Abstract
Lung carcinoma is the most widespread type of cancer worldwide, and is responsible for more deaths than other types of cancer. Lung cancer remains the chief cause of cancer-related deaths in both men and women worldwide, and is increasingly common in women. Each year, the number of deaths from lung cancer is greater than the number due to breast and colorectal cancer combined. Lung cancer accounted for 13% (1.6 million) of the total cases and 18% (1.4 million) of the deaths in 2008. In Iran, lung cancer is one of the five leading tumors. Among females, it was the fourth most commonly diagnosed cancer, and the second leading cause of cancer death.
Nanotechnology can be defined as the science and engineering involved in the design, characterization, and application of materials and devices whose smallest functional organization in at least one dimension is on the nanometer scale, i.e. one billionth of a meter. It is an exciting multidisciplinary field that involves the design and engineering of nano objects or nanotools with diameters less than 500 nanometers (nm), and it is one of the most interesting fields of the 21st century. Nanotechnology also offers the ability to detect diseases, such as tumors, much earlier than ever imaginable. This article presents nano devices for lung cancer detection and drug delivery systems.
Introduction
Lung carcinoma is the most widespread type of cancer in the world (CitationGao et al. 2012). Lung cancer is the most common fatal cancer in terms of both occurrence and death, with 1.04 million new cases per year and 921000 deaths in the world, with the highest rates presently observed in Europe and North America (CitationGuessous et al. 2007). Each year, the number of deaths due to lung cancer is greater than the number of deaths from breast, prostate, and colorectal cancer combined (CitationWarner et al. 2010). The 2 basic types of lung cancer are small cell lung cancer (SCLC) and non-SCLC (NSCLC). NSCLC accounts for nearly 85% of all cases of lung cancer (CitationMolina et al. 2008, CitationRay et al. 2009, CitationWang et al. 2012, CitationRamalingam and Belani 2008, CitationGaspar et al. 2012, CitationSher et al. 2008). SCLC accounts for nearly 15% of new cases of lung cancer diagnosed annually, and is responsible for up to 25% of lung cancer deaths each year (CitationSher et al. 2008). Among females, it is the fourth most universally recognized cancer and the second leading cause of cancer death (CitationJemal et al. 2011, CitationUkraintseva et al. 2008). Cigarette smoking has been identified as the single most dominant cause of the lung cancer epidemic (CitationGao et al. 2012, CitationSher et al. 2008, CitationAlberg et al. 2005, CitationAlberg and Samet 2003, CitationGanti and Mulshine 2006). However, other causes have been found, including workplace factors (e.g. exposure to asbestos, arsenic, chromium, nickel and radon) and other surrounding factors (exposure to passive smoking, indoor radon, and air pollution) (CitationJemal et al. 2011, CitationAlberg et al. 2005, CitationLam et al. 2001). In some developing countries, exposure to smoke from cooking stoves and fires is associated with the risk of lung cancer (CitationAlberg and Samet 2003).
In today's society, rapid and early diagnosis of lung cancer is a part of the therapeutic approach, and is important to facilitate rapid treatment (CitationPeng et al. 2008, CitationBiju et al. 2008). Researchers have sought out screening tests to detect lung cancer in the earliest stages, and several promising new approaches have been proposed for this purpose, such as computer-assisted chest radiographs, spiral computed tomography (CT) scanning, PCR-based assays of sputum, and fluorescence bronchoscopy. (CitationHorvath et al. 2009). Breath testing has been known as a non-intrusive medical method that might allow for the diagnosis of lung cancer (CitationPeng et al. 2008, CitationHorvath et al. 2009, CitationPreti et al. 1988, CitationGordon et al. 1985). Surgery is the preferred form of treatment for Stage I NSCLC (CitationKaskowitz et al. 1993). Effective treatment, such as photodynamic therapy, cryotherapy, YAG [yttriumaluminum- garnet] laser therapy, and electrocautery, which can eradicate carcinoma and microinvasive lung cancers in situ, is now available in addition to surgery. For stage I lung cancer, the Memorial, Hopkins, and Mayo studies showed that approximately 70% of the patients treated surgically survived more than 5 years compared with only 2% of those who did not have surgery. The 5-year survival of patients with stage IA lung cancer discovered by spiral CT is even better, at more than 80% (CitationPastorino 2006). However, current treatments for lung cancer have shown little success, because they cannot cure disseminated tumors with an acceptable level of toxicity. Thus, one alternative strategy that has shown promise in the treatment of lung cancer is targeted therapy. Drug delivery to the lungs appears to be an attractive proposition, on account of the large surface area of the alveolar region. It provides enormous opportunities to improve drug therapies using novel drug delivery systems (CitationKurmi et al. 2011). Nanoparticles could provide the advantage of sustained release in the lung tissue, and thus the systemic circulation, resulting in a reduction in dosage frequency, and improved patient compliance (CitationSung et al. 2007). Nanotechnology is an exciting multidisciplinary field that comprises the design and engineering of nano objects or nanotools less than 500 nanometers (nm) in size –(CitationCuenca et al. 2006, CitationSilva 2004, CitationCai et al. 2008, CitationTomoda et al. 2009). It is also one of the most rapidly rising fields in the 21st century (CitationMody 2011). The first time the concept of nanotechnology was introduced was in 1959 (CitationFanfair et al. 2007). In contrast to the tiny size of the structures studied, nanotechnology has become a large field of study involving chemistry, physics, engineering, computing, electronics, energy and biomedicine (CitationKurmi et al. 2011). Nanotechnology offers a range of materials and tools to diagnose and treat cancer, such as new imaging agents, and multifunctional targeted devices capable of bypassing biological barriers to deliver therapeutic agents directly (CitationSilva 2004, CitationConde et al. 2011, CitationBaptista 2009, CitationHeath and Davis 2008). Recent improvements in engineering at the nanoscale level have led to the advance of a variety of novel nanoscale platforms (quantum dots, nanoshells, gold nanoparticles, paramagnetic nanoparticles, carbon nanotubes), which are currently under development and investigation (CitationCuenca et al. 2006, CitationMozafari et al. 2009). shows the applications of these nanoparticles.
Table I. Uses of nanoparticles in nanotechnology.
Detection
Conventional lung cancer detection methods
Early detection of lung cancer has attracted the major interest in research because it considerably increases the survival rate (CitationGao et al. 2012). Early detection of cancer can be useful in curing the disease completely. Therefore, the necessity for techniques to detect the occurrence of cancer nodules in the early stage is increasing (CitationGomathi and Thangaraj 2010). Nowadays, some common biochemical and histopathological assays and graphical methods including sputum cytology, chest radiograph, fluorescence bronchoscopy, polymerase chain reaction (PCR), computed tomography (CT), and bronchial biopsy, are generally being used in lung cancer detection. However, the capacity to use these techniques is dependent on the size of the tumor and some special medical equipment, leading to cost escalation (CitationMa et al. 2010). Low-dose spiral (helical) CT methods are much more sensitive in detecting nodules than chest radiograph tomography (CT). Furthermore, the cost-effectiveness of CT screening for lung carcinoma will also need to be addressed if efficiency is confirmed. However, in some countries, open-access CT scans are accessible, including whole-body CT scanning (CitationFong et al. 2005).
Advantages and disadvantages
There is an increase of interest in lung cancer screening, as new technologies permit more precision and clarity in identifying people who are at risk. This enables us to give suitable treatment as soon as possible (CitationGanti and Mulshine 2006, CitationSutedja 2003). Screening is an approach in which one or more tests are used in a population, to detect a disease in individuals who do not have the symptoms of that disease. However, even though screening may lead to an earlier diagnosis in a minority of the screened subjects, it can cause injuries among the others. Overdiagnosis, misdiagnosis and the creation of a false sense of safety are some adverse effects of screening (CitationWarner et al. 2010). Unfortunately the most common ways currently used to diagnose lung cancer (e.g. bronchoscopy, biopsy and sputum cytology) can occasionally fail to spot tumors, because they are dependent on tumor size. The most recent methods for earlier detection, such as fluorescence bronchoscopes, spiral CT scanning and polymerase chain reaction-sputum assays, are expensive and are not amenable to prevalent screening because they are time consuming (CitationGanti and Mulshine 2006, CitationPeng et al. 2008).
Nanomaterials for lung cancer detection
Nanotechnology offers the ability to detect diseases, such as tumor, much earlier than ever imaginable. Often, patients diagnosed with breast, lung, colon, prostate and ovarian cancer have unknown or overt metastatic colonies. With the arrival of diagnostic nanotechnology, these numbers are expected to greatly lessen (CitationBiju et al. 2008). Increasing information suggests that the nanoparticles, whose crust contains a targeting particle that binds to receptors which are highly expressed in tumor cells, can act as cancer image contrast agents to increase sensitivity and specificity in cancer detection (CitationSajja et al. 2009, CitationShenoy et al. 2006). In contrast with other small-molecule contrast agents, the advantage of using nanoparticles is their large surface region and the possibility of surface modifications for further conjugation or encapsulation of huge amounts of therapeutic agents (CitationSajja et al. 2009). Nanoparticles as colloidal systems can be made-up from a multitude of materials in a range of compositions, including quantum dots (QDs), polymeric nanoparticles, gold nanoparticles (AuNPs), paramagnetic nanoparticles, and so on (CitationPark et al. 2009a).
