Abstract
The human genome is exposed to mutations during the life cycle because of many types of changes in the DNA. Viruses, radiation, transposons, mutagenic chemicals, or any errors that happen during DNA replication or the meiotic process in the cell, may cause the mutation. Many mutations have no effect on phenotype or health, while some mutations cause crucial diseases such as cancer or cardiac diseases; therefore, a better understanding of the effects of mutation on phenotype is a very important part of genetic studies. Biosensors based on DNA, RNA, and peptide nucleic acids are the most sensitive tools, due to a strong pairing of lined up nucleotide strands between bases in their complementary parts. These methods can provide information to assist clinicians in making successful treatment decisions and increase the patient survival rate. In this review, we discuss DNA biosensors based on peptide nucleic acids that have an important role in cancer diagnosis.
| Abbreviations | ||
| PCR | = | polymerase chain reaction |
| PNA | = | peptide nucleic acids |
| POCT | = | point of care testing |
| RAPD | = | random amplified polymorphic DNA |
| RFLP | = | restriction fragment length polymorphism |
Introduction
Recent processes in the expansion of DNA biosensors for nucleotide sequence-specific DNA hybridization and the detection of DNA damage are briefly reviewed (CitationPalecek et al. 1998). With the discovery of the double helix structure of deoxyribonucleic acid (DNA) in 1953, identifying and sequencing of DNA molecules got more attention (CitationCrick and Watson 1954). DNA is the carrier of genetic information, and the base material of biological heredity. Nucleic-acid sequences, which are different in every living organism, virus or pathogen, provide practical ways to recognize and discern diverse diseases (CitationLiu et al. 2012). After the recognition of DNA, DNA-based diagnostics have evolved, which include the isolation of DNA polymerase, RAPD, RFLP, and PCR techniques (CitationSingh et al. 2010).
As new genes are discovered and proved to be involved in disease, primary patient diagnosis, carrier detection, and prenatal diagnosis will continue to increase progressively (CitationChin et al. 2005). The human genome is exposed to mutations during the life cycle, owing to many types of changes in the DNA. The mutation may be created by viruses, radiations, transposons, mutagenic chemicals, or any errors that happen during DNA replication or the meiotic process in the cell. Many mutations have no effect on phenotype or health, while some mutations cause crucial diseases such as cancer or cardiac diseases; therefore, a better understanding of the effects of mutation on phenotype is a very important part of genetic studies (CitationAltintas and Tothill 2012, CitationBertram 2000, CitationAminetzach et al. 2005, CitationSawyer et al. 2007, CitationNna et al. 2010).
About 5%–10% of cancers are hereditary and due to single gene mutations. In the last decade, a number of different hereditary cancer syndromes and their causative genes have been determined, including the hereditary breast–ovarian cancer syndrome, due to the BRCA1/2 genes (CitationChin et al. 2005, CitationWooster et al. 1994). Evaluation of the genetic risk of cancer in clinics demonstrates the primary arm of prevention in oncology, in order to recognize high-risk patients for screening and prevention (CitationChin et al. 2005).
To improve patient care, molecular diagnostic laboratories have been involved in creating new tests that are dependable, affordable and low-cost. They are also used to optimize the existing protocols, by making them quicker and more economical (CitationLucarelli et al. 2004). According to the analysis of genomic sequences, molecular diagnostics have suggested a highly sensitive and quantitative method for the detection of infectious disease pathogens and genetic diversity (CitationLucarelli et al. 2004). Through the efforts of researchers, many DNA-testing technologies were created a few years ago (CitationBeaudet and Belmont 2008, CitationDing et al. 2009). Although these conventional technologies provide the gold standard for laboratory-based DNA diagnostics, they cannot meet the needs of point-of-care clinical diagnostics (CitationLiu et al. 2012, CitationTost and Gut 2005). The identification of DNA sequences and their sequence discriminations is difficult and time-consuming, and has low hybridization efficiency. To overcome these problems, DNA sensors/arrays/chips appeared in high throughput analysis, which in turn indicates a considerable decrease in effort, time and cost (CitationSeeman 1999). These biorecognition elements, in combination with different transduction methods, have assisted rapid extension in the fields of bioanalysis and related technologies, and are known as biosensors and biochips (CitationVo-Dinh and Cullum 2000).
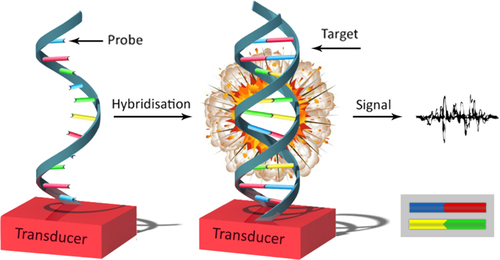
A biosensor is an analytical device that incorporates a biological sensing element with a transducer, to produce a signal used for the analyte concentration () (CitationLei et al. 2006, CitationClark 1987). In particular, DNA hybridization biosensors suggest significant promise for getting sequence-specific information in a faster, simpler, and cheaper method, in contrast to traditional hybridization assays (CitationWang 1998). The development of systems permitting DNA detection is stimulated by utilization in many areas like DNA diagnostics, gene analysis, fast detection of biological warfare agents, and legal applications. Detection of genetic mutations at the molecular level results in the possibility of performing accurate diagnostics even before any sign of a disease occurs (CitationSassolas et al. 2008). Depending on the nature of the biological process, biosensing devices may be divided into two general classes: biocatalytic sensors (based primarily on immobilized enzymes), and affinity devices (using antibodies, receptors and nucleic acids) (CitationWang 1999). Recent understanding of the structure–function of nucleic acids, specifically RNA, has created a new landscape in the development of new analytical and diagnostic methods (CitationMascini et al. 2005). With the increasing number of cancer cases that are growing throughout the world and the increased number of mortalities because of delayed detection, biosensors have an important role in the early diagnosis of cancer (CitationTothill 2009).
