Abstract
The purpose of the present work is to improve the antimalarial activity of the drug arteether (AE) by enhancing its solubility, by loading it into a nanostructured lipid carrier (NLC). The in vitro drug release profile of the NLC showed 93% drug release in 32 h. Complete eradication of the parasite from the blood and efficient anti-malarial pharmacological activity were observed, with the use of AE-loaded NLCs. These results suggest that nanolipid carriers of AE could be a promising carrier system to deliver the poorly soluble antimalarial drug through the oral route, by enhancing its solubility as well as preventing its degradation in the stomach.
Introduction
Malaria has had a greater impact on world history than any other infectious disease. More than 300 to 500 million individuals worldwide are infected with the Plasmodium species, and 1.5 to 2.7 million people a year, most of whom are children, die from this infection. Malaria is endemic in over 90 countries, which have a combined population of 2400 million people; this represents 40% of the world's population (CitationOshaghi et al. 2011). Malaria is a protozoan infection of the red blood cells, caused in humans by four species of the genus Plasmodium (Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale) (CitationCrawley and Nahlen 2004). P. falciparum causes the most severe disease, and is responsible for most malaria-related deaths (CitationGkrania-Klotsas and Lever 2007). The drug of choice for most of the last 300 years has been the cinchona alkaloid, quinine (CitationDondorp and Day 2007). Its replacement by chloroquine was reversed when widespread resistance to chloroquine precluded its further use for this indication. However, since Chinese scientists rediscovered and developed the artemisinin derivatives, antimalarial drug treatment has been changing at a fast pace. These drugs are rapidly parasitocidal, and unlike quinine, kill young circulating parasites, preventing their further maturation and sequestration (Dondorp et al. 2007). Arteether (AE) is one of the derivatives of artemisinin compounds, which induce a very rapid reduction of parasitemia, starting almost immediately after administration. AE is a semi-synthetic derivative of artemisinin, which is an active constituent of the plant, Artemisia annua (CitationKlayman 1985). It has a schizonticidal activity and is active against Plasmodium falciparum, with a low recrudescence rate. It is used in the case of chloroquine-resistant malaria, as well as in cerebral malaria (CitationBalint 2001), (CitationFerone 1977). The solubility of the drug is very low (17 μg/ml) in water, and moreover, 40% of the drug degradates in the stomach, which leads to poor bioavailability of the drug (CitationKrishna and Flanagan 1989). AE falls under the BCS Class II category. Its high solubility in oil and more lipophilic nature, mean that AE would also be able to readily cross the blood-brain barrier and effectively control cerebral malaria, which is primarily caused by the blockade of cerebral microcapillaries by the parasites (CitationDutta and Tripathi 2003). It has some gametocidal action too, which aids in cutting down the transmission of falciparum malaria. It has a long elimination half-life (> 20 h) and is more stable and more lipophilic than the other artemisinin compounds, and hence, is a potential drug for the treatment of cerebral malaria. As the solubility is limited, there is a need to increase the solubility of the drug, which ultimately leads to an increase in the bioavailability of the drug. For these reasons, it is necessary to enhance the solubility of AE. Nanostructured lipid carriers (NLCs) are considered a smart generation of nanoparticles, to enhance the solubility of poorly water-soluble drugs. Furthermore, they have improved properties for drug-loading, and also show prolonged release action. NLCs have an irregular structure, which offers more space for accommodation of the drug molecule.
Thus, the rationale of the study is to enhance the solubility by dissolving the AE in liquid lipid (oleic acid) separately, and using it in the preparation of NLCs. This will lead to an enhancement of the solubility of the drug, and hence an oral dosage form can be prepared.
Materials and methods
Materials
Glyceryl monostearate and Oleic acid were purchased from CDH (P) Ltd., New Delhi. Pluronic F-68 as surfactant was purchased from HiMedia. β– Cyclodextrin and Polyvinylpyrrolidone were purchased from HiMedia. Arteether pure drug was obtained from Cipla Ltd., Baddi. The P.berghei strain was obtained from National Institute of Malaria Research (NIMR), Delhi. Swiss albino mice were procured from the animal house of ISF College of Pharmacy, Moga (Punjab), India.