Quantum dots
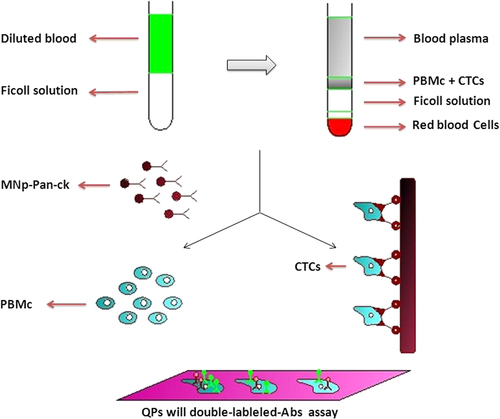
Quantum dots (QDs) are semiconductor nanocrystals (∼2–100 nm), with exclusive optical and electrical properties –(CitationGhasemi et al. 2009, CitationJamieson et al. 2007, CitationHardman 2006, CitationJin et al. 2011, CitationLeutwyler et al. 1996). They were first prepared in 1982, for use as probes for analysis of surface kinetics (CitationAzzazy et al. 2007). Structurally, QDs consist of a metalloid crystalline core and a “cap” or “shell” that shields the core and renders the QD bioavailable (CitationCuenca et al. 2006, CitationGhasemi et al. 2009, CitationHardman 2006, CitationAzzazy et al. 2007). One of the more exclusive properties of QDs is their fluorescence spectrum, which renders them optimal fluorophores for biomedical imaging (CitationHardman 2006). They are used in imaging, detection, and targeting. The research also indicates that QDs could be used to study cell differentiation and development in embryogenesis (CitationGhasemi et al. 2009). The application of QDs, as a novel technology for biosystems, has been frequently studied on mammalian cells. There is an increasing affinity to apply QDs as markers in plant science. The use of QDs as markers of the cells for plant bioimaging would be useful because of their tiny size, brilliant emission of the self-assembled QDs on the excitation wavelength, and stability under comparatively ruthless environments (CitationValizadeh et al. 2012). Such QDs have important advantages in chemical and biological research studies, contrary to traditional fluorescent organic dyes and green fluorescent proteins. These properties have made QDs a topic of high priority, mainly in cellular imaging and molecular profiling of pathological tissue specimen of cancer patients, for the diagnosis of types and stages of disease, prediction of prognosis, and directing the treatment strategy (CitationJin et al. 2011, CitationLiu et al. 2011a, CitationChan and Nie 1998, CitationDubertret et al. 2002, CitationKonkar et al. 2005). Aqueous solubility is the common problem for all types of QDs when they are in use in the biological research studies. To avoid this problem, ligand exchange and polymer coating are confirmed to be effective, besides the synthesis of QDs in aqueous solutions directly (CitationJin et al. 2011). However, toxicity is another big concern, particularly for in vivo studies (CitationBiju et al. 2008, CitationJin et al. 2011). Some QD-coating materials have themselves been found to be cytotoxic, such as mercaptoacetic acid. From this, it can be seen that the physicochemical properties of QDs are the main consideration in QD toxicity (CitationHardman 2006). However, QDs also establish their application in the near infrared (NIR) imaging (700–1000 nm) (CitationBiju et al. 2008, CitationTomoda et al. 2009). Despite the large size of QDs compared with organic dye molecules, their bright PL and high photochemical endurance are promising for in vivo fluorescence imaging. In 2002, Akerman et al. introduced the in vivo applications of QDs. They injected a peptide-coated CdSe/ZnS QD sample through the tail vein of a mouse, and demonstrated the specificity of the conjugate to endothelial cells in the blood vessels of the lungs (CitationBiju et al. 2008). Surgery is the preferred treatment technique for NSCLC patients. However, many patients remain at risk of recurrence and metastasis following surgery, and it is difficult for usual histopathologic and imaging methods to detect metastasis. Also, more sensitive and specific methods are needed to detect lung cancer earlier and recognize patients at highest risk for reappearance. A novel method of screening micrometastases of lung cancer in peripheral blood using magnetic nanoparticles (MNPs) and quantum dots (QDs), was developed to achieve early diagnosis and prevent recurrence. Pan-cytokeratin (pan-CK) is the common protein marker for the detection of epithelial tumor cells. Lunx, a new human lung-specific gene, has been reported to be an advanced diagnostic marker for the detection of micrometastases in marginal blood and lymph nodes of NSCLC patients. Surfactant protein-A (SP-A) is the most important of the four proteins in the pulmonary surfactant system. Moreover, expression of SP-A was also described for a portion of NSCLC, which facilitates a diagnostic marker for these carcinomas. In 2002, Bernards and Weinberg offered a new model of metastasis, where metastatic ability is gained early during primary tumor development. In this study, the MNPs synthesized were successfully fixed with pan-CK Ab, and two kinds of synthesized QDs were conjugated with Lunx and SP-A Abs. QDs with double-labeled Abs were first used together to recognize the CTCs of NSCLC patients collected by MNP-pan-ck (CitationWang et al. 2012). illustrates the processes by which the MNP-pan-ck enriches the CTCs from NSCLC patients, and QDs with double-labeled Abs are used at the same time to recognize CTCs.
Gold nanoparticle
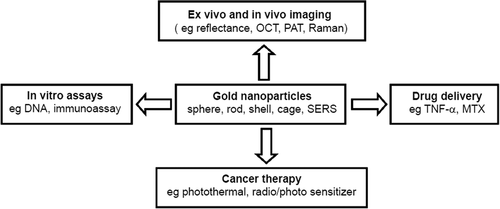
Lately, nanoparticles based on gold chemistry have attracted research and practical attention(CitationShenoy et al. 2006). Nanosized particles of noble metals, particularly gold nanoparticles (AuNPs), have received immense interest due to their advantageous electronic, optical, and thermal properties, as well as their catalytic property, and the possibility of application in the fields of physics, chemistry, biology, medicine, and material science, and their related interdisciplinary fields (CitationChen 2004, CitationTiwari et al. 2011). AuNPs are a suitable platform for progress of efficient delivery systems. They have a number of enviable properties that make them brilliant candidates for use in delivery applications. First, the gold core is in essence inert, non-toxic, and biocompatible, making it a perfect starting point for carrier assembly (CitationRana et al. 2012), and can be simply synthesized and functionalized, and is biocompatible (CitationDuncan et al. 2010). In cancer research, colloidal gold can be used to target tumors, and in present detection techniques using Surface Enhanced Raman Spectroscopy (SERS) in vivo (CitationQian et al. 2007). In many studies, AuNPs are used as ultrasensitive luminous probes to recognize cancer biomarkers in human blood cancer therapy, and in medicine delivery (CitationHuang et al. 2010). AuNPs have been investigated in different areas such as in vitro assay, as well as in vitro and in vivo imaging (CitationCai et al. 2008). In addition to drug and gene delivery, they are also being investigated for use in thermal ablation and radiotherapy improvement () (CitationCai et al. 2008, CitationConde et al. 2011, CitationShenoy et al. 2006, CitationDe Jong and Borm 2008) (CitationChen et al. 2009). Lately, the blend of organic building blocks with anti-cancer drugs and inorganic nanocrystals has been carefully studied as a new approach in cancer therapy and diagnosis (CitationQian et al. 2007). Methotrexate (MTX), an inhibitor of dihydrofolate reductase, is a chemotherapeutic factor for treating a variety of cancer types. An MTX-AuNP conjugate was prepared, and the cytotoxic/antitumor effect was examined in vitro and in vivo. Administration of the conjugate suppressed tumor growth in a mouse model of Lewis lung carcinoma, whereas an equal dose of free MTX had no antitumor effect (CitationCotugno et al. 2012). Following the first report of the exclusive ability of various inorganic nanomaterials for diagnosis, the potential capability of AuNPs for computed tomography imaging has recently been revealed (CitationPark et al. 2009b). Recently, AuNPs have also successfully been tested as sensors for discriminating and classifying the histology of different lung cancers. The sensor was able to distinguish between normal and cancerous cells, SCLC and NSCLC, and between two subtypes of NSCLCs. Peng et al. developed an AuNP-based biosensor system with the capacity to detect lung carcinoma by analyzing an individual's exhaled breath. The sensor uses a combination of an array of chemiresistors based on AuNPs and pattern recognition methods. Another research group reported the detection of picograms of enolase 1 (ENO1), an immunogenic antigen associated with NSCLC, by using an immunosensor that detects electrochemical signal probes of AuNP congregates (CitationIndira and Lakshmi 2010).
Figure 2. The versatile properties of AuNPs have been employed for biomedical applications in many areas (CitationGanti and Mulshine 2006).
Magnetic nanoparticles
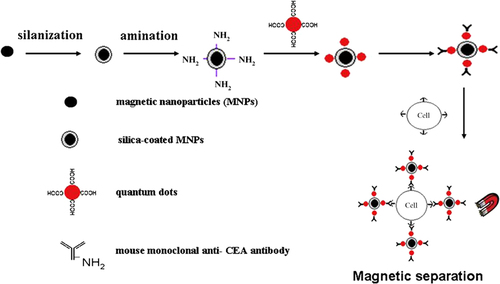
In the past decade, nanomaterials have shown vast potential in diagnosis and therapy; inorganic nanomaterials in particular, are increasing in their popularity in biomedical application. Much notice has been focused on nano-sized inorganic particles including magnetic nanoparticles (MNPs) and quantum dots (QDs), due to their exclusive optical, electrical and magnetic properties, which can be generally applied in disease diagnosis and therapy (CitationMa et al. 2010). Nowadays, MNPs are attracting notice because of their potential use as contrast agents for magnetic resonance imaging (MRI, and as heating mediators for cancer thermotherapy (hyperthermia) (CitationIndira and Lakshmi 2010, CitationIto et al. 2005). MNPs have a particle range within the nanoscale, with magnetic properties. Different metals can be used to pass on the magnetic properties of MNPs; nickel, cobalt, and iron have been established as such examples. Natural and inorganic polymers including RGD peptides, fibronectin, and dextran, can be used to encircle the magnetic center (CitationMarkides et al. 2012). The chief properties of MNPs for medical applications are nontoxicity, biocompatibility, injectability, and high-level gathering in the target tissue; the most important property among those mentioned above is nontoxicity (CitationIto et al. 2005). We can categorize the biomedical applications of MNPs according to their application inside (in vivo) or outside (in vitro) the body. In vivo applications could be further divided into therapeutic (hyperthermia and drug-targeting), and investigative applications (nuclear magnetic resonance (NMR) imaging), whereas for in vitro applications, the chief use is diagnostic (separation/selection, and magneto relaxometry) (CitationTartaj et al. 2003). For in vivo applications, the MNPs must be encapsulated within a biocompatible polymer during or after the training process, to avoid changes from the original structure (CitationAkbarzadeh et al. 2012). Each possible application of the MNPs requires having diverse properties for biomedical uses, the application of particles that present superparamagnetic activities at room temperature is preferred; moreover, applications in therapy, biology, and medical diagnosis need the magnetic particles to be stable in water at pH 7 and in a physiological environment (CitationAkbarzadeh et al. 2012). The most exclusive feature of magnetic particles is their reaction to a magnetic force, and this feature has been utilized in applications such as drug targeting and bioseparation, including cell sorting (CitationValizadeh et al. 2012, CitationAhmadi et al. 2014). For their use in biomedical applications, MNPs are desired to reveal superparamagnetic properties (SPIONs). SPIONs are usually small particles composed of either a magnetite (Fe3O4) or maghemite (γ-Fe2O3) core coated with a biocompatible organic/inorganic polymer. Iron (Fe) oxide-based MNPs are appropriate for biological application for the following reasons: the superparamagnetic nature implies that the particles will not be attracted to each other, and so the risk of agglomeration in a medical setting is minimized (CitationMarkides et al. 2012). MNPs exhibit the feature of superparamagnetism, not remaining magnetized after the action of magnetic field, to reduce the risk of particle aggregation (CitationIndira and Lakshmi 2010). The chief factors which determine toxicity and the biocompatibility of these materials, are the nature of the magnetically responsive components, such as magnetite, iron, nickel, and cobalt, and the final size of the particles, their core, and the coating condition. (CitationAkbarzadeh et al. 2012). Elevated magnetic materials, such as cobalt and nickel, are susceptible to oxidation and are toxic; thus they are of little attention (CitationMarkides et al. 2012, CitationAkbarzadeh et al. 2012). A new strategy to detect lung cancer cells is the utilization of magnetic and fluorescent bifunctional nanocomposites (BNPs) blended with monoclonal anti-carcinoembryonic antigen (CEA) antibodies. The BNPs, consisting of silica-covered superparamagnetic nanoparticles and quantum dots (QDs), exhibited prominent luminescence and were basically separated in an outer magnetic field. The binding specificity of the antibody-conjugated BNPs were definite, by incubating with human lung adenocarcinoma SPCA-1 cells, human leukemic K562 cells and human embryonic lung fibroblast MRC-5 cells (CitationCai et al. 2008). In this study, fabricated BNPs that incorporated magnetic nanoparticles in a silica shell and water-soluble QDs, were immobilized on the silica surface. The BNPs demonstrated their targeting effect to capture SPCA-1 cells after surface-modification with anti-CEA antibodies. These immuno-nanoparticles were then used to separate and detect cancer cells in the pleural effusions from the patients. Confocal laser scanning microscopy (CLSM) was conducted to have a qualitative assessment of cellular targeting by immuno-nanoparticles. The process described above is depicted simply in . Extra experiments proved that the immune nanoparticles, as prepared, can competently capture and detect cancer cells in pleural effusion from lung cancer patients (CitationMa et al. 2010). MNPs have also been used to overcome drug resistance. A cisplatin-resistant A549 lung tumor xenograft model was chemosensitized with cisplatin-loaded MNPs. Molecular studies illustrated that cisplatin-loaded MNP-treated tumors had a significant reduction in localization of lung resistance-related proteins and enhanced cytotoxicity of cisplatin (CitationPourhassan-Moghaddam et al. 2013).
