?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The present review briefly describes the nature, type and pathogenesis of ulcerative colitis, and explores the potential use of peptides and proteins in the treatment of inflammatory bowel disease, especially ulcerative colitis. Intestinal absorption and the barrier mechanism of peptide and protein drugs are also discussed, with special emphasis on various strategies which make these drugs better therapeutics having high specificity, potency and molecular targeting ability. However, the limitation of such therapeutics are oral administration, poor pharmacokinetic profile and decreased bioavailability. The recent findings illustrated in this review will be helpful in designing the peptide/protein drugs as a promising treatment of choice for ulcerative colitis.
Introduction
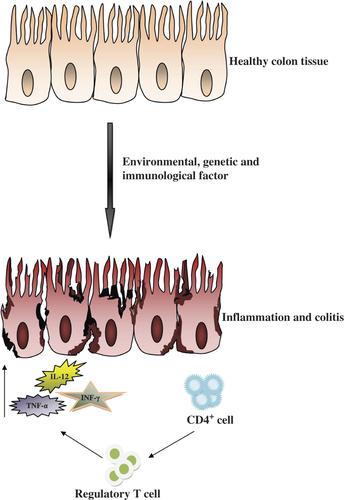
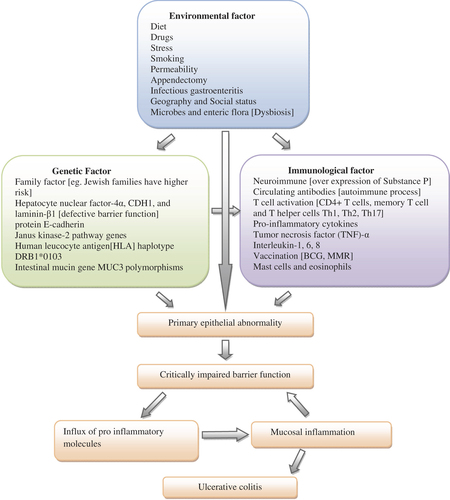
Ulcerative colitis (UC) and Crohn's disease (CD) are two forms of inflammatory bowel disease (IBD), which are characterized by chronic, persistent and relapsing ulceration of the bowel, resulting in weight loss, chronic diarrhea and bloody stool (CitationRabbi et al. 2014). Specific clinical manifestations discriminate both these diseases; however, the common symptoms include diarrhea, bloody stool, weight loss, abdominal pain, cramping, fatigue and fever (CitationCollnot et al. 2012). In UC, the inflammatory response is limited to the colon only, involving its mucosal and submucosal layers, while in CD, the inflammation process extends to any part of the gastrointestinal tract throughout the bowel wall layers, i.e. mucosa, submucosa, muscularis and serosa (CitationAmbrosini et al. 2007). UC is a destructive disease, mainly involving secretory and motility disorders of the large intestine (CitationStoyanova and Gulubova 2002, CitationCollnot et al. 2012). The precise etiology of these diseases is not yet available now, but some studies report that immune, genetic, environmental, and mainly microbial factors, are involved in the pathogenesis of these disorders (CitationKanwar et al. 2008). However, one study suggests that their pathogenesis involves an imbalance in the reaction between pro-inflammatory and anti-inflammatory responses, which is mainly due to the activation of CD4 T-helper 1 and T-helper 17 cells () (CitationKayhan et al. 2013). A number of animal models for colitis have recently been recognized, which provide new insights into the pathogenesis and immune retort of this disease (CitationHong et al. 2002).
IBD shows variability of its incidence across different countries; it is high in the United States and United Kingdom (1 in every 250) but low in Asia, South America and New Zealand Maori (CitationJess et al. 2002, CitationGearry et al. 2006). The worldwide incidence rate of UC, which is 0.5 to 24.5 per 100,000 inhabitants, is more than that of CD, which is 0.1 to 16 per 100,000 inhabitants (CitationMolodecky et al. 2012, CitationCosnes et al. 2011). In Asia, the prevalence rate of UC is more than that of CD (CitationNg et al. 2013). Both these diseases are due to the westernization in the diet and lifestyle of people, and are more prevalent in the developed countries as compared to the developing nations (CitationNg et al. 2013, CitationBolge et al. 2010).
The rates of prevalence and incidence of UC in India are much higher than in other Asian countries. In India, the incidence rate of UC is recorded as 6.02 per 100,000 (CitationSood et al. 2003), which is much higher than in the other Asian countries as a whole, where it ranges from 0.4 to 2.1 per 100,000 population (CitationYang et al. 2008, CitationLok et al. 2008, CitationChow et al. 2009, CitationMorita et al. 1995). Populations of Indian ethnic origin have a higher prevalence of UC as compared to those of Chinese or Malay origin (CitationLee et al. 2000, CitationLing et al. 2002, CitationTan and Goh 2005). All these data provide evidence that UC is a common disease in India, distressing a large range of population.
Although a wide variety of marketed preparations are available for this disease, they provide only symptomatic relief and need further improvement (CitationRandall et al. 2012).
Nature of the disease
This disease is predominant at a young age (< 35 years) (). Increased prevalence in population is due to diet habits involving either gluten-rich food, or the high intake of commercially prepared food. Moreover, UC is more prevalent among non-smokers/ex-smokers, as compared to smokers (CitationGunisetty et al. 2012). One study reports that males are a little more susceptible to UC as compared to females (CitationLi et al. 2012). An internet-based survey on UC-affected individuals revealed that more than 50% of them experienced flares at least one or more times per week or month (CitationBolge et al. 2010). It can be recognised that it is a familial disease, as 20% of IBD individuals have blood relatives suffering with the same or other forms of this disease. The genetic basis of this disease comes from the evidence that 36% of monozygotic twins share this disease, while the rate is only 4% in dizygotic twins (CitationJess et al. 2005, CitationHalfvarson et al. 2005). More than 163 genomic loci are associated with IBD, 90 with CD, 73 with UC, and 110 for both diseases, as reported by genome-wide associated studies (GWAS) (CitationDeuring et al. 2013). The risk of colorectal cancer (CRC) is linked with progressive inflammation in this disease, which increases by 0.5%–1%/year/, 8–10 years after diagnosis (CitationLi et al. 2012).
Table I. Types of ulcerative colitis (CitationConrad et al. 2014, CitationTravis et al. 2008, CitationBokemeyer et al. 2012, CitationBitton 2001, CitationLimbergen et al. 2008).
Microscopic colitis (MC) is another subtype of this disease, in which the colonic mucosa shows inflammatory disorder but the endoscopic result is near normal (CitationPardi and Kelly 2011). Smoking increases the risk of MC, but alcohol consumption and lifestyle factors have no effect on it (CitationRoth et al. 2014).
Pathogenesis of ulcerative colitis
A genetically prejudiced, blown up and continual response of immunity against the body's own gut flora is an important pathogenic factor of this malady (). Environmental factors directly and indirectly stimulate these two factors involved in the disease pathogenesis (CitationCarbonnel et al. 2009, CitationGibson 1997, CitationDanese et al. 2004, CitationStasikowska et al. 2012, CitationPearl et al. 2013, CitationHanai et al. 2013, CitationOrdas et al. 2012, CitationFarrell and Peppercorn 2002).
Oral delivery of peptide/protein drug
The use of peptide and protein drugs has increased noticeably in the last two decades (CitationMcCrudden et al. 2013). The parenteral route is generally used for delivering peptides and proteins, but in comparison to the oral route, it has a low patient compliance, consumes time and money, and is inconvenient, sometimes also dangerous (CitationDorkoosh et al. 2001).