Method
Preparation of the nanolipid carrier
The NLCs of AE were prepared by the emulsification and ultrasonication methods. Both the phases (lipid and aqueous) were prepared separately. The surfactant was dissolved in aqueous phase (25 ml) by adding it to water and stirring continuously under magnetic stirring. The lipid phase was prepared by mixing the required amount of solid and liquid lipids (glyceryl monostearate and oleic acid) at a temperature of 70°C. AE, being poorly water-soluble, was added to the lipid phase. Now, both the phases, having the same temperature, were combined by adding the aqueous phase into the lipid phase. The mixture was now homogenized for 10 min at 8000 rpm. The prepared emulsion was then sonicated for 2 min for further reduction in size. Finally, the formulation was refrigerated at 2°C–5°C for 15–20 min (CitationVohra et al. 2013).
Optimization
Nanolipid carrier
Different process parameters viz., homogenization time, concentration of surfactant and (solid/liquid) lipid ratio were optimized on the basis of the particle size, polydispersity index (PDI) and zeta potential of nanolipid carriers.
Characterization of the nanolipid carrier
Morphology
A scanning electron microscope (SEM) is a type of electron microscope that images a sample by scanning it with a beam of electrons in a raster scan pattern. The electrons interact with the atoms that make up the sample, producing signals that contain information about the sample's surface topography, composition, and other properties such as electrical conductivity. A drop of sample was placed on the SEM sample holder and sputter-coated with platinum. An accelerating voltage of 20 kV was employed to take the SEM images. The SEM images were then used for the determination of average diameter of the particles.
Particle size and distribution
The Particle size and polydispersity index (PDI) of the optimized formulation was determined by laser diffractometry using the Beckman Coulter DelsaNano C particle analyzer.
Zeta potential
The charge on the particle determines the physical stability of the nanolipid carrier. It is determined by measuring the mobility of particles in an electric field. Formulations having high zeta potential (negative or positive) are considered stable. It is measured using the Beckman Coulter DelsaNano C ZetaSizer.
Differential scanning calorimetry
Differential scanning calorimetry (DSC) is a technique in which the degree of crystallinity and the polymorphic transitions causing energy change, are studied. The transition of arteether-loaded nanolipid carriers was analyzed by DSC (DSC model, Netzsch DSC 204) in a perforated aluminum sealed pan at a heating rate of 10° C/min, over a temperature range of 30°C – 300°C, using a blanket gas.
Entrapment efficiency
The percentage of drug entrapment was determined and expressed as the ratio of experimentally measured amount of drug in the dispersion, and the initial amount used for entrapment. Five ml of NLC suspension was taken in a centrifugation tube. Then, after it was centrifuged at 10,000 rpm for 20 min, it was assayed for drug content by UV spectrophotometry at 254 nm, after acidic degradation.
In vitro drug release
In vitro drug release studies were done with a dialysis membrane, using a magnetic stirrer at a rotation speed of 50 rpm. A phosphate buffer having the pH 6.8 was used as the dissolution medium. The dialysis membrane of the size (12 kDa, Sigma Chemicals) was loaded with 2 ml of nanolipid carrier. The temperature of the dissolution medium was set to 37°C. The volume of the medium was 100 ml. Samples equivalent to 3 ml were withdrawn at predetermined time intervals and were replaced with same volume. The samples were assayed for drug content at 254 nm, after acidic degradation using the UV-visible spectrophotometer, as described earlier.
In vivo studies: evaluation of pharmacological antimalarial activity of arteether-loaded NLC
Five to six week-old mice (25–30 g) were procured and maintained in an animal house, as approved by the Animal Ethics Committee. The plasmodium strain Plasmodium berghei, purchased from National Institute of Malaria Research, was used in the activity. All the mice were treated with a RBCs infected with 106 P.berghei, intraperitoneally. On every alternate day, the mean percentage of parasitemia was calculated for each group, for up to 30 days. Blood samples were taken from the tail vein, fixed in methanol, and then stained with Giemsa stain. A minimum of 500 cells were counted.