Biosensor structures
There are many possible classification designs. Biosensors and biochips are categorized either by their bioreceptor or their transducer type. The sampling part of a biosensor consists of a biosensitive layer, and this layer can either have bioreceptors or be made of bioreceptors covalently joined to the transducer (CitationVo-Dinh and Cullum 2000, CitationKoyun et al. 2008, CitationThévenot et al. 2001, CitationVo-Dinh 2007). A biosensor is a simple device that is made up of two elements:
| 1) | A bioreceptor that is an immobilized sensitive biological component (e.g. enzyme, DNA probe, antibody) recognizing the analyte (e.g. enzyme substrate, complementary DNA, antigen). However, though antibodies and oligonucleotides are widely engaged, enzymes are still the most commonly used biosensing elements in biosensors. | ||||
| 2) | A transducer is used to turn a (bio) chemical signal, caused by the interplay of the analyte with the bioreceptor, into an electronic one. The intensity of the generated signal is directly or inversely proportional to the analyte concentration. | ||||
Biosensors contain three parts:
| 1) | The sensitive elements (biologically derived material). | ||||
| 2) | The transducer or detector element, to transform the detected signal into a readable, quantified output. | ||||
| 3) | The signal processor that represents the transformed signal in a user-friendly way (CitationLiu et al. 2012). | ||||
Classification of biosensors
Classification of transducers
In biosensors, distinct biological elements may be associated with different kinds of transducers, if the reaction of the biological element with the substrate can be monitored (CitationPaddle 1996). Electrochemical transducers are the most widely used transducers in sensor technology, but optical and QCM type of sensors have gained much attention, and their application is expanding in practical uses (CitationTothill 2009).
| a) | Optical measurements (CitationBalslev et al. 2005, CitationKovacs 1998, CitationBrecht and Gauglitz 1997) (i.e. luminescence, absorption, surface plasmon resonance) (CitationPattnaik 2005, CitationLiedberg et al. 1983): | ||||
Optical transducers used in biosensors include fluorescence, interferometry, and spectroscopy of optical wave guides and surface plasmon resonances (SPR) (CitationTothill 2009). SPR is used to detect nanomolar levels of PCR products from genetically modified organisms, to resolve human gene mutations (CitationVercoutere and Akeson 2002). | |||||
| b) | Electrochemical (CitationJunge et al. 1997, CitationPividori et al. 2000): | ||||
Electrochemical transducers have recently received significant attention in relation to the detection of DNA hybridization (CitationWang 2002, CitationWang et al. 2004). They use DNA probe molecules connected to an electrically active surface, and measure current or resistance changes created by the hybridization of target DNA (CitationVercoutere and Akeson 2002). They have been developed based on DNA hybridization, and are used to detect cancer gene mutation. The electrochemical devices used with the biosensors have been miniaturized to small pocket size instruments which make them applicable for the home use or the doctor's surgery. A range of electrochemical transducers are applied, and these include amperometric, potentiometric, and impedimetric/conductivity instruments (CitationTothill 2009). New reviews in the application of electrochemical biosensors for cancer diagnosis have been reported by Lin, Ju, and Wang (CitationTothill 2009, CitationWang 2006, CitationLin and Ju 2005). | |||||
| c) | Mass-sensitive measurements (CitationLange et al. 2002, CitationDickert et al. 1999) (i.e. surface acoustic wave, microbalance, etc.) (CitationVo-Dinh and Cullum 2000, CitationKoyun et al. 2008, CitationThévenot et al. 2001, CitationVo-Dinh 2007): | ||||
This type of transducer is also classified as being a label-free method. Piezoelectric sensors comprise a quartz crystal coated with a gold electrode, and are applied as microbalances (QCM) sensitive to changes in the mass on the sensor surface (CitationTothill 2009, CitationLin and Ju 2005). | |||||
Bioreceptor classification
The recognition element is an important component of biosensors. Primary biosensors use naturally occurring recognition elements which are extracted from biological or environmental systems. With progress in technology and synthetic chemistry, lots of biosensor recognition elements that are used today are made in the laboratories, to provide an improved stability and reproducibility of biosensor action. For instance, recognition elements including receptor proteins, antigens, antibodies, enzymes, and nucleic acids (CitationBohunicky and Mousa 2011).There are various biological interactions of ligand/bioreceptor, such as:
| 1) | Antibody/antigen interactions (CitationVo-Dinh and Cullum 2000, CitationThévenot et al. 2001). | ||||
| 2) | Nucleic acid interactions (CitationVo-Dinh and Cullum 2000, CitationVo-Dinh et al. 2001). | ||||
| 3) | Enzymatic interactions (CitationVo-Dinh and Cullum 2000, CitationVo-Dinh et al. 2001). | ||||
| 4) | Cellular interactions (CitationVo-Dinh and Cullum 2000, CitationVo-Dinh et al. 2001) (i.e. microorganisms, proteins). | ||||
| 5) | Interactions using biomimetic materials (i.e. synthetic bioreceptors (CitationVo-Dinh and Cullum 2000, CitationVo-Dinh et al. 2001)). | ||||
Basic of DNA biosensors
The necessity for selectivity of the sensors for their designated analytes in a matrix of other chemical or biological elements is probably the main reason for the interest in biosensors. This high selectivity, when compared to the selectivity of currently used chemical sensors, is obtained by the usage of naturally occurring biomolecules (enzymes, receptors, antibodies, or nucleic acids) as the sensory element (CitationPaddle 1996). They are good candidates for cheap diagnosis of genetic disease, and for the detection of pathogenic biological species in clinical research. The detection of specific DNA sequences related to genetically modified organisms is also monitored as an emerging task (CitationLucarelli et al. 2004). DNA biosensors apply immobilized DNA as diagnostic elements (CitationLucarelli et al. 2004). They convert the event of recognition of the Watson-Crick base pair into a readable analytical signal. The DNA duplex on the electrode surface is identified as a hybrid (CitationKerman et al. 2003).
Biosensors based on DNA, RNA and peptide nucleic acids are the most sensitive tools due to nucleotide strands lined up with strong pairing between bases and their complementary parts (CitationMonošík et al. 2012). Researchers from different fields such as physics, chemistry, biology, engineering, and medicine, want to develop, invent and manufacture novel sensing devices to gain more efficient and dependable information (Garipcan et al. 2017). Some biosensors are either commercially available or under development now (CitationPaddle 1996). Alongside the possibility of being very sensitive and selective, such nucleic acid-based sensors have some advantages over antibody-based biosensors. They are more stable and can be stored for longer periods, and the probe can be frequently regenerated for greater use by immersing in hot buffer, within a short time (CitationPaddle 1996).
Probe design
Immobilization of the probe is a basic step in the development of DNA biosensors (CitationMascini et al. 2005). As the properties of the hybridization reaction are essentially related to the properties of biorecognition of the captured oligonucleotide, planning the capture probe is surely the most important pre-analytical step. Therefore, many probes, with varying chemical composition and conformational arrangement, have been used to make DNA biosensors (CitationLucarelli et al. 2008). Extremely high sensibility and selectivity is needed to maximize the hybridization efficiency and minimize the non-specific binding. Probes are routinely short oligonucleotides that are capable of hybridization with individual areas of the target nucleotide sequence (CitationWang 2002). The amplicons obtained from PCR, and the DNA pieces obtained from enzymatic digestion, have a double helix structure, and the double strands should be denatured to permit the hybridization with the probe immobilized on the surface of the sensor (CitationMascini et al. 2005).