Peptides and proteins are good in many aspects, such as their high potency, good selectivity and low toxicity, but the limitation of oral administration is the enzyme degradation in the GI tract (GIT) and serum (CitationSobczak et al. 2014). Moreover the molecular weight and size also affect their diffusion through the epithelial layer, which decreases with increase in molecular mass beyond 700 Da (CitationAntosova et al. 2014). Until the mid-1960s, it was assumed that the GIT digests all the orally administered peptides and proteins entirely before their absorption takes place, while little reaches the systemic circulation, having no nutritional or clinical relevance (CitationGitler 1964). However, later studies proposed that small amounts of such molecules enter blood circulation in the intact form (CitationGardner 1987). Cyclic peptides, in comparison with linear peptides, have increased metabolic stability (chemical and enzymatic) and lipophilicity, resulting in good half-life and improved penetration across biological membranes (CitationPiekielna et al. 2013). The use of mucoadhesive microsphere preparations from polyanhydrides is another approach to target the drug to the mucous lining of the intestines, which increases its bioadhesive properties, thus resulting in increased bioavailability of the delivered proteins and peptides (CitationPettit and Gombotz 1998).
Cellular uptake mechanism of proteins and peptides
Detection of antibodies to food proteins in human blood circulation indicated absorption of proteins and peptides (CitationRundles 1987, CitationPaganelli and Levinsky 1980). Appropriate absorption of protein and peptide is possible in the colon, as it lacks digestive enzymes and proteolytic activity; moreover, it provides longer residence time (CitationSinha and Kumria 2003). The major transport mechanisms involved in the colon for uptake of protein and peptide are the paracellular, transcellular, carrier-mediated, and receptor-mediated transport ().
Figure 3. Integrated process of absorption mechanism and hindrance factor of peptide and protein in intestinal epithelial cell. [A, B, C and D stand for receptor-mediated, paracellular, transcellular and carrier-mediated transport of protein drug respectively, whereas E, F, G and H stand for luminal enzyme, mucin barrier, brush border enzyme and intracellular enzyme respectively].
![Figure 3. Integrated process of absorption mechanism and hindrance factor of peptide and protein in intestinal epithelial cell. [A, B, C and D stand for receptor-mediated, paracellular, transcellular and carrier-mediated transport of protein drug respectively, whereas E, F, G and H stand for luminal enzyme, mucin barrier, brush border enzyme and intracellular enzyme respectively].](/cms/asset/1f783a68-32c2-4f49-ad2d-4e8992a224e4/ianb_a_975239_f0003_oc.jpg)
Paracellular transport
Paracellular transport allows the movement of substances across an epithelium by passing through water filled pores/channels in between epithelial cells (CitationPappenheimer and Reiss 1987, CitationMadara 1998). It is a passive diffusion transport regulated by a tight junction, which limits the permeation of ions and larger substances (CitationMadara 1998). Peptide and protein molecules having a value of log P less than zero (Pappenheimer & Reiss) and a molecular radius less than 15 Å (approximately 3.5 kDa) enter via this route (CitationRubas et al. 1996). The tight junctional paracellular space has a dimension of 8–9 Å in the human colon (CitationTomita et al. 1998). Paracellular transport of polypeptides depends upon the physicochemical properties, ion concentration, pH, molecular dimension, and anomalous mole-fraction effects (CitationTang and Goodenough 2003, CitationPauletti et al. 1997, CitationMiller and Johnston 2005), but is not much affected by the lipophilicity and hydrogen bonding capacity. It constitutes about 0.01% of the total absorptive surface area of the intestine (CitationPappenheimer and Reiss 1987).
Transcellular transport
Transcellular transport involves the process of transcytosis, i.e. the cell's apical membrane takes up the particle endocytically, and it is liberated at the basolateral pole (CitationShakweh et al. 2004). Unlike the paracellular route, which is limited to only tiny hydrophilic molecules (CitationMorishita and Peppas 2006), transcytosis is suitable for macro-sized proteins and peptides. As peptides have a lipophilicity [log P] range of − 2.2 – + 2.8 (CitationConradi et al. 1991), the number of polar groups determine its permeability (CitationBurton et al. 1996), and energy is required for such absorption to break the water– peptide hydrogen bonds (CitationConradi et al. 1991). It is a widely accepted transport mechanism for lipophilic substances, as the biological cell membrane has a lipid bilayer structure (CitationRautio et al. 2008). The intestine contains M-cells (1% of the total intestinal surface) and enterocytes as transporter cells (CitationGiannasca et al. 1999). M-cells transport a wide range of oral proteins and peptides, as they have high endocytotic capacity (CitationFrey and Neutra 1997, CitationClark et al. 2000). Transcellular transport depends upon size, charge, lipophilicity, hydrogen bond and presence of ligands. Moreover, the physiology of the GIT and the type of animal model also bear an influence (CitationFlorence 2004 & CitationBurton et al. 1991).
Carrier-mediated transport
This process follows an active transport mechanism in which specific molecules are taken up by carriers on the cell surface. It starts after recognition of the target molecule by the carrier, and its transportation across the GI epithelium, against the concentration gradient (CitationBai and Amidon 1992). Small hydrophilic molecules (CitationBarthe et al. 1999) and di/tripeptides (CitationBai and Amidon 1992) are actively absorbed by this process. The presence of net charge on peptides affects their affinity for intestinal carrier transport. Neutral dipeptides show the highest affinity, as compared to dipeptides containing two positive charges (CitationWood et al. 1992), whereas negative charges are more damaging to the affinity of the carrier system as compared to positive charges (CitationWootton and Hazelwood 1989).
Receptor-mediated transport
In receptor-mediated transport, protein molecules facilitate entry by acting either as receptors for ligands or ligands for surface-attached receptors (CitationJones 2002). In this transport system, one can increase the oral bioavailability of proteins by the modification of receptors and ligands specific for proteins and peptides. This transport system is known as endocytosis, as it involves cell invagination and vesicle formation. It comprises pinocytosis, phagocytosis, clathrin-mediated (receptor-mediated), and non-clathrin-mediated (potocytosis) endocytosis (CitationSwaan 2002). G-protein coupled receptors [GPCR] play a significant role in exerting the action of a large number of gut peptides. GPCRs comprise a family of about 700 membrane proteins, out of which 87 are identified to have peptide ligands (CitationHarmar 2004).
Progression obstacles: efficacy and safety considerations
Protein drugs formulated for parenteral administration are easily available, but very few formulations for oral administrations are available because of the poor bioavailability profile, as it depends upon the ability to cross the intestinal mucosa and reach the systemic blood flow (CitationKwan 1997). Only dimensional fractions of orally administered peptides and proteins reach the systemic circulation intact. The systemic availability of orally administered peptides [F] is calculated as
Where, FF = dose fraction that is neither metabolised in the gut nor lost in the faeces, FG = dose fraction that escapes degradation within GIT wall and reaches the portal vein, FH = dose fraction that escapes liver metabolism (CitationLangguth et al. 1997). The several factors which are hurdles in achieving good bioavailability of proteins and peptides are gastric pH, enzymatic degradation (CitationIkesue et al. 1993), large molecular size (CitationDonovan et al. 1990), immunogenicity, short plasma half-life and other factors like adsorption, aggregation and denaturation (CitationSaffran et al. 1986, CitationFix 1996). The surface area, mucosal thickness, residence time and nature of drug govern site-specific GIT absorption (CitationKompella and Lee 2001). Thus, development of an effective formulation still remains a challenge to researchers. In the pathway of effective oral delivery of therapeutic protein for IBD, metabolic liability and tissue impermeability are two important barrier factors (CitationHong et al. 2012).