Treatment of lung cancer
Traditional methods
Surgical resection remains the single most consistent and successful option for the treatment for patients diagnosed with lung cancer (CitationMolina et al. 2008). In 1933, Graham reported the first successful resection of a lung cancer by pneumonectomy, and for the next 20 years, whole removal of the ipsilateral lung was the procedure of choice for lung cancer (CitationDienemann 2001). Unluckily, a substantial number of patients with stage I lung cancers are unable to tolerate surgery due to their poor physical condition. Radiation therapy may be given with therapeutic intent to patients who are unfit for surgery, but the prospects of cure are substantially less than with surgical resection (CitationOkunaka et al. 2004). Radical radiotherapy may also lead to considerable pulmonary damage, with significant loss of function, and so it may not be appropriate for patients whose pulmonary function has previously been severely compromised. There is, therefore, a need for local treatments that can destroy tumors effectively, but which are less invasive than surgery and less damaging to normal lung tissue than radiotherapy. A new choice for cancer cure is photodynamic therapy (PDT). PDT has been successfully used to treat early-stage (Tis) lung cancers involving the central airways, by delivering light to photosensitized tissues using a fiber optic bronchoscope (CitationOkunaka et al. 2004). The concept of preoperative chemotherapy (PCT) was born from the hypothesis that drug resistance appears as a result of genetic changes. Therefore, the best efficacy of chemotherapy should be obtained with small tumor burdens. PCT, studied in more than 30 phase II studies, has been confirmed to be feasible at the expense of mild postoperative morbidity and mortality (CitationDepierre et al. 2002).
Drug delivery base of nanotechnology
Imprecise anticancer chemotherapy has no tumor targeting ability and will kill normal and cancer cells, causing rigorous side effects in many patients. To resolve this problem, targeted drug delivery systems have received immense attention (CitationLiu et al. 2011a). Carriers could supplement sustained drug delivery to the lungs, extend the duration of action, decrease therapeutic dose, improve patient compliance, and reduce the bad effects of highly toxic drugs. The targeting of drugs to the lungs can deliver therapeutic agents to the diseased regions by reducing their allocation to the non-target organs (CitationKurmi et al. 2011). One of the best values of nanotechnology will be in the development of novel and effective medical diagnostics and treatments (i.e. nanomedicine) (CitationSajja et al. 2009). Nanotechnology provides a range of nanoscale tools for medicine. Among them, nanoparticles are revolutionizing the field of drug delivery. These drug nanocarriers have the possibility to increase the therapeutic efficiency of a drug, since they can be engineered to modify the release and the stability and to prolong the circulation time of a drug, protecting it from removal by phagocytic cells or early degradation (CitationConti et al. 2006). The lung is a suitable target for drug delivery, due to non-invasive ways to present not only local effects in the lung, but possibly high systemic bioavailability, avoidance of first-pass metabolism, more rapid onset of therapeutic action, and the availability of a huge surface area (CitationDavaran et al. 2013, CitationYang et al. 2008, CitationPatton and Byron 2007). By increasing colloidal delivery systems such as liposomes, micelles and nanoparticles, a novel frontier was opened for improving drug delivery. Nanoparticles, with their unique characteristics such as small particle size, big surface area, and the potential of changing their surface properties, have frequent advantages compared with other delivery systems Nanoparticles could provide the advantage of sustained release in the lung tissue and thus the systemic circulation, resulting in a reduction in dosage frequency and improved patient compliance. Insulin is an important example for which the delivery of the drug to the lungs has been shown to be beneficial. Kawashima et al. dosed PLGA nanoparticles prepared with insulin to guinea pig lungs, and illustrated a significant reduction in blood glucose level, with a prolonged efficacy over 48 h, as compared to insulin solution. Additionally, insulin-loaded nanoparticles using a different polymer, poly (butyl cyanoacrylate), delivered to the lungs of rats, were shown by Zhang et al. to extend the duration of a hypoglycemic effect over 20 h (glucose level below 80% of the original levels), as compared to pulmonary administration of insulin solution (CitationSung et al. 2007). In general, nanoparticle delivery to the lungs is an attractive idea, because it can cause retention of the particles in the lungs, accompanied with an extended drug release if large porous nanoparticle matrices are used (CitationAzarmi et al. 2008).
Liposomes
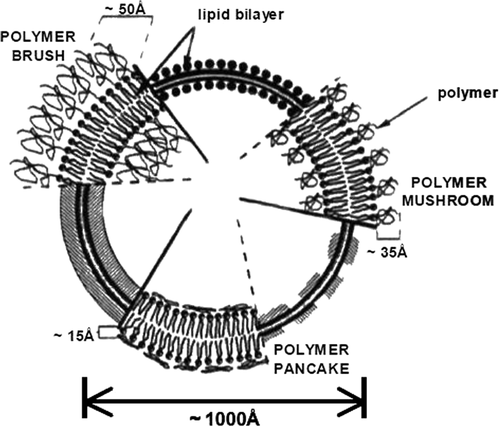
Although liposomes were at first developed as models of biological membranes, their potential as a drug delivery system have undergone exhaustive investigations for over 25 years (CitationChen 2004). Liposomal phospholipid vesicles were discovered by Banham in 1965, and were soon after recognized as promising drug carriers (CitationKaskowitz et al. 1993, CitationKonkar et al. 2005, CitationDuncan et al. 2010, CitationSadat et al. 2014, CitationDua et al. 2012). Liposomes are small spheres ranging in diameter from 50 nm to several microns (CitationBiju et al. 2008, CitationKonkar et al. 2005). They are self-assembling closed colloidal structures composed of lipid bilayers, and have a spherical figure in which an outer lipid bilayer encircles a central aqueous space (CitationPeng et al. 2008, CitationPastorino 2006, CitationIndira and Lakshmi 2010, CitationDua et al. 2012, CitationImmordino et al. 2006). Properties of the constituent phospholipids (CitationKurmi et al. 2011), include permeability, charge density and steric obstacle (CitationPeng et al. 2008). Because of their singular advantages over conventional drug therapy, including their ability to protect the drugs from degradation, target the drug to the site of action, and reduce the toxicity and side effects of such drugs, liposomes have been studied extensively as drug carriers, particularly for cancer therapy (CitationBiju et al. 2008). Their applications in drug delivery depend on physicochemical and colloidal characteristics, such as composition, size, loading effectiveness, and the stability of the carrier, as well as their biological interactions with the cells (CitationSadat et al. 2014). Liposomes are one of the most extensively investigated systems for the controlled delivery of drugs to the lung. They seem mainly suitable for transport of the therapeutic agent to the lung, since these vesicles can be prepared from endogenous compounds, such as the components of lung surfactants, and these properties render liposomes attractive candidates as vehicles for drug delivery. The first pharmaceutical liposomal products in market include the synthetic lung surfactant Alveofact®, for pulmonary instillation for the treatment of respiratory distress syndrome (RDS). Much interest has been focused on cationic liposomes for pulmonary gene delivery, because cationic liposomes offer the advantage of self-assembly with DNA material through favorable cationic–anionic electrostatic interactions. Additional benefits include evasion of complement inactivation after in vivo administration, the squat cost, and relative ease in producing nucleic acid–liposome complexes in a large scale (CitationDavaran et al. 2013, CitationJusto and Moraes 2003). Two kinds of problems in drug therapy (i.e. biodistribution all over the body and targeting to definite receptors) can be solved by using liposomal formulations: liposomes protect encapsulated molecules from degradation and can inactively target tissues or organs that have a discontinuous endothelium, such as the liver, spleen, and bone marrow (CitationImmordino et al. 2006). Liposome-based technology has progressed from the original generation of “conventional vesicles,” to stealth liposomes, targeted liposomes, and more recently, stimuli-sensitive liposomes (CitationIndira and Lakshmi 2010, CitationImmordino et al. 2006). Conventional liposomal formulations are mostly comprised of natural phospholipids or lipids such as 1,2-distearoryl-sn-glycero-3-phosphatidylcholine (DSPC), sphingomyelin, egg phosphatidylcholine, and monosialoganglioside. Because this formulation is made up of phospholipids only, liposomal formulations have encountered several challenges; one of the chief ones being the instability in plasma, which results in short half-life during movement in blood. Further attempts to overcome these challenges have been made, particularly in the handling of the lipid membrane. One of the attempts focused on the management of cholesterol. Calculation of cholesterol according to conventional formulations reduces rapid release of the encapsulated bioactive compound into the plasma (CitationIndira and Lakshmi 2010). Stealth liposome technology is one of the most often used liposome-based systems for the delivery of active molecules (CitationIndira and Lakshmi 2010, CitationImmordino et al. 2006). This strategy was developed to overcome most of the challenges encountered by conventional liposomes. Stealth liposome strategy was achieved basically by modifying the surface of the liposomal membrane, a method that was achieved by engineering hydrophilic polymer conjugates (CitationIndira and Lakshmi 2010, CitationImmordino et al. 2006). Natural or synthetic hydrophilic polymers such polyethylene glycol (PEG), chitosan, silk-fibroin, and polyvinyl alcohol (PVA), were exploited () (CitationIndira and Lakshmi 2010, CitationLiu et al. 2011b). PEGylated liposomal doxorubicin (DOXIL/Caelyx) is the exceptional pattern of stealth liposome technology to be proved by both the USA Food and Drug Administration (FDA) and the Europe Federation (CitationImmordino et al. 2006). Doxorubicin-loaded liposomes were surface- engineered with monoclonal antibody, and are now commercially available. The general advantage of this model of liposome is an increase in active molecules or the reach of drug into targeted cells via endocytosis (CitationIndira and Lakshmi 2010). To increase liposomal drug accumulation in the desired tissues, thereby producing more selective curative activity, the use of targeted liposomes has been suggested (CitationImmordino et al. 2006). One of the problems with liposomes as drug delivery carriers is their requirement of constancy in biological fluids. As a result, drug molecules leak to normal tissues and cause undesirable side effects (CitationChen 2004). The association of anticancer drugs with better delivery systems, in particular liposomes, has become a thriving strategy to advance the therapeutic effect of these molecules by increasing their concentration at the target site and decreasing the systemic toxicity. Anthracyclines, such as doxorubicin (DOX), are among the most usually used drugs for the treatment of both hematological and solid tumors. On the other hand, the clinical use of DOX is often limited by the risk of cumulative cardiac toxicity, which may lead to congestive heart failure and death. Liposomal