Mean percent parasitemia = infected RBCs × 100/Total no. of RBCs
The animals were divided into 3 groups comprising 6 animals (n = 6) each. Each animal was treated with a dose of (5 mg/kg) on the 4th day of PI for 7 days.
| 1) | Group 1- treated with CMC suspension | ||||
| 2) | Group 2- treated with arteether | ||||
| 3) | Group 3- treated with arteether-loaded NLC | ||||
Statistical Analysis
The data were expressed as mean ± S. D., and parasitemia as well as the in vitro drug release of the AE-NLCs were statistically assessed by one-way ANOVA, followed by Turkey's test, using the Jandel SigmaStat version 2.0.
Results and discussion
Optimization
The process parameters such as homogenization speeds, concentration of surfactant and ratio of solid and liquid lipid, were optimized at various levels, taking the particle size, PDI, and zeta potential as parameters ( and ).
Table I. Optimization of variable parameters.
Table II. Optimization of amount of drug. The optimized formulation F8 with lipid ratio (7:3), 200mg surfactant and homogenization speed 8000 rpm was furthur optimized for drug concentration.
Characterization of the nanolipid carrier
Morphology
The optimized formulation was visualized under SEM for surface morphology. SEM photographs revealed that nanolipid carriers are spherical in shape. The SEM image of the nanolipid carrier of AE is shown in .
Particle size and distribution
The particle size in the optimized formulation of AE was determined by the Zetasizer, mentioned in and . The particle size in the optimized formulation was found to be 227.4 nm, having a PDI of 0.275.
Particle charge
An observation of the particle charge is useful in the evaluation of stability of the formulation. The zeta potential of the optimized formulation was − 41.05 mV.
Differential Scanning Calorimetry
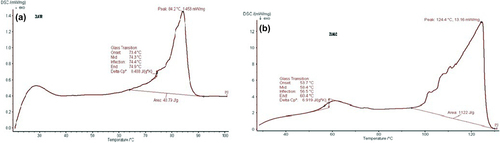
DSC gives information about the order of crystallinity, polymorphism and crystallization behavior of nanolipid carriers. The DSC of NLC () showed the complete absence of an AE peak, which indicates complete solubilization of AE inside the lipid matrix of NLC (CitationPople and Singh 2011).
Entrapment efficiency
The entrapment efficiency of the AE-loaded NLC was estimated four times (). Entrapment efficiency was found to be 84%, showing an excellent result. This might be due to imperfections in the lipid crystals, which offer more space to accommodate the drug. The addition of a lipophilic solubilizer might have resulted in the formation of imperfect crystals, causing complete immobilization of the drug, by avoiding the ejection of the drug during the cooling phase of the formulation (CitationWestesen et al. 1997).
In vitro drug release
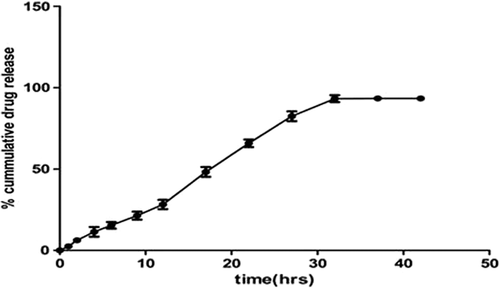
The cumulative release of AE from NLC, at 37° C, is shown in . In the first 12 h, about 28% of the drug was released from AE-NLC. Thereafter, the release rate of AE was faster for upto 32 h, when the maximum drug release of upto 93% was achieved, showing prolonged release action of the NLCs. shows that the drug release through the NLCs follow zero-order kinetics with an R2 of 0.964, thus ascertaining the controlled release.