Metabolic obstacle
In the GIT, metabolic enzymes are present everywhere including the lumen, brush border, cytosol, lysosomes and other cell organelles (CitationLangguth et al. 1997, CitationReis et al. 2006). Colonic tissue contains microvilli (1 mm high projection), but it lacks villi. Microvilli are a glycoprotein coating (glycocalyx), which forms a biochemical barrier at the gut surface. The glycocalyx consists of sulfated mucopolysaccharides, and being fuzzy and fibrous in nature, it forms a weakly acidic coat over the epithelial cells (CitationMacAdam 1993). It is associated with proteolytic activity of degrading proteins and peptides. The mucus layer present atop the glycocalyx consists of glands and goblet cells (CitationDaugherty and Mrsny 1999). The mucus secreted by goblet cells (CitationMacAdam 1993) is composed of mucin glycoproteins, enzymes, lipids, electrolytes [5%] and water [95%] (CitationPhelps 1978, CitationPonchel et al. 1987), and restricts the physical movement of proteins and peptides (CitationDaugherty and Mrsny 1999). The mucin glycoprotein imparts the hydrogel characteristics of mucus (CitationBakhru et al. 2013), and contributes to its cohesive and adhesive nature (CitationAllen and Garner 1980, CitationKompella and Lee 2001). Moreover, The GIT offers a swarm of diverse metabolic enzymes such as trypsin, chymotrypsin, and elastase, which act as endopeptidases, whereas aminopeptidase and carboxypeptidase A are exopeptidases in nature (CitationWoodley 1994). Being proteolytic in nature, these enzymes easily cleave orally administered proteins and peptides.
Tissue impermeability obstacle
Epithelial cell sheets represent tight apical junctions to most constituents, and work as a semipermeable barrier to ions, cells, solutes and water also. The epithelial cell sheet is identified and characterised as a transmembrane protein tight junction complex and includes proteins like claudins, junctional adhesion molecules (JAMs), occludin and tricellulin. These proteins contribute to the tight junctional nature of the barrier system (CitationChiba et al. 2008), and this is how the tight junctional structure acts as a physical barrier to peptide and protein transport (CitationMacAdam 1993). Occludin (60 kDa) was the first protein to be identified (CitationFuruse et al. 1993), whereas claudin (18–27 kDa) (CitationTsukita et al. 2001), is the major protein with barrier function, (CitationFuruse and Tsukita 2006) which the forms the yarn [backbone] for the tight junction (CitationFuruse et al. 1998). However, tight junctions have a role in maintaining homeostasis, and disturbance in their function leads to IBD and other disease conditions (CitationMankertz and Schulzke 2007). IBD-related diarrhea by a leak flux mechanism is an important characteristic of tight junction impairment (CitationMankertz and Schulzke 2007, CitationHering and Schulzke 2009). Changes in tight junctional proteins, especially increased expression of claudin-2, are noticed in UC (CitationSchulzke et al. 2009, CitationMankertz and Schulzke 2007).
Strategies to improve biological activity, specificity, stability and delivery of proteins and peptides
Physiochemical properties (molecular weight, pH stability, hydrophobicity, molecular size, and ionization constant) and biological barriers (proteolysis in stomach, variable pH, poor permeation and membrane efflux) are the two important factors to be considered carefully for effective oral delivery of proteins and peptides (CitationMahato et al. 2003). A number of strategies in this regard have been proposed, to avoid the degradation of protein and peptide drugs (CitationMorishita and Peppas 2006). Various approaches are summarized in . They include the opening of tight junctions to increase paracellular transport (CitationMiller and Johnston 2005), and the use of carrier systems such as microemulsions and various polymeric nanoparticles (NPs). Increasing the permeability of the cell membrane retention time in GI retention is another approach. All these techniques result in increased intestinal uptake and lymphatic absorption (CitationSchnurch 2013, CitationReis et al. 2006).
Table II. Various strategies for biotherapeutics of protein and peptide.
An ideal strategy should include the following points:-
Increase in intestinal contact time, resulting in increased bioavailability.
Prevent degradation by GIT microbial flora and increase absorption of poor drug.
Stability and integrity of peptides and proteins should not be altered.
Free from GIT irritation and cost effective also.
Various strategies in this regard include:-
Chemical modification
In last few decades, various structural modifications of peptides and proteins have been done to overcome the metabolic problem (proteolysis), which also increase potency and specificity. Chemical modification includes cyclization (CitationGangwar et al. 1997), lipophilic derivatization (CitationGoldberg and Orellana 2003), peptidomimetic synthesis (CitationAllemann et al. 1998) or alteration of the carbohydrate moiety (glycoprotein) linked to the protein (CitationCalceti et al. 2004). However, it should be noted that all these techniques should not affect the receptor- binding affinity and the intact form of the protein drugs. A study in the Caco-2 cell monolayer reveals that the conversion of a linear peptide into a cyclic form increases its intramolecular hydrogen bonding, which enhances its permeability. This is because the chances of hydrogen bond formation of peptide molecule with the aqueous solvent are reduced (CitationGangwar et al. 1997). Addition of functional groups is another approach in this regard, which includes acylation, PEGylation, glycosylation, and cross-linking. The hexyl-insulin monoconjugate (PEG-conjugated insulin) is such example, which shows acceptability in clinical phase for lowering the glucose level (CitationAl-Hilal et al. 2013). PEG conjugation of proteins shows a steric effect, which is helpful in protecting them from enzymatic degradation (CitationRekha and Sharma 2013).
Use of polymers
Advancement in the oral delivery of protein is incomplete without a significant polymer strategy, as it is a widely applied approach concerning the intestinal absorption, enzymatic degradation and loading capacity (CitationKadiyala et al. 2010). Various systems of formulation include the use of polymeric NPs, liposomes, bioconjugates, hydrogels and microparticles (CitationDi Marco et al. 2010, CitationYang and Frokjaer 2009). The choice of polymer should be suitable (composition and physicochemical properties), and according to the nature of peptide, so that optimised nanocomposites are achieved. Here too, PEG is the polymer best suited for the protein-polymer conjugate system, because of its good solubility, stability, biocompatibility and less toxicity (CitationSalmaso and Caliceti 2011). The size of the protein also affects its diffusion from the polymer matrix, as it is very fast in porous gels like polyacrylamide, which leads to tissue damage (CitationDai et al. 2005). The size of nanocomposites also determines their efficacy, and scientists have found that 600 nm-sized NPs are the best in treating IBD because of drug accumulation and low side effect due to reduced systemic entry (CitationWilson et al. 2010). Biocompatible polymers (Anionic dextrans, neutral water-soluble polymers, cationic polymer, hydrophobic polymers) release the drug in the intact form and prevent protein degradation, as compared to chemical bioconjugates (CitationSalmaso and Caliceti 2013).
Vesicular-mediated delivery
The vesicular system, being a novel method of drug delivery, provides targeted and controlled delivery of protein drugs with protection against the harsh environment in the GIT. Both hydrophilic and hydrophobic drugs can be loaded suitably in this system. Researchers have designed various carriers for such delivery, among which liposomes were designed first (CitationKumar and Rajeshwarrao 2011). Similarly, NPs are a part of one such carrier system, having good drug release profile and GIT stability (CitationRieux et al. 2006). Oral delivery of protein-loaded NPs is useful in targeting enterocytes, M-cells, L-cells and immune cells for treatment of inflammatory conditions and diseases (CitationRieux et al. 2013). Another novel approach in this regard includes the pH-sensitive and time-dependent vesicular system for inflammation of the colon (CitationPinto 2010). The most recently developed carriers in this regard include aquasomes (CitationUmashankar et al. 2010), niosomes (CitationKumar and Rajeshwarrao 2011), bilosomes (CitationSizer 1997), and biotinylated liposomes (BLPs) (CitationZhang et al. 2014). Aquasomes are self-assembled three-layered structures in which the drug loading is done in the central solid core. Aquasomes provide structural and biochemical stability to a wide variety of peptides such as insulin and hemoglobin (CitationPandey et al. 2011), which gives an assurance for their future therapeutic use in peptide delivery (CitationUmashankar et al. 2010). Similarly, bilosomes and biotinylated liposomes (BLPs) are conjugated with bile salt and biotin respectively, having good power of penetration and resulting in increased bioavailability (CitationSizer 1997, CitationZhang and Li 2014). The use of poly(lactic acid)-b-Pluronic-b-poly(lactic acid) (PLA-P85-PLA) vesicles is another such example for successful and effective delivery of insulin and other therapeutic peptides and proteins (CitationXiong et al. 2013).