formulations of DOX have shown prolonged times of systemic circulation in comparison with the free drug, reduced cardiotoxicity, improved solid tumor accumulation, and increased curative efficacy in numerous experimental models. Encouraged by these promising results, liposomal formulations are now being developed for new routes of delivery and with a higher degree of target specificity. Among these approaches are target-seeking vesicles that have antibodies or receptor ligands attached directly to the phospholipid head groups on the liposome or to the distal end of polyethylene glycol (PEG) chains. Transferrin (Tf), a glycoprotein responsible for cellular iron absorption, is one such molecule that is able to be utilized for active targeting. Tf is nonimmunogenic and can be conjugated without losing its biological activity, making it a promising cancer-targeting ligand. Previous in vitro studies have shown that Tf-conjugated, DOX-loaded liposomes revealed higher uptake levels and cytotoxicity in lung cancer cells, while they were relatively nontoxic to normal pneumocytes. Other studies using Tf attached to the distal terminal of PEG-chains of sterically stabilized-liposomes have concluded that these systems were able to suppress cancer growth more effectively than PEG-liposomes after i.v. administration into solid tumor-bearing animals. Inhalation of therapeutic aerosols is a long established method to deliver drugs topically to the lung or to the systemic circulation. In addition to drug solutions and particulates, liposomal formulations are being developed for aerosol administration. Due to their versatility and innoxious nature, liposomes are promising candidates for pulmonary drug delivery that allow delivery of compounds with formulation issues (e.g., lipophilic drugs and sensitive biopharmaceuticals), and to create a drug depot in the lung for sustained release (CitationGaspar et al. 2012). Other liposomal drugs used in clinical administration today include AmBisome (amphotericin B liposomes), DaunoXome (daunorubicin liposomes), DepoCyt (cytarabine liposomes), and Visudyne (verteporfin liposomes) (CitationZhang et al. 2007). Even though liposomes have been extensively studied for last few decades, the only efficient nanoformulation available in the market is Doxil® (ALZA, Mountain View, CA) (CitationHeath and Davis 2008). β- Elemene was found to play a major role in enhancing the effects of many anticancer drugs, and was generally used in the treatment of different kinds of malignancies and in reducing the side effects of chemotherapy. More study showed that it is also a promising anti-lung cancer drug, but the clinical application of β-Elemene was limited by its hydrophobic property, poor stability, and low bioavailability. With the development of novel excipients and novel technologies, the abundance of novel formulations of β-Elemene has improved considerably, which presents a positive view in terms of clinical application for β-Elemene. The liposome, as a drug delivery system, exhibits big advantages over conventional formulations for β-Elemene. Cisplatin has been demonstrated to have a definite impact on survival in advanced NSCLC (1995), but a problem is extensive systemic toxicity. Liposomal encapsulation of cisplatin has the attraction of potentially limited toxicity, as well as the potential for tumor targeting. ALZA Pharmaceuticals (Mountain View, CA,USA) developed a stealth (sterically stabilized) liposomal formulation of cisplatin (SPI-77) that showed promising results in preclinical models, with considerably greater delay in tumor growth than cisplatin (CitationWhite et al. 2006). Recently, a multicomponent liposomal drug delivery system, consisting of doxorubicin and antisense oligonucleotides, targeted to MRP1 mRNA and BCL2 mRNA to repress pump resistance and non–pump resistance respectively, have been developed. This liposomal system successfully delivered the antisense oligonucleotides and doxorubicin to the cell nucleus, inhibited MRP1 and BCL2 protein synthesis, and significantly potentiated the anticancer action of doxorubicin by stimulating the caspase-dependent pathway of apoptosis in multidrug-resistant human lung cancer cells (CitationKaskowitz et al. 1993).
Figure 4. Schematic diagrams of poly-(ethylene glycol) (PEG) configuration regimes (mushroom, brush, and pancake) for polymer grafted to the surface of liposome bilayer (CitationLiu et al. 2011b).
Dendrimers
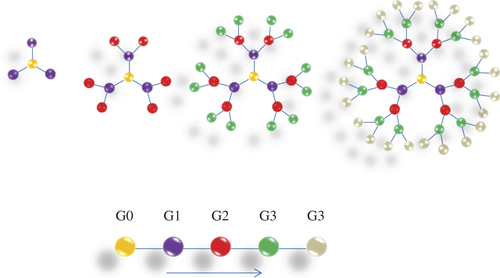
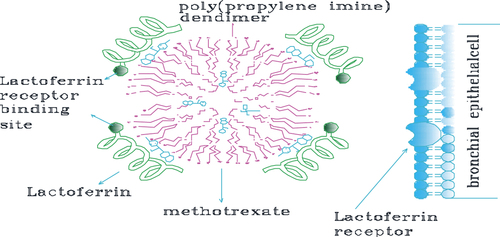
Dendrimers (the word dendron in Greek denotes tree, also called arborols and cascade molecules) are continually branched polymers that are normally 2–10 nm in diameter, with an almost spherical form. There are two kinds: the low-molecular weight type consists of monodisperse and highly symmetric dendrimers and dendrons, while the high-molecular weight type encompasses dendronized polymers, hyperbranched polymers, and brush polymers (also called bottle brushes). Due to the lack of molar mass, dendrimers and dendrons are considered macromolecules, but not polymers (CitationSajja et al. 2009). Dendrimers have three components: an initiator core, branches, and lethal functional groups. The initiator core is in the center of the molecule, and branches broaden out from it. The monomers attached to the center (G0), are called first generation monomers (G1), and two second generation monomers (G2) are close to each of the first generation monomers. Successive generations will organize in this same manner, with two monomers attached to the monomer from the prior generation () (CitationHeath and Davis 2008). They are attractive systems for drug delivery because of their nanometer size range, ease of preparation and functionalization, and their capability to display several copies of surface groups for biological reorganization processes (CitationSung et al. 2007, CitationQuintana et al. 2002). The special properties of dendrimers, such as their high degree of branching, multivalency, globular structure, and well-defined molecular weight, make them promising novel scaffolds for drug delivery (CitationGillies and Frechet 2005). In early studies, dendrimer-based drug delivery systems focused on encapsulating drugs. However, it was hard to control the release of drugs associated with dendrimers (CitationSingh and Lillard 2009). Dendrimers can be synthesized initially from the central core, working out toward the side-line (divergent synthesis), or in a top-down approach, starting from the farthest residues (convergent synthesis) (CitationBiju et al. 2008, CitationKaskowitz et al. 1993, CitationSung et al. 2007, CitationBlanco et al. 2010). Divergent methods were primarily introduced by Tomalia in the 1980's, when his group synthesized three-dimensional polyamidoamine (PAMAM) dendrimers by the growth of branches extending radially from a core site to the outside edge (CitationBiju et al. 2008). PAMAM dendrimers have attracted considerable research attention as drug carriers, due to their exclusive properties, i.e. hyperbranched and monodispersed tree-like structures with multifunctional surfaces, enabling the dendrimers to encapsulate or conjugate drug molecules (CitationLiu et al. 2011a). Besides, the terminal groups of PAMAM can be simply modified (e.g. by acetylation or modification with poly(ethylene glycol) [PEGylation]) to improve solubility and biocompatibility (CitationKurmi et al. 2011, CitationLiu et al. 2011a). PEGylated dendrimers are one of the junior-classes of dendrimers that draw the attention of numerous scientists, owing to their lengthened blood distribution time, smaller level of toxicity, and comparatively less accumulation in different organs. PEG-dendrimers are usually synthesized by the conjugation of PEG or polyethylene oxide (PEO) chains to a multifunctional dendritic chain (CitationHeath and Davis 2008). In order to improve the targeting ability of PAMAM, many targeting molecules, such as antibodies, folic acid, biotin, peptides, and carbohydrates, have been connected to PAMAM dendrimers (CitationLiu et al. 2011a). In 2002, Jesus and colleagues had explored the prospect of a 2, 2-bis (hydroxymethyl) propanoic acid-based dendritic scaffold as a delivery carrier for doxorubicin in vitro and in vivo. This dendritic nanoformulation, which contains doxorubicin covalently bound through a hydrazone linkage to a high molecular weight 3-arm polyethylene oxide, exhibits abridged cytotoxicity in vitro. However, in vivo biodistribution experiments showed least accumulation of the DOX dendrimer conjugate in critical organs, including the liver and heart, and increased half-life of doxorubicin compared to the free drug. Many investigators have also explored the possibility of cisplatin incorporation in dendrimers (CitationHeath and Davis 2008). Finally, chemotherapy drugs have been covalently bound to these dendrimers or encapsulated in the inside cavities of dendritic molecules to generate targeted drug delivery systems (CitationLiu et al. 2011a). A key factor of a targeted drug delivery systems is the finding of targeting molecules that can particularly identify tumor and cancer cells. Phage display technology is a potent approach to screening targeting molecules such as peptides, for cancer cells or tumor blood vessel endothelial cells. In order to develop a common drug carrier for non-small cell lung cancer chemotherapy, the Ph.D.-C7C ™ phage display library was utilized to display peptides specific for NSCLC. While studying the information, a novel peptide, lung cancer-targeting peptide (LCTP; RCPLSHSLICY) was discovered, which can exclusively target NSCLC. The LCTP was then conjugated with fluorescein isothiocyanate (FITC, a fluorescence labeling agent used as a tracer) and acetylated PAMAM to produce a targeted drug delivery carrier (PAMAM–Ac–FITC–LCTP). The routine of this drug carrier was evaluated by in vitro culturing of NCIH460 and 293T cells, and in vivo using athymic mice with lung cancer xenografts (CitationLiu et al. 2011a). Lactoferrin (Lf) is a non-heme iron-binding protein that is part of the transferrin protein family, along with serum transferrin, ovatransferrin, melanotransferrin and the inhibitor of carbonic anhydrase, and is cationic in nature. Lf was investigated as a targeting ligand for receptor-mediated gene delivery to human bronchial epithelial cells. A high number of Lf receptors (LfRs) were detected on bronchial epithelial cells (BEAS-2B) but not on alveolar epithelial (A549) cells, by fluorescence microscopy and FACS measurements, suggesting ability in targeting selectivity for bronchial epithelial cells. Previous studies have demonstrated that LfRs are expressed on the apical surface of bronchial epithelial cells. Thus, Lf may serve as a suitable targeting ligand for receptor-mediated delivery to the lung for treating specific lung cancers originating from bronchioles () (CitationKurmi et al. 2011). The in vitro drug release studies, and in vivo pharmacokinetic and tissue distribution studies, suggested that in the handling of lung cancer, Lf-conjugated 5.0G PPI dendrimers can be a promising drug-targeting carrier for anticancer bioactives. Encapsulation of MTX in Lf-conjugated 5.0G PPI dendrimers enhance the residence time as well as concentration of drug in lung, which could be helpful in reducing the dosing occurrence, as well as dosage (CitationKurmi et al. 2011).