The planning of probes to analyze samples sensitive to degradation (such as RNA) needs much attention, if a sandwich hybridization scheme is selected (CitationLucarelli et al. 2008). The design of linear probes has now gained many advantages during the years of experience, which has led to much commercially available software. However, a special challenge is still posed in the planning of a complete series of arrayed probes for the screening and identification of closely related and unrelated pathogens (CitationLucarelli et al. 2008, CitationTombelli et al. 2005, CitationBarlaan et al. 2005). The utilization of capture probes in the order of 18–25 nucleotides usually displays higher levels of property to the hybridization reaction (CitationLucarelli et al. 2008). However, excessively long capture oligonucleotides often show particularly unfavorable hybridization features and yields. Although a single or a few mismatches are impossible to markedly destabilize even a 30-mer probe–target duplex over a wide range of experimental conditions, the general hybridization efficiency of similar or longer probes might be particularly low, due to intra-molecular hydrogen bonding and consequent formation of non-reactive hairpin structures (CitationLucarelli et al. 2008).
Peptide nucleic acids
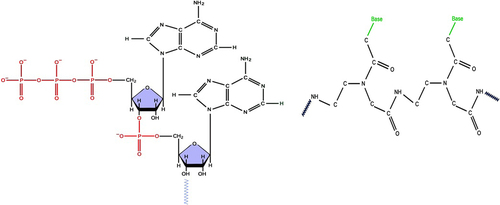
A peptide nucleic acid (PNA) is different from DNA, because the deoxyribose-phosphate backbone of DNA is replaced by a pseudo-peptide backbone, N-(2- aminoethyl) glycine (). The expansion of DNA sensors based on the sequence-specific hybridization between single-stranded nucleic acid probes tethered to solid substrates and their complementary DNA analytes in solution, has gained popularity over the past few years. In particular, the methods that can be followed in the absence of labels, such as electrochemical (CitationDharuman and Hahn 2007, CitationFang et al. 2008), optical (CitationSu et al. 2005, CitationNiu et al. 2007, CitationVikholm-Lundin et al. 2007), and piezoelectric (CitationTombelli et al. 2005, CitationDuman et al. 2003, CitationSkládal et al. 2004) methods, have gained significant attention (CitationAnanthanawat et al. 2009).
PNA forms complexes with DNA according to the Watson–Crick base-pairing rules. The PNA-DNA complexes exhibit high thermal stability and mismatch sensitivity (CitationHyrup and Nielsen 1996). The high stability of these hybrids has been illustrated by the absence of negative charges along the PNA backbone. Recently, many new PNA systems with modified structures have been developed, in efforts to improve the binding properties such as directional preference (parallel/antiparallel), selectivity between DNA and RNA, and binding specificity for different applications (CitationAnanthanawat et al. 2009, CitationAhn et al. 2003, CitationGovindaraju et al. 2003, CitationHuang et al. 2004, CitationTan et al. 2004, CitationSuparpprom et al. 2005, CitationVilaivan and Srisuwannaket 2006). PNA is a DNA analog with a neutral peptide backbone (CitationLucarelli et al. 2004). PNAs demonstrate unique features such as chemical stability, resistance to enzymatic degradation inside a living cell, recognition of specific nucleic acid sequences, formation of stable hybrid complexes, superior thermal stability and ionic strength, strand invasion, and unique hybridization relative to nucleic acids. These excellent physicochemical and biochemical features of PNA can provide a novel method of detection that is rapid, easy, and more dependable as an analytical process, and can be utilized in the field of molecular diagnostics (CitationSingh et al. 2010). High specificity of DNA binding can be achieved using new probes based on PNAs (CitationPalecek et al. 1998). PNAs are DNA mimics, in which the nucleobases are connected to a neutral N-(2 aminoethyl)-glycine pseudopeptide backbone. According to the Watson–Crick rules, PNA probes form stable hybrids by hydrogen bonding and base stacking. The backbone of PNA is neutral, due to which PNA/DNA hybrids demonstrate melting temperatures more than temperatures related to DNA/DNA duplexes, stability against nucleases and proteases, partial insensitivity to ionic strength, and generally higher selectivity against single base mismatches (CitationTost and Gut 2005). When compared with the conventional oligonucleotide probes, PNAs have received special interest in the development of electrochemical genosensing concepts, which is the major reason for the drastically different electrical characteristics of their molecular backbone (CitationLucarelli et al. 2008). Due to their neutral charge and appropriate interbase spacing, this synthetic DNA mimic leads to stronger attraction to complementary DNA sequences, and therefore promotes differentiation between complete matches and mismatches (CitationWang et al. 1997). The followers of PNAs contend that these probes are capable of binding their complementary sequence with comparably more affinity and specificity than the usual oligonucleotide probes, while discriminating mismatched targets to a larger extent. In spite of careful control of the hybridization conditions, many authors have observed partly strong non-specific signals for a number of SNP-containing targets (CitationLucarelli et al. 2008, CitationLiu et al. 2005, CitationSteichen et al. 2007).
The markedly better features of the PNA probe extensively improve the detection of single point mutation, found in several types of cancer (CitationWang et al. 1997). The capability to detect point mutations could be its most important advantage. PNA displays superior hybridization specifications and promotes chemical and enzymatic stability relative to nucleic acids. Its different molecular structure is also capable of novel ways of detection, particularly methods that avoid the introduction of a label (CitationBrandt and Hoheisel 2004).
The rearrangement of the molecules of PNA occur under alkaline conditions (CitationSchmidt et al. 1996). For more information about the affinity and characteristics, as well as the chemical and biophysical features of PNA molecules, we offer three views (CitationBrandt and Hoheisel 2004, CitationDemidov and Frank-Kamenetskii 2004, CitationUhlmann et al. 1998).
Biosensors for DNA sequence detection
The use of biosensors dates back to 1962, when Clark, known as the father of the biosensor concept, released an assay in which glucose oxidase (GOX) was trapped at a Clark oxygen electrode using a dialysis membrane (Clark Jr. and Lyons 1962) (CitationMonošík et al. 2012). The development of the Human Genome Project has had significant interest in the utilization of nucleic acid hybridization technologies to detect and recognize organisms and mutations. As known, cancer is a genetic disease caused by changes or modification of DNA sequences of key genes, and DNA analysis has a great potential for utilization in cancer diagnostics (CitationWang 2006). In order to identify the differences between a cancerous cell and a normal cell, it is necessary to assess a complicated network of pathways including gene regulation, signaling, cell metabolism, and changes in dynamics caused by various kinds of mutations which result in malignancy. For example, changes in the structure of the network through mutations or epigenetic effects, can result in changes in the dynamics that lead to different physiological features (CitationLaubenbacher et al. 2009). Since the network is normally complex, with multiple connections between pathways and important feedback loops, it is crucial to show it in the form of a computational model that can be used for a detailed analysis (CitationLaubenbacher et al. 2009). It is well appreciated that many different types of mutations must happen in a multi stage process before a cell becomes malignant, thereby altering different molecular pathways involving the affected genes (CitationLaubenbacher et al. 2009).