Other approaches
Mucolytics as good permeation enhancers are used for the oral delivery of protein-containing NPs. Absorption increases upto 6-fold by using N-acetyl-L-cysteine (NAC), which is the most common mucolytic for enhancing the bioavailability of oral peptide NPs, as tested in a murine model (CitationCarino et al. 2000).
The prodrug approach is another winning strategy that overcomes the metabolic problem of proteins, by altering the biopharmaceutical characteristics of the parent molecule. While designing the prodrug, covalent bonds between the carrier and parent drug play an important role in making the drug unalterable in upper part of the GIT, but allowing rapid release in the colon (CitationLautenschlager et al. 2014). This approach solves not only the metabolic problem, but it also includes the optimization of solubility, permeability, target delivery, elimination rate, and enzymatic and chemical stability (CitationGangwar et al. 1997).
Contact timing of the protein drug determines its absorption and bioavailability. A mucoadhesive delivery system extends contact time at the absorption site, resulting in increased concentration gradient and rapid absorption without intestinal damage [luminal dilution and degradation factor]. However, the gastrointestinal mucoadhesive patch system (GI-MAPS) is alternative form of such a delivery system gaining importance in the oral delivery of protein (CitationEiamtrakarn et al. 2002).
Nano and micro carriers of protein containing a protease inhibitor give promising results in oral delivery. The use of insulin microspheres (IMS) containing Eudragit L100 and aprotinin (AP) or the Bowman-Birk inhibitor (BBI) as a protease inhibitor, is one such example. This method shows excellent hypoglycemic effect by oral delivery of insulin in diabetic and normal murine models (CitationMorishita et al. 1992). However, protease inhibitors and other absorption enhancers are more effective in the colon as compared to the small intestine (CitationYamamoto and Muranishi 1997).
Cell-penetrating peptides (CPPs), as the name suggests, facilitate drug translocation across the intestinal cell. CPPs may be natural or synthetic and have a short cationic sequence (CitationFischer et al. 2005). They were discovered in 1988, and are helpful in the oral delivery of a number of small molecules like antibodies, nucleic acids, NPs, peptides, and proteins. Some of the most commonly used CPPs are penetratin, polyarginine, TAT, and transportan (CitationFonseca et al. 2009). Recent findings make it clear that they have good efficacy and safety profiles for future applications of peptides and proteins (CitationKhafagy and Morishita 2012).
Microfabricated patches are a novel method of oral and cutaneous delivery of protein and other drugs. They have a mucoadhesive property and increase the retention time at the intestinal epithelium. Unidirectional and controlled release are two important features of this delivery system (CitationSant et al. 2012).
Microgels and microcapsules provide a putative benefit to preserve the secondary and tertiary structure of proteins. Moreover, they also prevent the aggregation of molecules, with their enzymatic and chemical stability. Protein microgels coated with poly(ethylene terephtalate) (PET) reduce the production of pro-inflammatory cytokines (TNF-α, IL-1β, and MCP-1) in chronic inflammatory conditions (CitationBysell et al. 2011).
The use of protein and peptide drugs with a film coating is also a good treatment option for colon disorders. The film coating protects integrity and facilitates intestinal absorption of orally administered peptide and protein drugs (CitationMaroni et al. 2013).
Ceramic NPs are one of the unique nano-carriers for protein and polypeptide delivery (CitationSingh et al. 2013). They protect the structural integrity of the protein and are not affected by the pH change in intestinal delivery. Moreover, they can be rapidly prepared and have no swelling/porosity effect, which makes them better therapeutics (CitationYang et al. 2010). Ceramic NPs are widely used in the treatment of colitis and other diseases like diabetes, diarrhea, pain, etc. (CitationRaghvendra 2012).
Future of biotherapeutics
Recent findings make it possible to safely and effectively administer peptide and protein drugs for colitis () and various other disease conditions. This is attributed to the excellent selectivity, potency and effectiveness of such molecules at the site of delivery (CitationHussain et al. 2001, CitationKumar et al. 2006, CitationSato et al. 2006). In the last decades, The FDA has approved 30 therapeutically active protein and peptide drugs, while about a hundred such molecules were under clinical investigation (CitationYun et al. 2013). While one study report of 2005 clearly shows that more than 40 drugs of peptide and protein origin are available in the world market, there are 270 and 400 peptides are running in the clinical and advanced preclinical phase studies respectively (CitationRenukuntla et al. 2013). At present, about 160 protein molecules are established and licensed as therapeutics. Regulatory agencies are focused on further approval of numerous protein molecules in the coming years (CitationSalmaso and Caliceti 2013). All these outcomes are due to the better understanding and improvement in biotechnological knowledge (genomics, cell-line culture and pharmacological study), and incessant effort by researchers. However, just as everything in this world requires continuous improvement to remain sustainable, the same is applicable in this system also, to enable non- invasive delivery. Recent outcomes assure us that in the next five years, the existing problems will be overcome, and oral drugs will be made available, which is the most challenging form of therapeutics for colitis and other disease conditions.
Conclusions
In conclusion, protein and peptide drugs have therapeutic potential in the treatment of ulcerative colitis. Moreover, peptides from dietary sources are also seen to be useful in modulating the immune function of the intestine (CitationShimizu 2010). Various traditional and modern strategies have been proposed towards the betterment of protein delivery systems, which are somewhat similar to other conventional non-peptide formulations. No doubt, these approaches make the molecule more effective, and when applied, significantly improve the condition of colonic inflammation. Although the mechanism of action is different for different types of peptides employed, the effect is beneficial towards disease severity. A wide variety of animal species like rats, mice, piglets and also various cell-line studies prove the effectiveness of the use of protein delivery systems. Research outcomes from the last few decades ascertain that it is a wonderful and promising therapeutic approach towards ulcerative colitis, but some improvement is still required to make it rational and applicable in commercial therapeutics.
Table III. Proteins and peptides potentially used in colitis.
Acknowledgements
The authors are thankful to the Head of the Department, SLT Institute of Pharmaceutical Sciences, for providing necessary facilities and guidance. One of the authors, Mr Kantrol Kumar Sahu, is thankful to GGV for providing financial assistance in the form of a fellowship.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Al-Hilal AT, Alam F, Byun Y 2013. Oral drug delivery systems using chemical conjugates or physical complexes. Adv Drug Deliv Rev. 65:847.
- Allemann E, Leroux JC, Gurny R 1998. Polymeric nano- and microparticles for the oral delivery of peptides and peptidomimetics. Adv Drug Deliv Rev. 34:172.
- Allemann E, Leroux JC, Gurny R 1998. Polymeric nano- and microparticles for the oral delivery of peptides and peptidomimetics. Adv Drug Deliv Rev. 34:171–189.
- Allen A, Garner A 1980. Mucus and bicarbonate secretion in the stomach and their possible role in mucosal protection. Gut. 21:249–262.
- Al-Obeidi F, Castrucci AM, Hadley ME, Hruby VJ 1989. Potent and prolonged acting cyclic lactam analogues of alphamelanotropin: design based on molecular dynamics. J Med Chem. 32:2555–2561.