Polymeric micelle
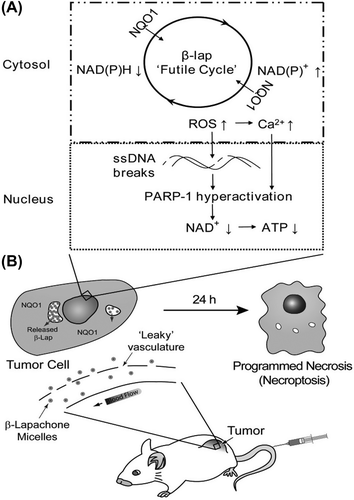
Polymeric micelles were one of the first polymer self- assemblies reported as a nano-DDS (CitationMiyata et al. 2010). Polymeric micelles, composed of amphiphilic block- copolymers, are promising as potent drug delivery vehicles for hydrophobic drugs (CitationBlanco et al. 2010). Amphiphilic block copolymers, containing a hydrophilic block and a hydrophobic block, are initially identified to construct those distinct domains in a micelle makeup through spontaneous self-assembly as a result of hydrophobic interactions in aqueous solutions. Therefore, hydrophobic drugs can be entrapped into the micelle center non-specifically, through hydrophobic interactions, or specifically, by covalent bonding with the block comprising the hydrophobic domain. In contrast, hydrophilic charged macromolecules including peptides, proteins, and nucleic acids can be loaded into the micelle core by using oppositely charged blocks to shape polyion complex (PIC) cores through electrostatic interactions and charge neutralization (CitationMiyata et al. 2010). Polymeric micelles have several advantages over usual surfactant micelles in that they have enhanced thermodynamic stability in physiological solutions, as indicated by their low critical micellar concentration, which makes polymeric micelles stable and prevents their rapid dissociation in vivo (CitationSung et al. 2007, CitationJones and Leroux 1999, CitationSavić et al. 2003). The size of polymeric micelles (less than ∼100 nm in diameter) not only makes them model drug delivery carriers because they evade renal exclusion and the RES, but also provides them with enhanced endothelial cell permeability in the vicinity of solid tumors, by passive diffusion (CitationSung et al. 2007, CitationNakanishi et al. 2001). Drugs can be partitioned in the hydrophobic center of micelles, and the outer hydrophilic layer forms a stable dispersion in aqueous media, which can then be administered intravenously (CitationSung et al. 2007). Efficient targeting of cytotoxic agents to solid tumors by polymeric micelles has been achieved by Kataoka et al., with a system based on doxorubicin (DOX) conjugated to poly(ethylene glycol)-poly(α,β-aspartic acid) block copolymer [PEG-PAsp (DOX)] (CitationSung et al. 2007). PEG-block-poly (aspartic acid) [PEG- b- P (Asp)] copolymers chemically conjugated with doxorubicin (DOX) instinctively form polymeric micelles with a diameter of 15–60 nm. This polymeric micelle can professionally entrap free DOX in the internal core, and the optimized formulation, called NK911, is now being studied in a phase II clinical trial at the National Cancer Center (NCC) Hospital in Japan (CitationNishiyama and Kataoka 2006). In this formulation, DOX chemically conjugated to the polymer area chain is pharmacologically inert, but contributes to the stable physical snare of free DOX into the micellar core, through π–π interaction of the anthracycline structure in DOX between the conjugated and unconjugated ones, also allowing its sustained release from the micellar core –(CitationNishiyama and Kataoka 2006, CitationYokoyama et al. 1993, Citation1994). Paclitaxel (Taxol) is one of the agents most usually used in mixture with platinum for the cure of advanced NSCLC. Though its use is restricted by the toxic effects associated with Cremophor EL (CrEL), the lipid-based solvent is used as a motor vehicle for paclitaxel. Due to its insolubility in water, paclitaxel is formulated with the micelle-forming vehicle CrEL, to improve drug solubility. However, the addition of CrEL has been shown to be the reason for hypersensitivity reactions and neuropathy. In addition, CrEL considerably alters the pharmacokinetics of paclitaxel. To avoid these drawbacks resulting from the use of CrEL, new CrEL-free formulations of paclitaxel are of huge interest for growth in the clinical setting. Genexol-PM (Samyang Co, Seoul, Korea) is a new formulation of paclitaxel, a sterile lyophilized polymeric micellar formulation without CrEL. Genexol-PM was found to have a three-fold maximum tolerated dose (MTD) in nude mice. In addition, the biodistribution of Genexol-PM showed two- to three-fold superior levels in a range of tissues including liver, spleen, kidney, and lung, and more prominently in tumors. Moreover, the in vivo antitumor value has been shown to be greater than that of Taxol (Bristol-Myers Squibb, Wallingford, CT) (CitationKim et al. 2007). β- Lapachone (β-lap) is a new anticancer agent whose mechanism of action is very dependent on the enzyme, NAD(P) H:quinone oxidoreductase-1 (NQO1), a flavoprotein found overexpressed in NSCLC. In cells overexpressing NQO1, β-lap undergoes an unsuccessful cycle resulting in reactive oxygen species (ROS) production. These ROS cause DNA single strand breaks (SSBs), hyper activation of poly (ADP-ribose) polymerase-1 (PARP-1), loss of NAD+ and ATP pools, and an exclusive outline of cell death referred to as “programmed necrosis” or “necroptosis”. Briefly, we highlight the clinical potential of a novel β-lap nanotherapuetic platform for the cure of lung cancers with elevations in NQO1. Polymeric micelles confirm a harmless delivery platform for β-lap, allowing them to avoid hemolytic anemia reactions with limited side effects and toxicity. Incorporation of the drug within micelles improved blood residence time, delicate tumor accumulation, and considerably lowered its toxicity. β-Lap micelles were greatly effective in treating both subcutaneous and orthotopic lung cancers that overexpress NQO1. The exclusive integration of nanotechnology and NQO1 specificity should result in improved value in future clinical applications () (CitationBlanco et al. 2010).
Carbon nanotubes
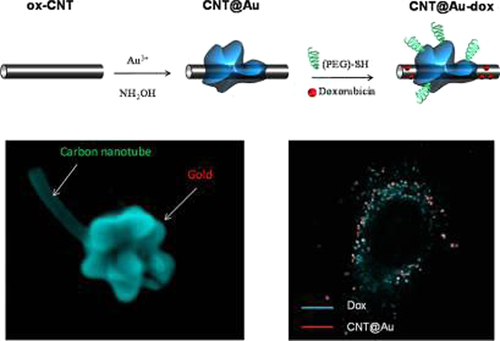
In current years, a wide range of diverse nanoscale drug delivery vectors have been evaluated. Remarkably, single-walled carbon nanotubes (SWCNTs) have attracted significant interest in this regard, as they offer potential advantages over the more generally studied metal nanoparticle systems, including their capability to carry a high cargo loading, their intrinsic stability, and structural flexibility, which could prolong the circulation time and thus the bioavailability of the drug molecules carried. Moreover, SWCNTs have been shown to enter mammalian cells, and due to their promising properties, SWCNT-based materials have already been investigated as potential delivery vehicles for intracellular transport of nucleic acids, proteins and drug molecules (CitationZhang et al. 2009). Carbon nanotubes (CNTs) are composed of a distinctive molecular shape of carbon atoms, and are 100 times stronger than steel, with only one-sixth of its weight, and exhibit unusual heat and conductivity properties (CitationSajja et al. 2009). They are considered one of the most promising nanomaterials, with the ability of both detecting the cancerous cells and delivering drugs or small therapeutic molecules to these cells (CitationJi et al. 2010). They are made up of thin sheets of benzene loop carbons rolled up into the shape of a seamless tubular structure (CitationJi et al. 2010). CNTs are commonly produced by three major techniques: electric arc discharge, laser ablation, and thermal or plasma-enhanced chemical vapor deposition (CVD) (CitationJi et al. 2010, CitationDigge et al. 2012). Based on their structure, CNTs can be classified into two common categories: single walled (SWNTs) which consist of one layer of cylinder graphene, and multi-walled (MWNTs), which contain numerous concentric graphene sheets (CitationJi et al. 2010, CitationDigge et al. 2012, CitationBianco et al. 2005, CitationHeister et al. 2009, CitationIijima 1991). Rare carbon nanotubes have very hydrophobic surfaces, and are not soluble in aqueous solutions. For biomedical applications, surface chemistry or functionalization is essential to solubilize CNTs, and improve biocompatibility and squat toxicity. Covalent and noncovalent are two forms of surface functionalization of carbon nanotubes (CitationDigge et al. 2012, CitationHou et al. 2002). Researchers have found that functionalized CNTs can cross the mammalian cell membrane by endocytosis or other mechanisms With the aid of specific peptides or ligands on their surface to recognize cancer-specific receptors on the cell surface, CNTs can carry healing drugs more steadily and efficiently into the cells that are previously unreachable, which makes them ideal candidates for drug delivery (CitationJi et al. 2010, CitationChen et al. 2008). Lately, novel SWNT-based tumor-targeted drug delivery systems (DDS) have been developed by numerous investigators. These delivery systems usually consist of three parts: functionalized SWNTs, cancer-targeting ligands, and anticancer drugs. When DDS interact with cancer cells, they can identify cancer-specific receptors on the cell surface and then induce receptor-mediated endocytosis. It has been confirmed that the complex was taken up professionally and particularly by cancer cells, with subsequent intracellular release of chemotherapeutic agents, which concealed proliferation of cancer cells more effectively than untargeted controls containing the same drug (CitationJi et al. 2010). Nanotubes were engaged as carriers for imaging and therapeutic agent delivery, and the biodistribution of radio-labeled nanotubes was investigated in mice by in vivo positron emission tomography (PET), ex vivo biodistribution, and Raman spectroscopy. Although current studies have shown that administration of nanotubes did not create apparent cytotoxic effects in mice, the outcome of the nanotubes inside the cells and tissues has yet to be investigated thoroughly before further development for human use (CitationSajja et al. 2009) SWCNTs have been functionalized with antibodies and low molecular weight targeting agents, providing a high effectiveness for nanotube internalization into cells. Such systems have been loaded with drug molecules such as doxorubicin (DOX) via π-π stacking interactions, and the release rate of DOX has even been shown to be controllable by using nanotubes with diverse diameters. Nevertheless, the first generation SWCNTs proved to be unsuitable as drug delivery vectors as they tend to form bundles that disperse poorly in aqueous solutions, which is inappropriate for pharmacological use. To vanquish this drawback, synthetic polymers such as poly (phenylacetylene) and natural polymers such as polysaccharides have been used to encase SWCNT via non-covalent interactions improving their compatibility with water and physiological environments more generally (CitationZhang et al. 2009). Ramified gold nanoparticles were grown on oxidized multiwalled carbon nanotubes by one-step reduction of gold chloride in water. The carbon nanotube/gold hybrids were used for the delivery of the anticancer drug doxorubicin hydrochloride into the A549 lung cancer cell line. Doxorubicin (DOX) can be adsorbed in high quantity on both inner and surfaces of oxidized carbon nanotubes by π–π stacking interactions between doxorubicin aromatic groups and carbon nanotube (CNT) backbone. In vitro cellular tests showed that the nanostructures can efficiently transport and deliver doxorubicin inside the cells () (CitationMinati et al. 2012).