Microarray chips that are based on detection of hybridization/interaction in short strands of nucleic acids propose operating systems for applications such as screening of genomes, detection of pathogenic organisms, and effective search in compound libraries to detect and survey potential therapeutic agents (CitationMascini et al. 2005). Such methods must provide early detection and selection of the treatment of diseases and result in increased patient survival rates (CitationWang 2006). DNA sequence detection is now a usual method, and is done using DNA chip microarrays. This class of tools allows researchers to measure global genome mRNA expression levels (CitationBrown and Botstein 1999, CitationWu et al. 2001, CitationVercoutere and Akeson 2002), and to characterize single nucleotide polymorphisms (SNPs) within individual organisms and populations (CitationVercoutere and Akeson 2002, CitationWang et al. 1998, CitationSyvanen 2001). Sample size and multiple preliminary steps hamper microarrays from easily assessing tissue-specific or cell-specific polynucleotide sequences, or providing real-time results (CitationVercoutere and Akeson 2002, CitationMeldrum 2000, CitationWang 2000, CitationEberwine et al. 2001, CitationGut 2001). These limitations also prevent the quick search for the effect of DNA or RNA levels, which are the techniques to diagnose bacterial food contamination, the genetically modified (transgene) organisms, or the tendency of biological warfare agents. An existing test, called the Enzyme Linked Immunosorbent Assay (ELISA) test, is not sensitive enough to detect proteins at levels associated with advanced stage of the disease. Therefore, smaller, simpler, faster, and much cheaper (one-step) devices are highly favorable for replacing time-consuming laboratory analyses (CitationWang 2006). Other technologies that address these issues by combining biological sensor molecules with signal transducers, are being developed (CitationVercoutere and Akeson 2002). In DNA biosensors, the sensitive element typically contains single-stranded DNA (ssDNA) molecules that permit hybridization of complementary single-stranded molecules (CitationGill et al. 2005). They have been expanded as alternatives to conventional DNA microarrays. Despite the wide variety of biosensors and biosensor-related techniques that have been introduced, the agreed definition for these devices has remained fairly constant – an analytical device made of a biological recognition element straightly jointed to a signal transducer, which together relate the concentration of an analyte (or group of related analytes) to a measurable response (CitationTurner 1989, CitationMarco and Barceló 1999, CitationLopez-Avila and Hill 1997, CitationRogers and Mascini 2004). The biological parts of biosensors are responsible for the selective recognition of the analyte, the generation of the physicochemical signal on the transducer, and finally, the sensitivity of the final device (CitationPaddle 1996). These devices pair with signal transduction directly to sequence recognition. Some of the most sensitive and functional technologies utilize fiber optics or electrochemical sensors in relation to DNA hybridization (CitationVercoutere and Akeson 2002).
It is essential to develop a DNA biosensor with low cost, high sensitivity and good selectivity (CitationBo et al. 2011). Expansion of DNA biosensors and DNA microarrays have increased greatly in the past few years, as illustrated by the great number of scientific studies in this area (CitationSassolas et al. 2008). Biosensor reader devices connected with electronic or signal processors, are initially responsible for showing the results in a user-friendly manner (CitationCavalcanti et al. 2008). Recently, a hybrid configuration of biosensors, which includes the features of both the high affinity (irreversible) binding of an antibody or DNA/RNA probe by amplifying the qualities of an enzyme, has been introduced (CitationPaddle 1996).
According to Downs (1991), electrochemical methods that can make use of the different redox behaviors of single- and double-stranded DNA to detect the hybridization of DNA, lack sensitivity and are prone to interference (CitationPaddle 1996). The basis of biodiversity is a particular chain of bases along a strand of DNA, and the special complementary nature of the pairing between the base pairs of neighboring strands in the double helix. The capability of a single-stranded nucleic acid molecule to find and adjoin itself to its complementary partner in a sample, has been used in genetic analyses and in a biosensor (CitationPaddle 1996).
Cancer biology
Cancer is a complex disease, due to the disruption of tissue architecture. Thus, tissues, and not individual cells, are the suitable levels of observation for the study of carcinogenesis (CitationBizzarri et al. 2011). Cancer has also been identified as the second major cause of death in most developing countries (CitationTothill 2009). A number of factors can cause cancer, including genetic or environmental factors such as the exposure to carcinogenic chemicals and radiations, or microbiological causes like bacterial (e.g. stomach cancer) or viral infections (e.g. cervical cancer) (CitationTothill 2009). Studies about cancer have been concentrated on the molecular entities (genes, enzymatic reactions, intracellular pathways), with the indirect assumption that molecules are autonomous and act like a biological system; that is, a collection of entities that work together to do a certain task. In many disease conditions, finding specific and sensitive indicators that are associated with just one type of the disease can be difficult. Moreover, based on different disease conditions and phases, the level of biomarkers in biological fluids will be different. Novel and emerging molecular techniques are used to study cancer and its results, with a better understanding of the disease, as well as the discovery of potential new genomic and proteomic biomarkers. Multi-analyte analysis based on lab-on-a-chip point-of-care devices (POC) are needed to overcome the challenges in cancer diagnosis. A more sensitive and rapid technology platform is needed to perform rapid diagnosis in cancer detection. Rapid diagnosis will help in providing better health care and reducing the waiting time for results, which can be highly stressful to the patients (CitationTothill 2009, CitationAhn et al. 2004). Thus, many of the molecular markers usually assess cancer diagnosis, and these can include proteins, peptides, over/under expression of gene markers, and gene mutations (CitationTothill 2009). However, a system is not simply a collection of genes and proteins, and we cannot understand its qualities completely just by drawing diagrams of their interconnections (CitationBizzarri et al. 2011). Highly sensitive methods are immediately needed for measuring cancer diagnosis that markers show at ultra-low levels during early stages of the disease (CitationWang 2006). Development in molecular biology leads to much attention to potential biomarkers that can be used for cancer diagnosis (CitationWang 2006). For example, DNA point mutations of the p53 gene, which are engaged in cell cycle control and regulation of apoptosis, were checked using piezoelectric biosensor detection of PCR amplicons, as described by Mascini and co-workers. PCR point mutation analysis of K-ras, which is related to colorectal cancers and uses a PCR microfluidics microchip, has been described by Soper and co-workers. Wide-scale POC diagnostic systems offer major promise for early detection of cancer at a curable stage of the disease (CitationWang 2006). A variety of biosensor platforms have been stated in research on cancer diagnosis. However, introducing this technology to commercial devices may need more time and investment (CitationTothill 2009). Modern electrochemical bioaffinity sensors, such as DNA-sensors or immunosensors, offer significant sensitivity necessary for rapid testing of cancer (CitationWang 2006). During the past three decades, we have observed an enormous amount of effort in the enhancing the scope of biosensors (CitationWang 2006).