In Vivo antimalarial activity of AE-Loaded NLC
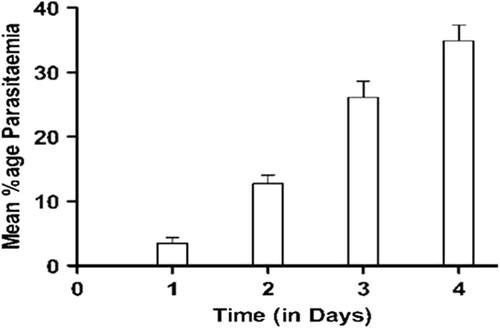
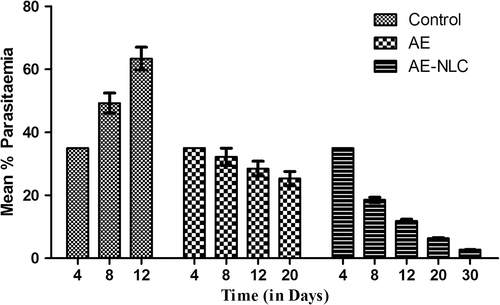
In vivo anti-malarial activity was tested with respect to mean percentage parasitemia and survival rate. Mice treated with AE-NLC have significantly prolonged survival periods when compared to control and AE groups. The control group showed the highest parasitemia and the control mice died within 10–12 days. On the other hand, AE-NLC resulted in the survival of almost 85% of infected mice even after 30 days. AE-NLC resulted in almost complete clearance of the parasite from the mice. On administration of AE alone, the mice survived for 14–19 days only ( and ). Thus, the study depicted that AE-NLC was the most effective, with lowest mean parasitemia and a prolonged survival period of more than 30 days ().
Table III. Mean percent parasitemia in mice infected with P. berghei after treatment.
Conclusion
In the present study, we developed an NLC system for the drug arteether, to solve the issue related to its poor solubility. According to the data, it was found that the release of AE from NLC occurred for more than 42 h, showing a sustained release action. By first incorporating AE into the liquid lipid of NLC, there is an enhancement in the solubility. By comparing the antimalarial activity of AE-NLC with that of the plain drug, it was found that the formulation had excellent antimalarial activity. The study showed that the survival period of the animal group which received the developed formulation was prolonged, as complete eradication of parasite was observed. This study has shown a successful approach for the development and optimization of arteether administered orally.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Balint GA. 2001. Artemisinin and its derivatives: an important new class of antimalarial agents. Pharmacol Ther. 90:261–265.
- Crawley J, Nahlen B. 2004. Prevention and treatment of malaria in young African children. Semin Pediatr Infect Dis. Elsevier.169–180.
- Dondorp AM, Day NP. 2007. The treatment of severe malaria. Trans R Soc Trop Med Hyg. 101:633–634.
- Dutta G, Tripathi R. 2003. New Antimalarial Drug Development in India: Arteether oz/[5-a Blood Schizontocide. Proc Indian natn Sci Acad. B69 No 861–870.
- Ferone R. 1977. Folate metabolism in malaria. Bull World Health Organ. 55:291.
- Gkrania-Klotsas E, Lever AM. 2007. An update on malaria prevention, diagnosis and treatment for the returning traveller. Blood Rev. 21:73–87.
- Klayman DL. 1985. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 228:1049–1055.
- Krishna AK, Flanagan DR. 1989. Micellar solubilization of a new antimalarial drug, β-arteether. J Pharma Sci. 78:574–576.
- Oshaghi M, Vatandoost H, Gorouhi A, Abai M, Madjidpour A, Arshi S, et al. 2011. Anopheline species composition in borderline of Iran–Azerbaijan. Acta Trop. 119:44–49.
- Pople PV, Singh KK. 2011. Development and evaluation of colloidal modified nanolipid carrier: application to topical delivery of tacrolimus. Eur J Pharm Biopharm. 79:82–94.
- Vohra T, Kaur I, Heer H, Murthy RR. 2013. Nanolipid carrier-based thermoreversible gel for localized delivery of docetaxel to breast cancer. Cancer Nanotechnol. 4:1–12.
- Westesen K, Bunjes H, Koch M. 1997. Physicochemical characterization of lipid nanoparticles and evaluation of their drug loading capacity and sustained release potential. J Control Release. 48:223–236.