Figure 8. Carbon nanotube/gold hybrids for the delivery of doxorubicin (CitationMinati et al. 2012).
Gold nanoparticles
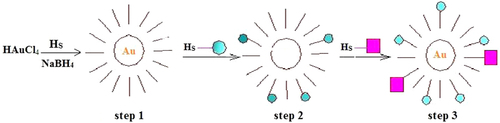
Gold nanoparticles (AuNPs) offer attractive vehicles for delivery of drugs, genetic materials, proteins, and small molecules. Lately, AuNPs have emerged as a promising delivery system for capable transport and release of pharmaceuticals into diverse cell types (CitationRana et al. 2012). They have a number of agreeable properties that make them vigorous candidates for use in delivery applications. First, the gold core is basically inert, non-toxic, and biocompatible, making it a model starting point for carrier construction. Secondly, AuNPs with a wide range of core sizes (1–150 nm) can be fabricated simply with controlled dispersity (CitationRana et al. 2012, CitationDuncan et al. 2010). A simple, systematic, and bottom-up synthesis of colloidal AuNPs was introduced by Turkevitch in 1951. In this synthesis, narrow size-dispersed (ca. 20 nm) AuNPs were obtained by reducing AuCl_4 ions with sodium citrate. This method was subsequently modified in different ways, among which a phase-transfer and reduction method introduced by Brust and coworkers is widely accepted. In this method, an aqueous solution of HAuCl4 (30 mL, 30 mM) was mixed with a solution of tetraoctylammonium bromide (80 mL, 50 mM) in toluene. This organic-aqueous two-phase mixture was vigorously stirred until the yellowish-orange color was transferred from the aqueous to organic phase, indicating the transfer of AuCl4 from the water to toluene layer by tetraoctylammonium ions. This was followed by the addition of 170 mg of dodecane thiol and a freshly prepared aqueous solution of sodium borohydride (NaBH4, 25 mL, 0.4 M), that resulted in the reduction of AuCl4 into Au NPs and the formation of dodecane thiol-capped NPs. This method has been modified over the years, and different physical and chemical methods have been introduced for the size- and shape controlled synthesis of AuNPs () (CitationPeng et al. 2008, CitationBiju et al. 2008). Both size and dispersity are key aspects for drug delivery systems. Lastly, the greatly tunable and multivalent surface structures of AuNPs offer the diversity to incorporate multiple therapeutic drugs or bio macromolecules by covalent or non-covalent conjugation on the surface of a nanoparticle. One chief aspect of AuNPs is their ease of functionalization. This capability to tailor the surface has made AuNPs useful in both active and passive targeting. Similarly, a diversity of functional monolayers can be produced to offer payload release strategies using internal or external stimuli, such as glutathione, pH, heat, and light (vide infra). As a result, the flexibility of the AuNP monolayer platform is central to the appeal of using AuNPs as drug and biomolecule delivery systems (CitationRana et al. 2012). At this point, the therapeutic capability of a novel AuNP carrier system composed of a poly (ethylene glycol) (PEG) shell, per-6-thio-b-cyclodextrin (SH-CD) as a drug pocket, and an anti-epidermal growth factor receptor antibody (anti-EGFR antibody) as a targeting ligand, is described. PEG derivatives are biocompatible polymers and can protect nanocarriers and their payloads from enzymatic degradation. Cyclodextrins (CDs) have been used as drug-delivery agents because of their ability to protect drugs from physical, chemical, and enzymatic degradation and also to solubilize hydrophobic drugs. Therefore, SH-CDs on the surface of AuNPs can present sites to accommodate hydrophobic molecules. B-Lapachone, which can be encapsulated into the cavity of b-CD (association constant: Kc ¼ (1.23 0.01) 103 M1), was adopted as a chemotherapeutic drug, because it exhibits higher activity against various cancer cells in breast, lung, and prostate tissue (CitationPark et al. 2009b).
Magnetic nanoparticles
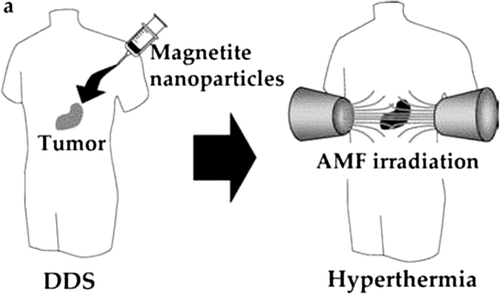
Magnetic nanoparticles (MNPs) are usually used in bio applications such as imaging (MRI), targeted delivery (drugs/genes) and cell transfection (magnetofection) (CitationValizadeh et al. 2012). The possibilities for the use of iron oxide magnetic nanoparticles in drug targeting have considerably improved in current years. MNPs, in cooperation with an external magnetic field and/or magnetizable implants, permit the delivery of particles to the ideal target area, attach them at the local site while the medication is released, and take action locally (magnetic drug targeting).Transportation of drugs to a specific site can remove side effects and also decrease the dosage required (CitationAkbarzadeh et al. 2012). Using MNPs either for diagnostic or treatment purposes was the centre of interest throughout the last two decades, but there were only two articles published on the specific delivery of nanoparticles to the lung (CitationAzarmi et al. 2008). In one of the early studies, Mykhaylyk and others evaluated the pharmacokinetics of doxorubicin magnetic conjugate (DOX-M) nanoparticles in a mouse model. They investigated the efficiency of a non-uniform magnetic field on the clearance of the magnetic DOX-M. In this work, they injected DOX-M suspensions into the sinus vein in the eye of adult male mice, and applied a magnetic field centered over the left lung. They showed that a non-uniform magnetic field was a strong factor in modifying the pharmacokinetics of the DOX-M conjugate. The magnetic field application resulted in considerable enrichment of DOX-M in the lungs, and a depletion of the magnetic carrier in the liver, compared to a reference without a magnetic field. They showed that the application of a magnetic field can significantly increase the bioavailability of DOX-M in the lungs (CitationAzarmi et al. 2008). Magnetic hyperthermia is a noninvasive therapeutic approach for lung cancer that entails the heat-induced ablation of selected cancer tissue. When subjected to alternating currents, the magnetic material, such as superparamagnetic iron oxide (SPIO) nanoparticles generate sublethal heat that causes local tissue damage. Sadhukha et al. evaluated the effectiveness of tumor-targeted SPION for hyperthermic destruction of NSCL in a mouse model () (CitationPourhassan-Moghaddam et al. 2013). Although their work in mice showed some promising results, the result of using magnetic fields in humans for increasing the localization of a drug in the lungs, using MNPs, has not yet been proven. The delivery of magnetic nanoparticles to the lungs might be valuable and worthy of more detailed research, to be used as a useful drug delivery system or as a safe diagnostic tool (CitationAzarmi et al. 2008, CitationIijima 1991).
Solid lipid nanocapsules
Since the beginning of 1990s, studies on the solid lipid nanocapsules (SLNs) have been focused on an alternative route to polymeric nanoparticles (CitationDavaran et al. 2013). Solid lipid nanocapsules (SLNs) are another class of vehicles for drug and gene delivery. SLNs are superior to their lipid counterparts in their enhanced stability, high drug loading, improved biocompatibility, and facility of large-scale manufacture and production (CitationPourhassan-Moghaddam et al. 2013). The advantages of drug release from SLNs in the lung are control of the release profile, attainment of a prolonged release, and having a faster in vivo degradation compared to particles made from PLA or PLGA. In addition, SLNs proved to have a higher tolerability in the lungs compared to particles made from some polymeric materials (CitationDavaran et al. 2013). Zara et al. studied the pharmacokinetics of doxorubicin-loaded SLNs after intravenous injection, and compared the results with a doxorubicin solution. They demonstrated that the drug concentration in the lungs was higher in animals treated with doxorubicin nanoparticles compared those injected with the doxorubicin solution () (CitationDavaran et al. 2013). Choi et al. transfected p53-null H1299 lung cancer cells with the SLN-carrier p53.The authors were able to demonstrate efficient expression of the p53 protein compared to commercially available Lipofectin, suggesting that SLNs could be used as highly efficient gene therapy vehicles in lung cancer (CitationPourhassan-Moghaddam et al. 2013). Prior studies also have demonstrated the ability to entrap amiodarone, ibuprofen tripentone, etoposide, docetaxel and paclitaxel into LNCs (CitationHureaux et al. 2009).