Cancer markers
Molecular biology and associated technologies lead to much better understanding of human cancer and malignancy, and of potential biomarkers which may be used for diagnosis (CitationSoper et al. 2006). Biomarkers can either be seen inside the cancer cells or be extracellular. If the markers are inside the tumor cells, they need to be collected and enriched with the cells, needing to be lysed in order to release the biomarkers before analysis. Enrichment methods for selecting tumor cells have been reported, and these methods include immunomagnetic separation using magnetic beads, and centrifugation using a density gradient (CitationTothill 2009, CitationAhn et al. 2004, CitationLara et al. 2004, CitationChoesmel et al. 2004). We show potential biomarkers in (CitationAkbarzadeh et al. 2014, CitationAkbarzadeh et al. 2012b, Citation2012c, Citation2012d, CitationValizadeh et al. 2012,).
Table I. Potential biomarkers for cancer diagnosis (CitationTothill 2009, CitationBohunicky and Mousa 2011, CitationSmith et al. 2000, CitationMeyer and Rustin 2000).
Application of biosensors
Arrays of biosensors can be utilized to detect the signature patterns of several proteins and multiple DNA mutations to guide and assist in treatment and screening (CitationWang 2006). The new solutions will enable the biosensors to perform cancer tests quickly, cheaply and reliably, in a decentralized environment (CitationWang 2006). The introduction of biosensor technology to the market, and its establishment as a measurement technique, requires a lot of focus on the interactions that occur between industry and academia (CitationConnolly 1995).
To understand the link between cancer and genetics more thoroughly, DNA analysis has become increasingly important in the diagnosis and treatment of cancer [l]. Various techniques such as electrophoresis, hybridization of probes with DNA, and in vitro amplification methods, are used to recognize the various cancers. (CitationWang et al. 1997). Recent studies have shown the hybridization of electrochemical biosensors for the detection of unique DNA sequences (CitationWang et al. 1997). DNA biosensors have been identified as a screening tool for the bioanalysis of environmental pollution studies and DNA-drug reactions. The changes in the DNA redox features (i.e. the oxidation of the guanine base) were reviewed, to study the interactions between DNA and analytes (CitationBagni et al. 2006). Several biosensor applications for cancer diagnostics are described. Rasooly and Jacobson reviewed some of the elements of cancer for cancer diagnostics, including proteins and DNA variations. Other useful cancer protein biomarkers are used by several researchers, including prostate-specific membrane antigen (PSMA), HER2/nue as a breast cancer marker, and cytokeratins to identify circulating tumor cells in peripheral blood of breast cancer patients. The new ultimate aim for biosensor utilization is point of care testing (POCT). POCT, which is diagnostic testing is done on site, makes is possible to significantly improve the delivery of health services and health care (CitationRasooly 2006). The POC clinical programs offer great potential for clinical cancer trials. POC systems are considered as integrated systems that can process clinical samples for many different types of biomarkers in a diversity of settings, such as clinical laboratories, doctors’ offices and finally, at home (CitationSoper et al. 2006). The development of POC methods will provide chances for better screening of patients at risk, tighter surveillance of disease recurrence, and better monitoring of treatment. Moreover, POC technologies are, by their very nature, low cost in their implementation, enabling large scale screening for disease prevention, and are more attractive to health care insurers (CitationSoper et al. 2006). There are several challenges in moving biosensors to POCT for cancer, including:
Development of reproducible biomarker assays.
Advances in recognition ligands, including non-antibody recognition ligands.
Development of multi-channel biosensors.
Advances in sample preparation and cancer cell enrichment.
Miniaturization and integration.
Development of new, more sensitive transducers.
Microfluidics integration.
Advanced manufacturing techniques.
Cost reduction (CitationRasooly and Jacobson 2006).
Biosensors should be compared with these procedures with regard to assay times, sensitivities, and low prices (CitationConnolly 1995). The following points may help the formative stage of the project:
Carefully identify target users and required performance.
Base parameter selection on diagnostic needs.
Develop prototypes for a panel of parameters: thyroid, fertility, etc.
Interact with clinicians at an early stage of the project.
Be realistic in terms of the practical use of the sensor (CitationConnolly 1995).
As clinical diagnostics and other applications (e.g., environmental screening) do not commonly need the massive gathering of data typical of gene chips, alternative technologies that promise to provide flexible and economical alternatives for utilizations that need relatively fewer measurements are developing. Other methods, such as electrochemical and piezoelectric transductions, are the most interesting methods because of their simplicity, low cost instrumentation, real-time and label-free detection, and commonly high sensitivity (CitationLucarelli et al. 2008). In the level of basic research, biosensors for clinical diagnostics will profit typically from research in the growing field of bioelectronics, but there is a special need for contributions in the following areas (CitationConnolly 1995) that are shown in .
Table II. Many areas that need biosensors.
Achieving the same accuracy in an in vitro technique would create a revolution in de novo DNA or RNA sequence detection, so considerable effort has been devoted to develop such a device (CitationVercoutere and Akeson 2002).
Advantages and disadvantages
Some researchers have tabulated the working features, cost, the advantages and disadvantages of these various technologies (CitationPaddle 1996). The two main prerequisites for a good action of a DNA biosensor are high specificity and high sensitivity (CitationWang 2002). The time consuming preparative steps in gene probe evaluation make it hard for them to be considered as the basis of biosensors for in situ detection of pathogenic microorganisms. Although large effort gone in to develop biosensors, a still relatively small number of analytes, particularly toxic materials, can be measured by commercially available devices (CitationPaddle 1996). Evaluations involving antibody or DNA based on biosensors are time consuming when we have to work in a dangerous environment. Despite this, biosensors are capable of being used for highly sensitive and specific onsite measurements of contamination by specific toxic materials (CitationPaddle 1996). Biosensors have also been offered for application in the detection of fatigue (Dambrot, 1992), and studying vigilance status (Dittmar et al., 1992) (CitationPaddle 1996).