- Ambrosini R, Barchiesi A, Mizio VD, Terlizzi MD, Leo L, Filippone A, et al 2007. Inflammatory chronic disease of the colon: How to image. Eur J Radiol. 61:442.
- Antosova Z, Mackova M, Kral V, Macek T 2014. Therapeutic application of peptides and proteins: parenteral forever? Trends Biotechnol. 27:628.
- Bai JPF, Amidon GL 1992. Structural specificity of mucosal-cell transport and metabolism of peptide drugs: implication for oral peptide drug delivery. Pharm Res. 9:969–978.
- Bakhru SH, Furtado S, Morello AP, Mathiowitz E 2013. Oral delivery of proteins by biodegradable nanoparticles. Adv Drug Deliv Rev. 65:812.
- Barthe L, Woodley J, Houin G 1999. Gastrointestinal absorption of drugs: methods and studies. Fundam Clin Pharmacol. 13:154–168.
- Bitton A 2001. Medical management of ulcerative proctitis, proctosigmoiditis, and leftsided colitis. Semin Gastrointest Dis. 12:263–274.
- Bokemeyer B, Hommes D, Gill I, Broberg P, Dignass A 2012. Mesalazine in left-sided ulcerative colitis: Efficacy analyses from the PODIUM trial on maintenance of remission and mucosal healing. J Crohn's Colitis. 6:476–482.
- Bolge SC, Waters H, Piech CT 2010. Self-reported frequency and severity of disease flares, disease perception, and flare treatments in patients with ulcerative colitis: results of a national internet-based survey. Clin Ther. 32:238.
- Burton PS, Conradi RA, Hilgers AR 1991. (B) Mechanisms of peptide and protein absorption (2) transcellular mechanism of peptide and protein absorption: passive aspects. Adv Drug Deliv Rev. 7:365–385.
- Burton PS, Conradi RA, Ho NF, Hilgers AR, Borchardt RT 1996. How structural features influence the biomembrane permeability of peptides. J Pharm Sci. 85:1336–1340.
- Bysell H, Mansson R, Hansson P, Malmsten M 2011. Microgels and microcapsules in peptide and protein drug delivery. Adv Drug Deliv Rev. 63:1172–1185.
- Calceti P, Salmaso S, Walker G, Schnürch AB 2004. Development and in vivo evaluation of an oral insulin-PEG delivery system. Eur J Pharm Sci. 22:315–323.
- Carbonnel F, Jantchou P, Monnet E, Cosnes J 2009. Environmental risk factors in Crohn's disease and ulcerative colitis: an update. Gastroenterol Clin Biol. 33:S145–157.
- Carino GP, Jacob JS, Mathiowitz E 2000. Nanosphere based oral insulin delivery. J Control Release. 65:261–269.
- Chiba H, Osanai M, Murata M, Kojima T, Sawada N 2008. Transmembrane proteins of tight junctions. Biochim Biophy Acta. 1778: 588–600.
- Chow DK, Leong RW, Tsoi KK, Ng SS, Leung WK, Wu JC, et al 2009. Long-term follow-up of ulcerative colitis in the Chinese population. Am J Gastroenterol. 104:647–654.
- Clark MA, Hirst BH, Jepson MA 2000. Lectin-mediated mucosal delivery of drugs and microparticles. Adv Drug Deliv Rev. 43:207–223.
- Collnot EM, Ali H, Lehr CM 2012. Nano- and microparticulate drug carriers for targeting of the inflamed intestinal mucosa. J Control Release. 161:236.
- Conrad K, Roggenbuck D, Laass MW 2014. Diagnosis and classification of ulcerative colitis. Autoimmunity Rev. 13:463–466.
- Conradi RA, Hilgers AR, Ho NF, Burton PS 1991. The influence of peptide structure on transport across Caco-2 cells. Pharm Res. 8:1453–1460.
- Cosnes J, Rousseau CG, Seksik P, Cortot A 2011. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 140:1785–1794.
- Dai C, Wang B, Zhao H 2005. Microencapsulation peptide and protein drugs delivery system. Colloids Surf B Biointerfaces. 41:118.
- Danese S, Sans M, Fiocchi C 2004. Inflammatory bowel disease: the role of environmental factors. Autoimmun Rev. 3:394–400.
- Daugherty AL, Mrsny RJ 1999. Transcellular uptake mechanisms of the intestinal epithelial barrier part one. Pharm Sci Technolo Today. 2:145.
- Dave SH, Tilstra JS, Matsuoka K, Li F, Karrasch T, Uno JK, et al 2007. Amelioration of Chronic Murine Colitis by Peptide-Mediated Transduction of the IκB Kinase Inhibitor NEMO Binding Domain Peptide1. J Immunol. 179:7852–7859.
- Deuring JJ, Haar C, Kuipers EJ, Peppelenbosch MP, Woude CJ 2013. The cell biology of the intestinal epithelium and its relation to inflammatory bowel disease. Int J Biochem Cell Biol. 45:800.
- Di Marco M, Shamsuddin S, Razak KA, Aziz AA, Devaux C, Borghi E, et al 2010. Overview of the main methods used to combine proteins with nanosystems: absorption, bioconjugation, and encapsulation. Int J Nanomed. 5:37–49.
- Donovan MD, Flynn GL, Amidon GL 1990. Absorption of polyethylene glycols 600 through 2000: the molecular weight dependence of gastrointestinal and nasal absorption. Pharm Res. 7:863–68.
- Dorkoosh FA, Verhoef JC, Borchard G, Tehrani MR, Junginger HE 2001. Development and characterization of a novel peroral peptide drug delivery system. J Control Release. 71:308.
- Eiamtrakarn S, Itoh Y, Kishimoto J, Yoshikawa Y, Shibata N, Murakami M, Takada K 2002. Gastrointestinal mucoadhesive patch system (GI-MAPS) for oral administration of G-CSF, a model protein. Biomaterials. 23:145–152.
- Farrell RJ, Peppercorn MA 2002. Ulcerative colitis. Lancet. 359: 331–340.
- Fischer R, Mleczek MF, Hufnagel H, Brock R 2005. Break on through to the other side-biophysics and cell biology shed light on cell-penetrating peptides. Chembiochem. 6:2126–2142.
- FitzGerald AJ, Pu M, Marchbank T, Westley BR, May FEB, Boyle J, et al 2004. Synergistic effects of systemic trefoil factor family 1 (TFF1) peptide and epidermal growth factor in a rat model of colitis. Peptides. 25:793–801.
- Fix JA 1996. Oral controlled release technology for peptides: status and future prospects. Pharm Res. 13:1760–1764.
- Florence AT 2004. Issues in oral nanoparticle drug carrier uptake and targeting. J Drug Target. 12:65–70.
- Fonseca SB, Pereira MP, Kelley SO 2009. Recent advances in the use of cell-penetrating peptides for medical and biological applications. Adv Drug Deliv Rev. 61:953–964.
- Frey A, Neutra MR 1997. Targeting of mucosal vaccines to Peyer's patch M cells. Behring Inst Mitt. 98:376–389.
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S 1993. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 123:1777–1788.
- Furuse M, Sasaki H, Fujimoto K, Tsukita S 1998. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occluding in fibroblasts. J Cell Biol. 143:391–401.
- Furuse M, Tsukita S 2006. Claudins in occluding junctions of humans and flies. Trends Cell Bio. 16:181–188.
- Gangwar S, Pauletti GM, Wang B, Siahaan TJ, Stella VJ, Borchardt RT 1997. Prodrug strategies to enhance the intestinal absorption of peptides. Drug Discov Today. 2:148–154.
- Gardner MLG 1987. Passage of intact peptides across the intestine. Adv Biosci. 65:99–106.