Inhalable nanocomposites
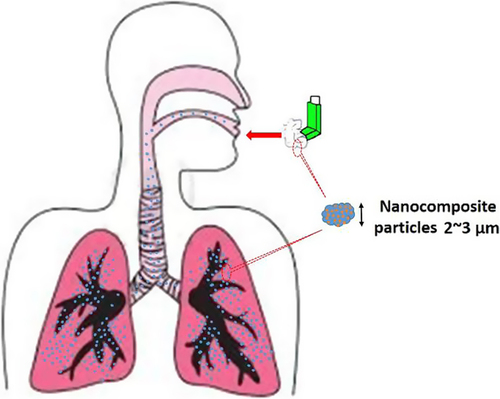
Recently, novel drugs for treatment of lung sarcoma including, camptothecin analogs, taxanes, antimetabolites and vinca alkaloids have been developed. They are radiation sensitizers, making multiple treatment with radiotherapy more effective (furthermore, their multiple doses in combination with radio-sensitizers lead to much more effective results). However, many novel drugs have strong toxicity, mostly in resulting in myelosuppression. Therefore, it is necessary to construct a new system that improves therapeutic efficiency and minimize side effects. As one of the new systems, inhalation is considered to be efficient. Inhalation is a non-invasive advancement for both local and systemic drug delivery. If anticancer drugs are able to be delivered straight to lung cancer cells, the dose can be minimized. Furthermore, toxic side effects observed in other tissues can also be minimized (CitationTomoda et al. 2009). TAS-103 is a new topoisomerase inhibitor, which acts by the inhibiting catalytic activity of both topoisomerase I and II in the small micromolar dose range. TAS-103 has a tough binding affinity for DNA. The drug has two binding modes. One is an outer surface binding from major-groove and the other is an intercalation of DNA. The drug inhibits topoisomerase-1 activity by the drug-induced DNA unwinding. On the contrary, the drug decreases the general catalytic activity of topoisomerase-2 through an inhibition of enzyme-mediated DNA religation. TAS-103 has marked efficacy against various lung metastatic tumors, and a vast antitumor spectrum in human tumor xenografts (derived from lung, colon, breast, and pancreatic cancer) (CitationTomoda et al. 2009). Poly (lactic-co-glycolic acid) (PLGA) is one of the most successfully developed biodegradable polymers. Amongst the different polymers developed to prepare polymeric nanoparticles, PLGA has attracted significant attention due to its attractive properties: (i) biodegradability and biocompatibility, (ii) FDA and European Medicine Agency agreement in use as drug delivery systems for parenteral administration, (iii) well-described formulations and methods of production adapted to various types of drugs, e.g. hydrophilic or hydrophobic, tiny molecules or macromolecules, (iv) protection of drug from degradation, (v) possibility of sustained release, (vi) possibility to change surface properties to give stealth and/or better interaction with biological materials, and (vii) possibility to target nanoparticles to specific organs or cells (CitationDanhier et al. 2012). An in vivo drug biodistribution study demonstrated that by inhalation of TAS-103-loaded PLGA nanocomposite particles, the drug collection in lungs was higher than the drug concentration in plasma. Also in the lungs, intravenous administration of free drug and nanocomposites showed that the cytotoxicity of nanocomposite against A549 cells was higher than that of free drug. Therefore, it is suggested that inhalable nanocomposite system is helpful for treatment of the lung cancer (CitationTomoda et al. 2009) ().
Conclusion and future prospects
Lung cancer is the most widespread type of cancer in the world, and each year, the number of lung cancer deaths is increasing. It seems, therefore, that there is a need for early detection and treatment. Considering the disadvantages of conventional methods for lung cancer detection, such as bronchoscopy, biopsy, and sputum cytology, we need to identify new ways and technologies for lung cancer detection. One of the new methods for the task is the use of nano devices. They are used for both identification and drug delivery. Types of nano devices used include: gold nanoparticles, liposomes, quantum dots, polymeric micelles, magnetic nanoparticles, carbon nanotubes and dendrimers. The most important features of these nano devices are that they are amenable, low cost, and time saving. Another feature of these nano devices is their large surface area, which gives rise to the possibility of surface modifications for further conjugation or encapsulation of huge amounts of therapeutic agents. In general, nanoparticles permit design in drug delivery of poorly water soluble molecules, as well as impart the ability to conquer biological barriers and selectively target desired sites within the body. Such capability of nano devices could help early detection and treatment of lung cancer. However, many challenges must be conquered in order to expedite the translation of nanoparticle-based therapies from the bench to the bedside.
Authors’ contributions
AA and FB conceived of the study and participated in its design and coordination. MR participated in the sequence alignment and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors thank the Department of Medical Nanotechnology, Faculty of Advanced Medical Science of Tabriz University for all support provided. This work is funded by 2014 Drug Applied Research Center Tabriz University of Medical Sciences Grant.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahmadi A, Shirazi H, Pourbagher N, Akbarzadeh A, Omidfar K. 2014. An Electrochemical Immunosensor for Digoxin Using Core-Shell Gold Coated Magnetic Nanoparticles as Labels. Mol Biol Rep. 41:1659–1668.
- Akbarzadeh A, Samiei M, Davaran S. 2012. Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res Lett. 7:144.
- Alberg AJ, Brock MV, Samet JM. 2005. Epidemiology of lung cancer: looking to the future. J Clin Oncol. 23:3175–3185.
- Alberg AJ, Samet JM. 2003. Epidemiology of lung cancer. Chest. 123:21S–49S.
- Azarmi S, Roa WH, Löbenberg R. 2008. Targeted delivery of nanoparticles for the treatment of lung diseases. Adv Drug Deliv Rev. 60: 863–875.
- Azzazy HME, Mansour MMH, Kazmierczak SC. 2007. From diagnostics to therapy: prospects of quantum dots. Clin Biochem. 40:917–927.
- Baptista PV. 2009. Cancer Nanotechnology-Prospects for Cancer Diagnostics and Therapy. Curr Can Ther Rev. 5:80–88.
- Bianco A, Kostarelos K, Prato M. 2005. Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol. 9:674.
- Biju V, Itoh T, Anas A, Sujith A, Ishikawa M. 2008. Semiconductor quantum dots and metal nanoparticles: syntheses, optical properties, and biological applications. Anal Bioanal Chem. 391:2469–2495.
- Blanco E, Bey EA, Khemtong C, Yang SG, Setti-Guthi J, Chen H, et al. 2010. β-lapachone micellar nanotherapeutics for non–small cell lung cancer therapy. Cancer Res. 70:3896–3904.
- Cai W, Gao T, Hong H, Sun J. 2008. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol Sci Appl. 1:17–32.
- Chan WCW, Nie S. 1998. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science. 281:2016–2018.
- Chen J, Chen S, Zhao X, Kuznetsova LV, Wong SS, Ojima I. 2008. Functionalized single-walled carbon nanotubes as rationally designed vehicles for tumor-targeted drug delivery. J Am Chem Soc. 130:16778–85.
- Chen L. 2004. Development and characterization of controlled drug delivery using nanoparticles. Thesis (M.S.) University of New Orleans.
- Chen Y-S, Hung YC, Liau I, Huang GS. 2009. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res Lett. 4:858–864.
- Conde J, Doria G, Baptista P. 2011. Noble metal nanoparticles applications in cancer. J Drug Deliv. 2012 :751075.
- Conti M, Tazzari V, Baccini C, Pertici G, Serino LP, De Giorgi U. 2006. Anticancer drug delivery with nanoparticles. In Vivo. 20: 697–701.
- Cotugno R, Fortunato R, Santoro A, Gallotta D, Braca A, De Tommasi N, Belisario MA. 2012. Effect of sesquiterpene lactone coronopilin on leukaemia cell population growth, cell type‐specific induction of apoptosis and mitotic catastrophe. Cell Prolif. 45:53–65.
- Cuenca AG, Jiang H, Hochwald SN, Delano M, Cance WG, Grobmyer SR. 2006. Emerging implications of nanotechnology on cancer diagnostics and therapeutics. Cancer. 107:459–466.
- Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. 2012. PLGA-based nanoparticles: An overview of biomedical applications. J Control Release. 161:505–522.
- Davaran S, Akbarzadeh A, Nejati-Koshki K, Alimohammadi S, Ghamari MF, Soghrati MM, Rezaei A, Khandaghi AA. 2013. In Vitro Studies of NIPAAM-MAA-VP Copolymer-Coated Magnetic Nanoparticles for Controlled Anticancer Drug Release. J Encapsul Adsorption Sciences. 3:108–115.
- De Jong WH, Borm PJA. 2008. Drug delivery and nanoparticles: applications and hazards. Inter J Nanomedicine. 3:133.
- Depierre A, Milleron B, Moro-Sibilot D, Chevret S, Quoix E, Lebeau B, et al. 2002. Preoperative chemotherapy followed by surgery compared with primary surgery in resectable stage I (except T1N0), II, and IIIa non–small-cell lung cancer. J Clin Oncol. 20:247–253.
- Dienemann H. 2001. Principles of surgical treatment in localized non-small cell lung cancer. Lung Cancer. 33:S3–S8.
- Digge MS, Moon RS, Gattani SG. 2012. Application of Carbon Nanotubes in Drug Delivery: A Review. Int J Pharm Tech Res. 4:839–847.
- Dua JS, Rana AC, Bhandari AK. 2012. Liposome: Methods of Preparation and Applications. IJPSR. 3:14–20.
- Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. 2002. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 298:1759–1762.
- Duncan B, Kim C, Rotello VM. 2010. Gold nanoparticle platforms as drug and biomacromolecule delivery systems. J Control Release. 148:122–127.
- Fanfair D, Desai S, Kelty C. 2007. The early history of nanotechnology. Connexions. 6.
- Fong KM., Yang IA, Zimmerman PV, Bowman RV. 2005. Cochrane systematic reviews of treatments for lung cancer. Respir Med. 99:1071–1078.
- Ganti AK, Mulshine JL. 2006. Lung cancer screening. Oncologist. 11:481–487.
- Gao L, Wang Z, Li F, Hammoudi AA, Thrall MJ, Cagle PT, Wong ST. 2012. Differential Diagnosis of Lung Carcinoma With Coherent Anti-Stokes Raman Scattering Imaging. Arch Pathol Lab med. 136:1502–1510.
- Gaspar MM, Radomska A, Gobbo OL, Bakowsky U, Radomski MW, Ehrhardt C. 2012. Targeted delivery of transferrin-conjugated liposomes to an orthotopic model of lung cancer in nude rats. J Aerosol Med Pulm Drug Deliv. 25:310–318.
- Ghasemi Y, Peymani P, Afifi S. 2009. Quantum dot: magic nanoparticle for imaging, detection and targeting. Acta Biomed. 80:156–165.
- Gillies ER, Frechet JMJ. 2005. Dendrimers and dendritic polymers in drug delivery. Drug Discovery Today. 10:35–43.
- Gomathi M, Thangaraj P. 2010. A computer aided diagnosis system for detection of lung cancer nodules using extreme learning machine. International Journal of Engineering Science and Technology. 2:5770–5779.
- Gordon SM, Szidon JP, Krotoszynski BK, Gibbons RD, O’Neill HJ. 1985. Volatile organic compounds in exhaled air from patients with lung cancer. Clin Chem. 31:1278–1282.
- Guessous I, Cornuz J, Paccaud F. 2007. Lung cancer screening: current situation and perspective. Swiss Medical Weekly. 137:304–11.
- Hardman R. 2006. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect. 114:165.
- Heath JR, Davis ME. 2008. Nanotechnology and cancer. Annu Rev Med. 59:251–265.
- Heister E, Neves V, Tîlmaciu C, Lipert K, Beltrán VS, Coley HM, et al. 2009. Triple functionalisation of single-walled carbon nanotubes with doxorubicin, a monoclonal antibody, and a fluorescent marker for targeted cancer therapy. Carbon. 47:2152–2160.
- Horvath I, Lázár Z, Gyulai N, Kollai M, Losonczy G. 2009. Exhaled biomarkers in lung cancer. Eur Respir J. 34:261–275.
- Hou PX, Bai S, Yang QH, Liu C, Cheng HM. 2002. Multi-step purification of carbon nanotubes. Carbon. 40:81–85.
- Huang X, El-Sayed IH, El-Sayed MA. 2010. Applications of gold nanorods for cancer imaging and photothermal therapy. Methods Mol. Biol. 624:343–357.