Although the biosensor reduces 65%– 85% of its original activity after 3–7 days in buffer, the dried enzyme membranes maintain their original activity even after 3 years of storage at 4°C. The benefit of this method is the low cost and easy preparation and replacement of the enzyme membrane. Therefore, there is no need to reactivate and reuse the membranes after enzyme inhibition; they may be arranged after each use (CitationPaddle 1996). Commercialization of biosensors is another important factor limiting the stability of the biological component during both storage and application. Naturally occurring protein molecules (enzymes, receptors and antibodies) are dependent on denaturation, and commonly need an amount of bound water for integrity of their performance. Low moisture and high ambient temperatures would inappropriately affect biosensors operating in the gas phase (CitationPaddle 1996). In an effort to develop a method considerably more rapid and sensitive than microarrays, most biosensors are at the proof-of-principle stage (CitationVercoutere and Akeson 2002). The most difficult obstacle in DNA biosensor development is the move to clinical use, but methods such as the nanoparticle-assisted electrochemical detections seem encouraging (CitationVercoutere and Akeson 2002).
Conclusion and future prospects
Cancer is the second most prominent cause of death in developing countries. At the present, due to the increasing number of cancer patients, it has attracted a lot of attention. Various techniques and markers are used to detect cancer-causing genes. As noted above, one of these new techniques is the use of DNA biosensors. The main features of this technique are simplicity, cheapness, portability and reliability. DNA biosensors are good candidates for cheap diagnosis of genetic diseases and for the detection of pathogenic biological species in clinical search. Their high specificity and sensitivity also led to detection of cancer markers in the early stages of the disease. Several biosensor applications for cancer diagnostics are described. Many researchers have reviewed some of the elements of cancer for cancer diagnostics, including proteins and DNA variations. Biosensors and micro-array chips that are based on detection of hybridization/interaction in short strands of nucleic acids propose an operating system for applications such as screening of genomes, detection of pathogenic organisms, and effective search in compound libraries to detect and survey potential therapeutic agents. (CitationAbbasi et al. 2014a, CitationAbbasi et al. 2014b, CitationAhmadi et al. 2014, CitationAkbarzadeh et al. 2012a, CitationAkbarzadeh et al. 2013a)
Authors’ contributions
NS conceived of the study and participated in its design and coordination. AA participated in the sequence alignment and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors thank the Department of Medical Nanotechnology, Faculty of Advanced Medical Science of Tabriz University, for all support provided.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
This work is funded by the 2014 Drug Applied Research Center Tabriz University of Medical Sciences Grant.
References
- Abbasi E, Aval SF, Akbarzadeh A, Milani M, Nasrabadi HT, Joo SW, et al. 2014a. Dendrimers: synthesis, applications and properties. Nanoscale Res Lett. 9:247.
- Abbasi E, Milani M, Aval SF, Kouhi M, Akbarzadeh A, Nasrabadi HT, et al. 2014b. Silver nanoparticles: synthesis, properties, bioapplications and limitations. Crit Rev Microbiol. 1–8.
- Ahmadi A, Shirazi H, Pourbagher N, Akbarzadeh A, Omidfar K. 2014. An electrochemical immunosensor for digoxin using core-shell gold coated magnetic nanoparticles as labels. Mol Biol Rep. 41:1659–1668.
- Ahn CH, Choi JW, Beaucage G, Nevin JH, Lee JB, Puntambekar A, Lee JY. 2004. Disposable Smart lab on a chip for point-of-care clinical diagnostics. Proc IEEE. 92:154–173.
- Ahn DR, Mosimann M, Leumann CJ. 2003. Synthesis of cyclopentane amide DNA (cpa-DNA) and its pairing properties. J Org Chem. 68:7693–7699.
- Akbarzadeh A, Asgari D, Zarghami N, Mohammad R, Davaran S. 2012a. Preparation and in vitro evaluation of doxorubicin-loaded Fe3O4 magnetic nanoparticles modified with biocompatible co-polymers. Int J Nanomedicine. 7:511–526.
- Akbarzadeh A, Mikaeili H, Zarghami N, Mohammad R, Barkhordari A, Davaran S. 2012b. Preparation and in-vitro evaluation of doxorubicin-loaded Fe3O4 magnetic nanoparticles modified with biocompatible copolymers. Int J Nanomedicine. 7:511–526.
- Akbarzadeh A, Nejati-Koshki K, Soghrati MM, Alimohammadi S, Ghamari MF, Davaran S. 2013a. In vitro studies of NIPAAM-MAA-VP copolymer-coated magnetic nanoparticles for controlled anticancer drug release. J Encapsulation Adsorpt Sci. 3:108–115.
- Akbarzadeh A, Rezaei A, Nejati-Koshki K, Alimohammadi S, Davaran S. 2014. Synthesis and physicochemical characterization of biodegradable star-shaped poly lactide-co-glycolide–b-cyclodextrin copolymer nanoparticles containing albumin. Adv Nanoparticles. 3:14–22.
- Akbarzadeh A, Samiei M, Davaran S. 2012c. Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res Lett. 7:144.
- Akbarzadeh A, Samiei M, Joo SW, Anzaby M, Hanifehpour Y, Nasrabadi HT, Davaran S. 2012d. Synthesis, characterization and in vitro studies of doxorubicin-loaded magnetic nanoparticles grafted to smart copolymers on A549 lung cancer cell line. J Nanobiotechnology. 10:46.
- Akbarzadeh A, Zarghami N, Mikaeili H, Asgari D, Goganian AM, Khiabani HK, et al. 2012b. Synthesis, characterization, and in vitro evaluation of novel polymer-coated magnetic nanoparticles for controlled delivery of doxorubicin. Nanotechnol Sci Appl. 5:13–25.
- Altintas Z, Tothill IE. 2012. DNA-based biosensor platforms for the detection of TP53 Mutation. Sensor Actuat B: Chem. 169:188–194.
- Aminetzach YT, Macpherson JM, Petrov DA. 2005. Pesticide resistance via transposition-mediated adaptive gene truncation in Drosophila. Science. 309:764–767.
- Ananthanawat C, Vilaivan T, Hoven VP. 2009. Synthesis and immobilization of thiolated pyrrolidinyl peptide nucleic acids on gold-coated piezoelectric quartz crystals for the detection of DNA hybridization. Sensor Actuat B Chem. 137:215–221.
- Bagni G, Osella D, Sturchio E, Mascini M. 2006. Deoxyribonucleic acid (DNA) biosensors for environmental risk assessment and drug studies. Anal Chim Acta. 573:81–89.
- Balslev S, Jorgensen AM, Bilenberg B, Mogensen KB, Snakenborg D, Geschke O, et al. 2005. Lab-on-a-chip with integrated optical transducers. Lab Chip. 6:213–217.