- Gearry RB, Richardson A, Frampton CM, Collett JA, Burt MJ, Chapman BA, Barclay ML 2006. High incidence of Crohn's disease in Canterbury, NewZealand: results of an epidemiologic study. Inflamm Bowel Dis. 12:936–943.
- Giannasca PJ, Giannasca KT, Leichtner AM, Neutra MR 1999. Human intestinal M cells display the sialyl Lewis A antigen. Infect Immun. 67:946–953.
- Gibson PR 1997. Ulcerative colitis: an epithelial disease? Bailliere's Clin Gastroenterol. 11:19.
- Gitler C 1964. Protein digestion and absorption in nonruminants. In: Munro HN, Allison JB, Eds. Mammalian Protein Metabolism. New York: Academic Press, pp. 35–69.
- Goldberg M, Orellana IG 2003. Challenge for the oral delivery of macromolecules. Nat Rev Drug Dis. 2:289–295.
- Gunisetty S, Tiwari SK, Bardia A, Phanibhushan M, Satti V, Habeeb MA, Khan AA 2012. The epidemiology and prevalence of Ulcerative colitis in the South of India. Open J Immunol. 2:144–148.
- Halfvarson J, Bresso F, D’Amato M, Jarnerot G, Pettersson S, Tysk C 2005. CARD15/NOD2 polymorphisms do not explain concordance of Crohn's disease in Swedish monozygotic twins. Dig Liver Dis. 37:768–772.
- Hanai H, Iida T, Ikeya K, Abe J, Maruyamab Y, Shimura T, et al 2013. A new paradigm in ulcerative colitis: Regulatory T cells are key factor which induces/exacerbates UC through an immune imbalance Molecular. Immunology, 54:173–180.
- Harmar AJ 2004. Clinical endocrinology and metabolism. Receptors for gut peptides. Best Pract Res Clin Endocrinol Metab. 18:463.
- Hering NA, Schulzke JD 2009. Therapeutic options to modulate barrier defects in inflammatory bowel disease. Dig Dis. 27:450–454.
- Hong S, Yum S, Yoo HJ, Kang S, Yoon JH, Min D, et al 2012. Colon-targeted cell-permeable NFκB inhibitory peptide is orally active against experimental colitis. Mol Pharm. 7:1310–1319.
- Hong T, Jin GB, Yoshino G, Miura M, Maeda Y, Cho S, Cyong JC 2002. Protective effects of Polygalae root in experimental TNBS-induced colitis in mice. J Ethnopharmacol. 79:341.
- Hussain N, Jaitley V, Florence AT 2001. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv Drug Deliv Rev. 50:107–142.
- Hwang JW, Lee SJ, Kim YS, Kim EK, Ahn CB, Jeon YJ, et al 2012. Purification and characterization of a novel peptide with inhibitory effects on colitis induced mice by dextran sulfate sodium from enzymatic hydrolysates of Crassostrea gigas. Fish Shellfish Immunol. 33:993–999.
- Ikesue K, Kopeckova P, Kopecek J 1993. Degradation of proteins by guinea pig intestinal enzymes. Int J Pharm. 95:171–179.
- Jess T, Riis L, Jespersgaard C, Hougs L, Andersen PS, Orholm MK, et al 2005. Disease concordance, zygosity, and NOD2/CARD15 status: follow-up of a population-based cohort of Danish twins with inflammatory bowel disease. Am J Gastroenterol. 100: 2486–2492.
- Jess T, Riis L, Vind I, Winther KV, Borg S, Binder V, et al 2002. Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: a population-based study from Copenhagen, Denmark. Inflamm Bowel Dis. 13:481–489.
- Jones GJR 2002. The potential use of receptor-mediated endocytosis for oral drug delivery. Adv Drug Deliv Rev. 20:83–97.
- Kadiyala I, Loo Y, Roy K, Rice J, Leong KW 2010. Transport of chitosan-DNA nanoparticles in human intestinal M-cell model versus normal intestinal enterocytes. Eur J Pharm Sci. 39:103–109.
- Kanwar B, Gao DW, Hwang AB, James P, Grenert JP, Williams SP, et al 2008. In vivo imaging of mucosal CD4 + T cells using single photon emission computed tomography in a murine model of colitis. J Immunol Methods. 329:21–22.
- Kato E, Yamane S, Nomura R, Matsumoto K, Tashima K, Horie S, et al 2012. Dysfunction of neurogenic VIP-mediated relaxation in mouse distal colon with dextran sulfate sodium-induced colitis. Pharmacol Res. 65:204–212.
- Kayhan GE, Gul M, Kayhan B, Gedik E, Ozgul U, Kurtoglu EL, et al 2013. Dexmedetomidine ameliorates TNBS-induced colitis by inducing immunomodulator effect. J Surg Res. 183:733–734.
- Khafagy ES, Morishita M 2012. Oral biodrug delivery using cell- penetrating peptide. Adv Drug Deliv Rev. 64:531–539.
- Kim CJ, Nolan JAK, Yang C, Archbold T, Fan MZ, Mine Y 2010. L-Tryptophan exhibits therapeutic function in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J Nutrit Biochem. 212:468–475.
- Kim CJ, Nolan JK, Yang C, Archbold T, Fan MZ, Mine Y 2009. L-cysteine supplementation attenuates local inflammation and restores gut homeostasis in a porcine model of colitis. Biochim Biophys Acta. 1790:1161–1169.
- Kim MS, Park SJ, Gu BK, Kim CH 2012. Ionically crosslinked alginate– carboxymethyl cellulose beads for the delivery of protein therapeutics. Appl Surf Sc.i, 262:28–33.
- Kompella UB, Lee VHL 2001. Delivery systems for penetration enhancement of peptide and protein drugs: design considerations. Adv Drug Deliv Rev. 46:211–245.
- Kumar GP, Rajeshwarrao P 2011. Nonionic surfactant vesicular systems for effective drug delivery-an overview. Acta Pharm Sinica B. 1:208–219.
- Kumar TR, Soppimath K, Nachaegari SK 2006. Novel delivery technologies for protein and peptide therapeutics. Curr Pharm Biotechnol. 7:261–276.
- Kwan KC 1997. Oral bioavailability and first-pass effects. Drug Metab Dispos. 25:1329–1336.
- Langguth P, Bohner V, Heizmann J, Merkle HP, Wolffram S, Amidon GL, Yamashita S 1997. The challenge of proteolytic enzymes in intestinal peptide delivery. J Control Release. 46:39–57.
- Lautenschlager C, Schmidt C, Fischer D, Stallmach A 2014. Drug delivery strategies in the therapy of inflammatory bowel disease. Adv Drug Deliv Rev. 71:58–76.
- Lee M, Nolan JK, Archbold T, Fan MZ, Juneja LR, Okubo T, Mine Y 2009. Therapeutic potential of hen egg white peptides for the treatment of intestinal inflammation. J Funct Foods. 1:161–169.
- Lee YH, Fock K, See SJ, Ng TM, Khor C, Teo EK 2000. Racial differences in the prevalence of ulcerative colitis and Crohn's disease in Singapore. J Gastroenterol Hepatol. 15:622–625.
- Li Y, Haar CD, Peppelenbosch MP, Van der Woude CJ 2012. SOCS3 in immune regulation of inflammatory bowel disease and inflammatory bowel disease-related cancer. Cytokine Growth Factor Rev. 23:128.
- Li Z, Chen J, Sun W, Xu Y 2010. Investigation of archaeosomes as carriers for oral delivery of peptides. Biochem Biophys Res Commun. 394:412–417.