- Hureaux J, Lagarce F, Gagnadoux F, Vecellio L, Clavreul A, Roger E, et al. 2009. Lipid nanocapsules: ready-to-use nanovectors for the aerosol delivery of paclitaxel. Eur J Pharm Biopharm. 73:239–246.
- Iijima S. 1991. Helical microtubules of graphitic carbon. Nature. 354:56–58.
- Immordino ML, Dosio F, Cattel L. 2006. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 1:297.
- Indira TK, Lakshmi PK. 2010. Magnetic nanoparticles—a review. Inter J Pharm Sci Nanotechnol. 3.
- Ito A, Shinkai M, Honda H, Kobayashi T. 2005. Medical application of functionalized magnetic nanoparticles. J Biosci Bioeng. 100:1–11.
- Jamieson T, Bakhshi R, Petrova D, Pocock R, Imani M, Seifalian AM. 2007. Biological applications of quantum dots. Biomaterials. 28:4717–4732.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. 2011. Global cancer statistics. CA Cancer J Clin. 61:69–90.
- Ji S-R, Liu C, Zhang B, Yang F, Xu J, Long J, et al. 2010. Carbon nanotubes in cancer diagnosis and therapy. Biochim Biophys Acta. 1806:29–35.
- Jin, S., Hu YX, Gu ZJ, Liu L, Wu HC. 2011. Application of quantum dots in biological imaging. Journal of Nanomaterials. 2011:12.
- Jones M-C, Leroux J-C. 1999. Polymeric micelles–a new generation of colloidal drug carriers. Eur J Pharm Biopharm. 48:101–111.
- Justo OR, Moraes ÂM. 2003. Incorporation of antibiotics in liposomes designed for tuberculosis therapy by inhalation. Drug Deliv. 10: 201–207.
- Kaskowitz L, Graham MV, Emami B, Halverson KJ, Rush C. 1993. Radiation therapy alone for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 27:517–523.
- Kim DW, Kim SY, Kim HK, Kim SW, Shin SW, Kim JS, et al. 2007. Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Annals of oncology. 18:2009–2014.
- Konkar A, Lu S, Madhukar A, Hughes SM, Alivisatos AP. 2005. Semiconductor nanocrystal quantum dots on single crystal semiconductor substrates: high resolution transmission electron microscopy. Nano Lett. 5:969–973.
- Kurmi BD, Gajbhiye V, Kayat J, Jain NK. 2011. Lactoferrin‐conjugated dendritic nanoconstructs for lung targeting of methotrexate. J Pharm Sci. 100:2311–2320.
- Lam S, Lam B, Petty TL. 2001. Early detection for lung cancer. New tools for casefinding. Can Fam Physician. 47:537–544.
- Leutwyler WK, Bürgi SL, Burgl HB. 1996. Semiconductor clusters, nanocrystals, and quantum dots. Science. 271:933.
- Liu J, Chu L, Wang Y, Duan Y, Feng L, et al. 2011a. Novel peptide– dendrimer conjugates as drug carriers for targeting nonsmall cell lung cancer. Inter J Nanomedicine. 6:59.
- Liu R, Gan L, Yang X, Xu H. 2011b. Chitosan as a condensing agent induces high gene transfection efficiency and low cytotoxicity of liposome. J Biosci Bioeng. 111:98–103.
- Ma J, Fan Q, Wang L, Jia N, Gu Z, Shen H. 2010. Synthesis of magnetic and fluorescent bifunctional nanocomposites and their applications in detection of lung cancer cells in humans. Talanta. 81:1162–1169.
- Markides H, Rotherham M, El Haj AJ. 2012. Biocompatibility and Toxicity of Magnetic Nanoparticles in Regenerative Medicine. J Nanomater.
- Minati L, Antonini V, Dalla Serra M, Speranza G. 2012. Multifunctional Branched Gold–Carbon Nanotube Hybrid for Cell Imaging and Drug Delivery. Langmuir. 28:15900–15906.
- Miyata K, James Christie R, Kataoka K. 2010. Polymeric micelles for nano-scale drug delivery. React Funct Polym.
- Mody HR. 2011. Cancer nanotechnology: Recent trends and developments. Internet J Med Update-EJOURNAL. 6.
- Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. 2008. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
- Mozafari M, Pardakhty A, Azarmi S, Jazayeri JA, Nokhodchi A, Omri A. 2009. Role of nanocarrier systems in cancer nanotherapy. J Liposome Res. 19:310–321.
- Nakanishi T, Fukushima S, Okamoto K, Suzuki M, Matsumura Y, Yokoyama M et al. 2001. Development of the polymer micelle carrier system for doxorubicin. J Control Release. 74: 295–302.
- Nishiyama N, Kataoka K. 2006. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol Ther. 112:630–648.
- Okunaka T, Kato H, Tsutsui H, Ishizumi T, Ichinose S, Kuroiwa Y. 2004. Photodynamic therapy for peripheral lung cancer. Lung Cancer. 43:77–82.
- Park K, Lee S, Kang E, Kim K, Choi K, Kwon IC. 2009a. New generation of multifunctional nanoparticles for cancer imaging and therapy. Adv Funct Mater. 19:1553–1566.
- Park C, Youn H, Kim H, Noh T, Kook YH, Oh ET, et al. 2009b. Cyclodextrin-covered gold nanoparticles for targeted delivery of an anti-cancer drug. J. Mater. Chem. 19:2310–2315.
- Pastorino U. 2006. Early detection of lung cancer. Respiration. 73: 5–13.
- Patton JS, Byron PR. 2007. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 6:67–74.
- Peng G, Trock E, Haick H. 2008. Composites of carbon nanotubes and non-polymeric materials for diagnosing lung cancer via breath samples. International Society for Optics and Photonics.
- Pourhassan-Moghaddam M, Rahmati-Yamchi M, Akbarzadeh A, Daraee H, Nejati-Koshki K, Hanifehpour Y, Joo SW. 2013. Protein Detection through Different Platforms of Immuno-Loop-Mediated Isothermal Amplification. Nanoscale Res Lett. 8:485.
- Preti G, Labows JN, Kostelc JG, Aldinger S, Daniele R. 1988. Analysis of lung air from patients with bronchogenic carcinoma and controls using gas chromatography-mass spectrometry. J Chromatogr. 432:1–11.
- Qian X, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, et al. 2007. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat Biotechnol. 26:83–90.
- Quintana A, Raczka E, Piehler L, Lee I, Myc A, Majoros I, et al. 2002. Design and function of a dendrimer-based therapeutic nanodevice targeted to tumor cells through the folate receptor. Pharm Res. 19:1310–1316.
- Ramalingam S, Belani C. 2008. Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. Oncologist. 13:5–13.
- Rana S, Bajaj A, Mout R, Rotello VM. 2012. Monolayer coated gold nanoparticles for delivery applications. Adv Drug Deliv Rev. 64:200–216.
- Ray M, Salgia R, Vokes EE. 2009. The role of EGFR inhibition in the treatment of non-small cell lung cancer. Oncologist. 14:1116–1130.
- Sadat TMF, Akbarzadeh A, Zarghami N, Zeighamian V, Rahimzadeh A, Alimohammadi S. 2014. PLGA-cased nanoparticles as cancer drug delivery systems. Asian Pac J Cancer Prev. 15:517–535.
- Sajja HK, East MP, Mao H, Wang YA, Nie S, Yang L. 2009. Development of Multifunctional Nanoparticles for Targeted Drug Delivery and Non-invasive Imaging of Therapeutic Effect. Curr Drug Discov Technol. 6:43.
- Savić R, Luo L, Eisenberg A, Maysinger D. 2003. Micellar nanocontainers distribute to defined cytoplasmic organelles. Science. 300: 615–618.
- Shenoy D, Fu W, Li J, Crasto C, Jones G, DiMarzio C, et al. 2006. Surface functionalization of gold nanoparticles using hetero-bifunctional poly (ethylene glycol) spacer for intracellular tracking and delivery. Inter J Nanomedicine. 1:51.
- Sher T, Dy GK, Adjei AA. 2008. Small cell lung cancer. Mayo Clin Proc. 83:355–367
- Silva GA. 2004. Introduction to nanotechnology and its applications to medicine. Surg Neurol. 61:216–220.
- Singh R, Lillard JW Jr. 2009. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 86:215–223.
- Sung JC, Pulliam BL, Edwards DA. 2007. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 25:563–570.
- Sutedja G. 2003. New techniques for early detection of lung cancer. Euro Respi J. 21:57s–66s.
- Tartaj P, del Puerto Morales M, Veintemillas-Verdaguer S, González-Carreno T, Serna CJ. 2003. The preparation of magnetic nanoparticles for applications in biomedicine. J Phys D Appl Phys. 36:R182.
- Tiwari PM, Vig K, Dennis VA, Singh SR. 2011. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials. 1:31–63.
- Tomoda K, Ohkoshi T, Hirota K, Sonavane GS, Nakajima T, Terada H, et al. 2009. Preparation and properties of inhalable nanocomposite particles for treatment of lung cancer. Colloids Surf B Biointerfaces. 71:177–182.
- Ukraintseva SV, Arbeev KG, Yashin AI. 2008. Epidemiology of hormone-associated cancers as a reflection of age. Adv Exp Med Biol. 57–71.
- Valizadeh A, Mikaeili H, Samiei M, Farkhani SM, Zarghami N, Kouhi M. 2012. Quantum dots: synthesis, bioapplications, and toxicity. Nanoscale Res Lett. 7:480.
- Wang Y, Zhang Y, Du Z, Wu M, Zhang G. 2012. Detection of micrometastases in lung cancer with magnetic nanoparticles and quantum dots. Int J Nanomedicine. 7:2315.
- Warner E, Jotkowitz A, Maimon N. 2010. Lung cancer screening–are we there yet? Eur J Intern Med. 21:6–11.
- White SC, Lorigan P, Margison GP, Margison JM, Martin F, Thatcher N. 2006. Phase II study of SPI-77 (sterically stabilised liposomal cisplatin) in advanced non-small-cell lung cancer. Br J Cancer. 95:822–828.
- Yang W, Peters JI, Williams RO. 2008. Inhaled nanoparticles—a current review. Int J Pharm. 356:239–247.
- Yokoyama M, Okano T, Sakurai Y, Kataoka K. 1994. Improved synthesis of adriamycin-conjugated poly (ethylene oxide)-poly (aspartic acid) block copolymer and formation of unimodal micellar structure with controlled amount of physically entrapped adriamycin. J Controlled Release. 32:269–277.
- Yokoyama M, Sugiyama T, Okano T, Sakurai Y, Naito M, Kataoka K. 1993. Analysis of micelle formation of an adriamycin-conjugated poly (ethylene glycol)–poly (aspartic acid) block copolymer by gel permeation chromatography. Pharm Res. 10:895–899.
- Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. 2007. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 83:761–769.
- Zhang X, Meng L, Lu Q, Fei Z, Dyson PJ. 2009. Targeted deli- very and controlled release of doxorubicin to cancer cells using modified single wall carbon nanotubes. Biomaterials. 30: 6041–6047.