- Barlaan EA, Sugimori M, Furukawa S, Takeuchi K. 2005. Electronic microarray analysis of 16 S rDNA amplicons for bacterial detection. J Biotechnol. 115:11–21.
- Beaudet AL, Belmont JW. 2008. Array-based DNA diagnostics: let the revolution begin. Annu Rev Med. 59:113–129.
- Bertram JS. 2000. The molecular biology of cancer. Mol Aspects Med. 21:167–223.
- Bidan G. 1992. Electroconducting conjugated polymers: new sensitive matrices to build up chemical or electrochemical sensors. A review. Sensor Actuat B Chem. 6:45–56.
- Bizzarri M, Giuliani A, Cucina A, D’Anselmi F, Soto AM, Sonnenschein C. 2011. Fractal analysis in a systems biology approach to cancer. Semin Cancer Biol. 21:175–182.
- Bo Y, Yang H, Hu Y, Yao T, Huang S. 2011. A novel electrochemical DNA biosensor based on graphene and polyaniline nanowires. Electrochimica Acta. 56:2676–2681.
- Bohunicky B, Mousa SA. 2011. Biosensors: the new wave in cancer diagnosis. Nanotechnol Sci Appl. 4:1–10.
- Brandt O, Hoheisel JD. 2004. Peptide nucleic acids on microarrays and other biosensors. Trends Biotechnol. 22:617–622.
- Brecht A, Gauglitz G. 1997. Recent developments in optical transducers for chemical or biochemical applications. Sensor Actuat B Chem. 38:1–7.
- Brown PO, Botstein D. 1999. Exploring the new world of the genome with DNA microarrays. Nat Genet. 21(1 Suppl):33–37.
- Cavalcanti A, Shirinzadeh B, Zhang M, Kretly LC. 2008. Nanorobot hardware architecture for medical defense. Sensors. 8:2932–2958.
- Chin TM, Tan SH, Lim SE, Iau P, Yong WP, Wong SW, Lee SC. 2005. Acceptance, motivators, and barriers in attending breast cancer genetic counseling in Asians. Cancer Detect Prev. 29:412–418.
- Choesmel V, Pierga JY, Nos C, Vincent-Salomon A, Sigal-Zafrani B, Thiery JP, Blin N. 2004. Enrichment methods to detect bone marrow micrometastases in breast carcinoma patients: clinical relevance. Breast Cancer Res. 6:R556.
- Clark LC. 1987. Biosensors: Fundamentals and Applications. New York: Oxford University Press.
- Connolly P. 1995. Clinical diagnostics opportunities for biosensors and bioelectronics. Biosens Bioelectron. 10:1–6.
- Crick FHC, Watson JD. 1954. The complementary structure of deoxyribonucleic acid. Proc R Soc Lond A Math Phys Sci. 223:80–96.
- Demidov VV, Frank-Kamenetskii MD. 2004. Two sides of the coin: affinity and specificity of nucleic acid interactions. Trends Biochem Sci. 29:62–71.
- Dharuman V, Hahn JH. 2007. Effect of short chain alkane diluents on the label free electrochemical DNA hybridization discrimination at the HS-ssDNA/diluent binary mixed monolayer in presence of cationic intercalators. Sensor Actuat B Chem. 127:536–544.
- Dickert FL, Tortschanoff M, Bulst WE, Fischerauer G. 1999. Molecularly imprinted sensor layers for the detection of polycyclic aromatic hydrocarbons in water. Anal Chem. 71:4559–4563.
- Ding C, Zhang Q, Lin JM, Zhang SS. 2009. Electrochemical detection of DNA hybridization based on bio-bar code method. Biosens Bioelectron. 24:3140–3143.
- Du H, Strohsahl CM, Camera J, Miller BL, Todd D. 2005. Krauss Sensitivity and specificity of metal surface-immobilized “molecular beacon” biosensors. J Am Chem Soc. 127:7932–7940.
- Duman M, Saber R, Pişkin E. 2003. A new approach for immobilization of oligonucleotides onto piezoelectric quartz crystal for preparation of a nucleic acid sensor for following hybridization. Biosens Bioelectron. 18:1355–1363.
- Eberwine J, Kacharmina JE, Andrews C, Miyashiro K, McIntosh T, Becker K, et al. 2001. mRNA expression analysis of tissue sections and single cells. J Neurosci. 21:8310–8314.
- Fang B, Jiao S, Li M, Qu Y, Jiang X. 2008. Label-free electrochemical detection of DNA using ferrocene-containing cationic polythiophene and PNA probes on nanogold modified electrodes. Biosens Bioelectron. 23:1175–1179.
- Garipcan B, Çağlayan MO, Demirel G. 2011. New generation biosensors based on ellipsometry. In: Andrea Serra, Ed. New Perspectives in Biosensors Technology and Applications. Chapter 9. InTech; pp. 197–214.
- Gill R, Patolsky F, Katz E, Willner I. 2005. Electrochemical control of the photocurrent direction in intercalated DNA/CdS nanoparticle systems. Angew Chem Int Ed Engl. 44:4554–4557.
- Govindaraju T, Gonnade RG, Bhadbhade MM, Kumar VA, Ganesh KN. 2003. (1 S, 2 R/1 R, 2 S)-Aminocyclohexyl Glycyl Thymine PNA: Synthesis, Monomer Crystal Structures, and DNA/RNA Hybridization Studies. Org Lett. 5:3013–3016.
- Grisel A, Francis C, Verney E, Mondin G. 1989. Packaging technologies for integrated electrochemical sensors. Sensor Actuat. 17:285–295.
- Gut IG. 2001. Automation in genotyping of single nucleotide polymorphisms. Hum Mutat. 17:475–492.
- Hierlemann A, Brand O. 2003. Microfabrication techniques for chemical/biosensors. Proc IEEE. 91:839–863.
- Huang Y, Dey S, Zhang X, Sönnichsen F, Garner P. 2004. The α-helical peptide nucleic acid concept: Merger of peptide secondary structure and codified nucleic acid recognition. J Am Chem Soc. 126:4626–4640.
- Hyrup B, Nielsen PE. 1996. Peptide nucleic acids (PNA): synthesis, properties and potential applications. Bioorg Med Chem. 4:5–23.
- Junge W, Lill H, Engelbrecht S. 1997. ATP synthase: an electrochemical transducer with rotatory mechanics. Trends Biochem Sci. 22:420.
- Kerman K, Kobayashi M, Tamiya E. 2003. Recent trends in electrochemical DNA biosensor technology. Meas Sci Technol. 15:R1.
- Kovacs GTA. 1998. Micromachined Transducers Sourcebook. New York, NY: WCB/McGraw-Hill.