- Limbergen JV, Russell RK, Drummond HE, Aldhous MC, Round NK, Nimmo ER, et al 2008. Definition of phenotypic characteristics of childhood- onset inflammatory bowel disease. Gastroenterology. 135:1114–1122.
- Ling KL, Ooi CJ, Luman W, Cheong WK, Choen FS, Ng HS 2002. Clinical characteristics of ulcerative colitis in Singapore, a multiracial city-state. J Clin Gastroenterol. 356:144–148.
- Lok KH, Hung HG, Ng CH, Kwong KC, Yip WM, Lau SF, et al 2008. Epidemiology and clinical characteristics of ulcerative colitis in Chinese population: experience from a single center in Hong Kong. J Gastroenterol Hepatol. 23:406–410.
- MacAdam A 1993. The effect of gastro-intestinal mucus on drug absorption. Adv Drug Deliv Rev. 11:201–220.
- Madara JL 1998. Regulation of the movement of solutes across tight junctions. Annu Rev Physiol. 60:143–159.
- Mahato RI, Narang AS, Thoma L, Miller DD 2003. Emerging trends in oral delivery of peptide and protein drugs. Crit Rev Ther Drug Carrier Syst. 20:153–214.
- Mankertz J, Schulzke JD 2007. Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol. 23:379–383.
- Maroni A, Curto MDD, Zema L, Foppoli A, Gazzaniga A 2013. Film coatings for oral colon delivery. Int J Pharm. 457:372–394.
- McCrudden MT, Singh TR, Migalska K, Donnelly RF 2013. Strategies for enhanced peptide and protein delivery. Ther Deliv. 4:593–514.
- van der Merwe SM, Verhoef JC, Kotze AF, Junginger HE 2004. N-Trimethyl chitosan chloride as absorption enhancer in oral peptide drug delivery. Development and characterization of minitablet and granule formulations. Eur J Pharm Biopharm. 57:85–91.
- Miller NS, Johnston TP 2005. Current strategies used to enhance the paracellular transport of therapeutic polypeptides across the intestinal epithelium. Int J Pharm. 294:201–216.
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al 2012. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 142:46–54.
- Morishita I, Morishita M, Takayama K, Machida Y, Nagai T 1992. Hypoglycemic effect of novel oral microspheres of insulin with protease inhibitor in normal and diabetic rats. Int J Pharm. 78:9–16.
- Morishita M, Peppas NA 2006. Is the oral route possible for peptide and protein drug delivery? Drug Discov Today. 11:905–910.
- Morita N, Toki S, Hirohashi T, Minoda T, Ogawa K, Kono S, et al 1995. Incidence and prevalence of inflammatory bowel disease in Japan: nationwide epidemiological survey during the year 1991. J Gastroenterol. 30:1–4.
- Ng SC, Bernstein CN, Vatn MH, Lakatos PL, Loftus EV Jr, Tysk C, et al 2013. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 62:630–649.
- Nolan JK, Zhang H, Ibuki M, Nakamori T, Yoshiura K, Turner PV, et al 2012. The PepT1-transportable soy tripeptide VPY reduces intestinal inflammation. Biochim Biophys Acta. 1820:1753–1763.
- Ordas I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ 2012. Ulcerative colitis. Lancet. 380:1606–1619.
- Paganelli R, Levinsky RI 1980. Solid phase radioimmunoassay for detection of circulating food protein antigens in human serum. J Immunol Methods. 37:333–341.
- Pandey RS, Sahu S, Sudheesh MS, Madan J, Kumar M, Dixit VK 2011. Carbohydrate modified ultrafine ceramic nanoparticles for allergen immunotherapy. Int Immunopharmacol. 11:925–931.
- Pappenheimer JR, Reiss KZ 1987. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J Membr Biol. 100:123–136.
- Pardi DS, Kelly CP 2011. Microscopic colitis. Gastroenterology. 140:1155–1165.
- Pauletti GM, Gangwar S, Knipp GT, Nerurkar MM, Okumu FW, Tamura K, et al 1996. Structural requirements for intestinal absorption of peptide drugs. J Control Release. 41:3–17.
- Pauletti GM, Okumu FW, Borchardt RT 1997. Effect of size and charge on the passive diffusion of peptides across Caco-2 cell monolayers via the paracellular pathway. Pharm Res. 14:164–168.
- Paulsen AF, Hoglund P, Lundin S, Paulsen O 1993. Pharmacokinetics of 1-deamino-8-D-arginine vasopressin after various routes of administration in healthy volunteers. Clin Endocrinol (Oxf). 38:177–182.
- Pearl DS, Shah K, Whittaker MA, Smith HN, Brown JF, Shute JK, Trebble TM 2013. Cytokine mucosal expression in ulcerative colitis, the relationship between cytokine release and disease activity. J Crohn's Colitis. 7:481–489.
- Pettit DK, Gombotz WR 1998. The development of site-specific drug-delivery systems for protein and peptide biopharmaceuticals. Tibtech. 16:345–346.
- Phelps CF 1978. Biosynthesis of mucus glycoprotein. Br Med Bull. 34:43–48.
- Piekielna J, Perlikowska R, Gach K, Janecka A 2013. Cyclization in opioid peptides. Curr Drug Targets. 14:798.
- Pinto JF 2010. Site-specific drug delivery systems within the gastro- intestinal tract: From the mouth to the colon. Int J Pharm. 395: 44–52.
- Ponchel G, Touchard F, Duchene D, Peppas A 1987. Bioadhesive analysis of controlled release systems. I. Fracture and interpenetration analysis in poly(acrylic acid)-containing systems. J Control Release. 5:129–141.
- Qi K, Wu J, Wan J, Men X, Xu Z 2014. Purified PEGylated porcine glucagon-like peptide-2 reduces the severity of colonic injury in a murine model of experimental colitis. Peptides. 52:11–18.
- Rabbi MF, Labis B, Metz-Boutigue MH, Bernstein CN, Ghia JE 2014. Catestatin decreases macrophage function in two mouse models of experimental colitis. Biochemical Pharmacology. 89: 386–398.
- Raghvendra SR 2012. Nano-clays and drug delivery system: an overview. Int J Pharm World Rev. 3:1–16.
- Randall C, Vizuete J, Wendorf G, Ayyar B, Constantine G 2012. Current and emerging strategies in the management of Crohn's disease. Best Pract Res Clin Gastroenterol. 26:601–610.
- Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jarvinen T, Savolainen T 2008. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 7:255–270.
- Reis CP, Neufeld RJ, Ribeiro AJ, Veiga F 2006. Nanoencapsulation II. Biomedical applications and current status of peptide and protein nanoparticulate delivery systems. Nanomedicine. 2:53–65.
- Rekha MR, Sharma CP 2013. Oral delivery of therapeutic protein/ peptide for diabetes – Future perspectives. Int J Pharm. 440:48–62.
- Renukuntla J, Vadlapudi AD, Patel A, Boddu SHS, Mitra AK 2013. Approaches for enhancing oral bioavailability of peptides and proteins. Int J Pharm. 447:76.
- Rieux A, Fievez V, Garinot M, Schneider YJ, Preat V 2006. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J Control Release. 116:1–27.
- Rieux A, Pourcelle V, Cani PD, Brynaert JM, Preat V 2013. Targeted nanoparticles with novel non-peptidic ligands for oral delivery. Adv Drug Deliv Rev. 65:833–844.
- Roth B, Gustafsson R J, Jeppsson B, Manjer J, Ohlsson B 2014. Smoking- and alcohol habits in relation to the clinical picture of women with microscopic colitis compared to controls. BMC Women's Health. 14:8.
- Rubas W, Cromwell ME, Shahrokh Z, Villagran J, Nguyen TN, Wellton M, et al 1996. Flux measurements across Caco-2 monolayers may predict transport in human large intestinal tissue. J Pharm Sci. 85:165–169.
