (ISCOMs) with lipid vinyl sulfones and their application in immunological techniques and therapy. Int J Nanomed. 7:5941–5956.
- Gagandeep Garg T., Malik B, Rath G, Goyal AK. 2014. Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur J Pharm Sci. 53:10–16.
- Garg T. 2014. Current nanotechnological approaches for an effective delivery of bio-active drug molecules in the treatment of acne. Artif Cells Nanomed Biotechnol. 1–8.
- Garg T, Goyal AK. 2012. Iontophoresis: Drug delivery system by applying an electrical potential across the skin. Drug Deliv Lette. 2:270–280.
- Garg T, Goyal AK. 2014a. Biomaterial-based scaffolds–current status and future directions. Expert Opin Drug Deliv. 11:767–789.
- Garg T, Goyal AK. 2014b. Liposomes: Targeted and controlled delivery system. Drug Deliv Lett. 4:62–71.
- Garg T, Goyal AK, Arora S, Murthy R. 2012a. Development, optimization & evaluation of porous chitosan scaffold formulation of gliclazide for the treatment of Type-2 diabetes mellitus. Drug Deliv Lett. 2:251–261.
- Garg T, Rath G, Goyal AK. 2014. Ancient and advanced approaches for the treatment of an inflammatory autoimmune disease-psoriasis. Crit Rev Ther Drug Carrier Syst. 31:331–364.
- Garg T, Singh O, Arora S, Murthy R. 2012b. Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst. 29:1–63.
- Goyal AK, Rath G, Garg T. 2013a. Nanotechnological approaches for genetic immunization. In: Erdmann VA & Barciszewski J, Eds. DNA and RNA Nanobiotechnologies in Medicine: Diagnosis and Treatment of Diseases Berlin: Springer, pp. 67–120.
- Goyal G, Garg T, Malik B, Chauhan G, Rath G, Goyal AK. 2013b. Development and characterization of niosomal gel for topical delivery of benzoyl peroxide. Drug Deliv.
- Granfors K. 1979. Measurement of immunoglobulin M (IgM), IgG, and IgA antibodies against Yersinia enterocolitica by enzyme-linked immunosorbent assay: persistence of serum antibodies during disease. J Clin Microbiol. 9:336–341.
- Kaur M, Garg T, Rath G, Goyal AK. 2014a. Current nanotechnological strategies for effective delivery of bioactive drug molecules in the treatment of tuberculosis. Crit Rev Ther Drug Carrier Syst. 31:49–88.
- Kaur M, Malik B, Garg T, Rath G, Goyal AK. 2014b. Development and characterization of guar gum nanoparticles for oral immunization against tuberculosis. Drug Deliv.
- Kaur R, Garg T, Das Gupta U., Gupta P, Rath G, Goyal AK. 2014c. Preparation and characterization of spray-dried inhalable powders containing nanoaggregates for pulmonary delivery of anti-tubercular drugs. Artif Cells Nanomed Biotechnol. 1–6.
- Kirby DJ, Rosenkrands I, Agger EM, Andersen P, Coombes AG, Perrie Y. 2008. PLGA microspheres for the delivery of a novel subunit TB vaccine. J Drug Target. 16:282–293.
- Ko YT, Bickel U. 2012. Liposome-encapsulated polyethylenimine/ oligonucleotide polyplexes prepared by reverse-phase evaporation technique. AAPS PharmSciTech. 13:373–378.
- Modgill V, Garg T, Goyal AK, Rath G. 2014. Permeability study of ciprofloxacin from ultra-thin nanofibrous film through various mucosal membranes. Artif Cells Nanomed Biotechnol. 1–6.
- Morie A, Garg T, Goyal AK, Rath G. 2014. Nanofibers as novel drug carrier – An overview. Artif Cells Nanomed Biotechnol. 1–9.
- Oda K, Matsuda H, Murakami T, Katayama S, Ohgitani T, Yoshikawa M. 2000. Adjuvant and haemolytic activities of 47 saponins derived from medicinal and food plants. Biol Chem. 381:67–74.
- Orme IM. 2013. Vaccine development for tuberculosis: current progress. Drugs. 73:1015–1024.
- Parnami N, Garg T, Rath G, Goyal AK. 2013. Development and characterization of nanocarriers for topical treatment of psoriasis by using combination therapy. Artif Cells Nanomed Biotechnol.
- Singh H, Sharma R, Joshi M, Garg T, Goyal AK, Rath G. 2014. Transmucosal delivery of Docetaxel by mucoadhesive polymeric nanofibers. Artif Cells Nanomed Biotechnol.
- Sylvester PW. 2011. Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Methods Mol Biol. 716:157–168.
- Tang X, Deng W, Xie J. 2012. Novel insights into Mycobacterium antigen Ag85 biology and implications in countermeasures for M. tuberculosis. Crit Rev Eukaryot Gene Expr. 22:179–187.