- Rundles CC 1987. Failure of antigen exclusion. In: Brostoff J, Challacombe SJ, Ed. Food Allergy and Intolerance, London: Tindall B, pp. 223–236.
- Saffran M, Kumar G, Savariar C, Burnham J, Williams F, Neckers D 1986. A new approach to the oral administration of insulin and other peptide drugs. Science. 233:1081–1084.
- Sakuma S, Suzuki N, Kikuchi H, Hiwatari K, Arikawa K, Kishida A, Akashi M 1997. Oral peptide delivery using nanoparticles composed of novel graft copolymers having hydrophobic backbone and hydrophilic branches. Int J Pharm. 149:93–106.
- Salmaso S, Caliceti P 2011. A useful tool to improve the biological performance of biotech drugs. In: Van der Walle C, Ed. Peptide and Protein Delivery. Boston: Elsevier Health Sciences, 247–290.
- Salmaso S, Caliceti P 2013. Self assembling nanocomposites for protein delivery: Supramolecular interactions of soluble polymers with protein drugs. Int J Pharm. 440:111–123.
- Salmaso S, Caliceti P 2013. Self assembling nanocomposites for protein delivery: Supramolecular interactions of soluble polymers with protein drugs. Int J Pharm. 440:111.
- Sant S, Tao SL, Fisher OZ, Xu Q, Peppas NA, Khademhosseini A 2012. Microfabrication technologies for oral drug delivery. Adv Drug Deliv Rev. 64:496–407.
- Sato AK, Viswanathan M, Kent RB, Wood CR 2006. Therapeutic peptides: technological advances driving peptides into development. Curr Opin Biotechnol. 17:638–642.
- Schnurch AB 2013. Nanocarrier systems for oral drug delivery: Do we really need them? Eur J Pharm Sci. 49:272–277.
- Schulzke JD, Ploeger S, Amasheh M, Fromm A, Zeissig S, Troeger H, et al 2009. Epithelial tight junctions in intestinal inflammation. Ann N Y Acad Sci. 11:294–300.
- Shakweh M, Ponchel G, Fattal E 2004. Particle uptake by Peyer's patches: a pathway for drug and vaccine delivery. Expert Opin Drug Deliv. 1:141–163.
- Shimizu M 2010. Interaction between food substances and the intestinal epithelium. Biosci Biotechnol Biochem. 74:232–241.
- Singh D, Dubey P, Pradhan M, Singh MR 2013. Ceramic nanocarriers: versatile nanosystem for protein and peptide delivery. Expert Opin Drug Deliv. 10:241–259.
- Sinha VR, Kumria R 2003. Microbially triggered drug delivery to the colon. Eur J Pharm Sci. 18:3–18.
- Sizer PJH 1997. Towards an oral influenza vaccine. Trends Biotech. 15:282–285.
- Sobczak M, Zakrzewski PK, Cygankiewicz AI, Mokrowiecka A, Chen C, Sałaga M, et al 2014. Anti-inflammatory action of a novel orally available peptide 317 in mouse models of inflammatory bowel diseases. Pharmacol Rep. 66:741–750.
- Sood A, Midha V, Sood N, Bhatia AS, Avasthi G 2003. Incidence and prevalence of ulcerative colitis in Punjab, North India. Gut. 52: 1587–1590.
- Stasikowska KO, Danilewicz M, Głowacka A, Wągrowska DM 2012. Mast cells and eosinophils are involved in activation of ulcerative colitis. Adv Medical Sci. 57:230–236.
- Stoyanova II, Gulubova MV 2002. Mast cells and inflammatory mediators in chronic ulcerative colitis. Acta Histochem. 104:185.
- Swaan PW 2002. Recent advances in intestinal macromolecular drug delivery via receptor-mediated transport pathways. Pharm Res. 15:826–834.
- Tan TM, Goh KL 2005. Ulcerative colitis in a multiracial Asian country: racial differences and clinical presentation among Malaysian patients. World J Gastroenterol. 11:5859–5862.
- Tang VW, Goodenough DA 2003. Paracellular ion channel at the tight junction. Biophys J. 84:1660–1673.
- Tomita M, Shiga M, Hayashi M, Awazu S 1998. Enhancement of colonic drug absorption by the paracellular permeation route. Pharm Res. 5:341–346.
- Travis SPL, Stange EF, Lemann M, Oresland T, Bemelman WA, Chowers Y, et al 2008. European evidence-based Consensus on the management of ulcerative colitis: Current management. J Crohn's Colitis. 2:24–62.
- Tsukita S, Furuse M, Itoh M 2001. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2:285–293.
- Umashankar MS, Sachdeva RK, Gulati M 2010. Aquasomes: a promising carrier for peptides and protein delivery. Nanomedicine. 6:419–426.
- Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin D, Murthy N 2010. Orally delivered thioketal nanoparticles loaded with TNF-α-siRNA target inflammation and inhibit gene expression in the intestines. Nat Mater. 9:923–928.
- Wood C, Miller J, Wilson G, Hidalgo IJ 1992. Effect of charge on affinity for the intestinal di-/tripeptide transporter. Pharm Res. 9:S–254.
- Woodley JF 1994. Enzymatic barriers for GI peptide and protein delivery. Crit Rev Ther Drug Carrier Syst. 11:61–95.
- Wootton R, Hazelwood R 1989. Relative affinity of a series of charged dipeptides for the peptide carrier of rabbit intestinal brush-border membranes. Biochem Soc Trans. 17:691–692.
- Xing L, Dawei C, Liping X, Rongqing Z 2003. Oral colon-specific drug delivery for bee venom peptide: development of a coated calcium alginate gel beads-entrapped liposome. J Control Release. 93:293–300.
- Xiong XY, Li QH, Li YP, Guo L, Li ZL, Gong YC 2013. Pluronic P85/poly(lactic acid) vesicles as novel carrier for oral insulin delivery. Colloids Surf B Biointerfaces. 111:282–288.
- Yamamoto A, Muranishi S 1997. Rectal drug delivery systems Improvement of rectal peptide absorption by absorption enhancers, protease inhibitors and chemical modification. Adv Drug Deliv Rev. 28:275–299.
- Yang L, Sheldon BW, Webster TJ 2010. Nanophase ceramics for improved drug delivery: current opportunities and challenges. Am Ceramic Soc Bull. 89:24–33.
- Yang M, Frokjaer S 2009. Novel formulation approaches for peptide and protein injectables. In: Jorgensen L, Nielsen H, Eds. Delivery Technologies for Biopharmaceuticals. Chichester: Wiley & Sons, pp. 9–28.
- Yang SK, Yun S, Kim JH, Park JY, Kim HY, Kim YH, et al 2008. Epidemiology of inflammatory bowel disease in the Songpa- Kangdong district, Seoul, Korea, 1986–2005: a KASID study. Inflamm Bowel Dis. 14:542–549.
- Yin B, Hu X, Wang J, Liang H, Li X, Niu N, et al 2011. Blocking TNF-α by combination of TNF-α- and TNFR-binding cyclic peptide ameliorates the severity of TNBS-induced colitis in rats. Eur J Pharmacol. 656:119–124.
- Yun Y, Cho YW, Park K 2013. Nanoparticles for oral delivery: Targeted nanoparticles with peptidic ligands for oral protein delivery. Adv Drug Deliv Rev. 65:822–832.
- Zhang X, Qi J, Lu Y, He W, Li X, Wu W 2014. Biotinylated liposomes as potential carriers for the oral delivery of insulin. Nanomedicine. 10:167–176.
- Zhang YZ, Li YY 2014. Inflammatory bowel disease: Pathogenesis. World J Gastroenterol. 20:91–99.