- Koyun A, Ahlatcıoğlu E, İpek YK. 2008. Biosensors and their principles. Methods 6:57–64.
- Lange D, Hagleitner C, Hierlemann A, Brand O, Baltes H. 2002. Complementary metal oxide semiconductor cantilever arrays on a single chip: mass-sensitive detection of volatile organic compounds. Anal Chem. 74:3084–3095.
- Lara O, Tong X, Zborowski M, Chalmers JJ. 2004. Enrichment of rare cancer cells through depletion of normal cells using density and flow-through, immunomagnetic cell separation. Exp Hematol. 32:891–904.
- Laubenbacher R, Hower V, Jarrah A, Torti SV, Shulaev V, Mendes P, et al. 2009. A systems biology view of cancer. Biochim Biophys Acta. 1796:129–139.
- Lauks IR. 1998. Microfabricated biosensors and microanalytical systems for blood analysis. Acc Chem Res 31:317.
- Lei Y, Chen W, Mulchandani A. 2006. Microbial biosensors. Anal Chim Acta. 568:200–210.
- Liedberg B, Nylander C, Lunström I. 1983. Surface plasmon resonance for gas detection and biosensing. Sensor Actuat. 4:299–304.
- Lin J, Ju H. 2005. Electrochemical and chemiluminescent immunosensors for tumor markers. Biosens Bioelectron. 20:1461–1470.
- Liu A, Wang K, Weng S, Lei Y, Lin L, Chen W, et al. 2012. Development of electrochemical DNA biosensors. TrAC Trends Anal Chem. 37:101–111.
- Liu J, Tian S, Nielsen PE, Knoll W. 2005. In situ hybridization of PNA/DNA studied label-free by electrochemical impedance spectroscopy. Chem Commun. 21:2969–2971.
- Lopez-Avila V, Hill HH. 1997. Field analytical chemistry. Anal Chem 69:289–305.
- Lowery TJ, Garcia S, Chavez L, Ruiz EJ, Wu T, Brotin T, et al. 2005. Optimization of xenon biosensors for detection of protein interactions. ChemBioChem. 7:65–73.
- Lucarelli F, Marrazza G, Turner AP, Mascini M. 2004. Carbon and gold electrodes as electrochemical transducers for DNA hybridisation sensors. Biosens Bioelectron. 19:515–530.
- Lucarelli F, Tombelli S, Minunni M, Marrazza G, Mascini M. 2008. Electrochemical and piezoelectric DNA biosensors for hybridisation detection. Anal Chim Acta. 609:139–159.
- Marco MP, Barceló D. 1999. Environmental applications of analytical biosensors. Meas Sci Technol. 7:1547.
- Mascini M, Tombelli S, Palchetti I. 2005. New trends in nucleic acid based biosensors: University of Florence (Italy) 25–28 October 2003. Bioelectrochemistry. 67:131–133.
- Mauriz E, Calle A, Manclús JJ, Montoya A, Escuela AM, Sendra JR. 2006. Single and multi-analyte surface plasmon resonance assays for simultaneous detection of cholinesterase inhibiting pesticides. Sensor Actuat B Chem. 118:399–407.
- Meldrum D. 2000. Automation for genomics, part two: sequencers, microarrays, and future trends. Genome Res. 10:1288–1303.
- Meyer T, Rustin GJS. 2000. Role of tumour markers in monitoring epithelial ovarian cancer. Br J Cancer. 82:1535.
- Monošík R, Streďanský M, Šturdík E. 2012. Biosensors-classification, characterization and new trends. Acta Chimica Slovaca. 5: 109–120.
- Niu S, Singh G, Saraf RF. 2007. Label-less fluorescence-based method to detect hybridization with applications to DNA micro-array. Biosens Bioelectron. 23:714–720.
- Nna E, Tothill IE, Ludeman L, Bailey T. 2010. Endogenous control genes in prostate cells: evaluation of gene expression using ‘real-time’ quantitative polymerase chain reaction. Med Princ Pract. 19:433–439.
- Paddle BM. 1996. Biosensors for chemical and biological agents of defence interest. Biosens Bioelectron 11:1079–1113.
- Palecek E, Fojta M, Tomschik M, Wang J. 1998. Electrochemical biosensors for DNA hybridization and DNA damage. Biosens Bioelectron. 13:621–628.
- Pattnaik P. 2005. Surface plasmon resonance. Appl Biochem Biotechnol. 126:79–92.
- Pividori MI, Merkoci A, Alegret S. 2000. Electrochemical genosensor design: immobilisation of oligonucleotides onto transducer surfaces and detection methods. Biosens Bioelectron. 15:291–303.
- Quinn CP, Pathak CP, Heller A, Hubbell JA. 1995. Photo-crosslinked copolymers of 2-hydroxyethyl methacrylate, poly (ethylene glycol) tetra-acrylate and ethylene dimethacrylate for improving biocompatibility of biosensors. Biomaterials. 16:389–396.
- Rasooly A, Jacobson J. 2006. Development of biosensors for cancer clinical testing. Biosens Bioelectron. 21:1851–1858.
- Rasooly A. 2006. Moving biosensors to point-of-care cancer diagnostics. Biosens Bioelectron. 21:1847.
- Rogers KR, Mascini M. 2004. Biosensors for analytical monitoring. United States Environmental Protection Agency
- Sassolas A, Leca-Bouvier BD, Blum LJ. 2008. DNA biosensors and microarrays. Chem Rev. 108:109.
- Sawyer SA, Parsch J, Zhang Z, Hartl DL. 2007. Prevalence of positive selection among nearly neutral amino acid replacements in Drosophila. Proc Natl Acad Sci U S A. 104:6504–6510.
- Schalkhammer TGM. 2002. Biosensors. Anal Biotechnol. 2002:167–219.
- Schmidt J, Orgel L, Nielsen P. 1996. Separation of “uncharged” oligodeoxynucleotide analogs by anion-exchange chromatography at high pH. Anal Biochem. 235:239–241.
- Seeman NC. 1999. DNA engineering and its application to nanotechnology. Trends Biotechnol. 17:437–443.
- Singh RP, Oh BK, Choi JW. 2010. Application of peptide nucleic acid towards development of nanobiosensor arrays. Bioelectrochemistry. 79:153–161.
- Skládal P, dos Santos Riccardi C, Yamanaka H, da Costa PI. 2004. Piezoelectric biosensors for real-time monitoring of hybridization and detection of hepatitis C virus. J Virol Methods. 117:145–151.
- Smith DS, Humphrey PA, Catalona WJ. 2000. The early detection of prostate carcinoma with prostate specific antigen. Cancer. 80:1852–1856.
- Soper SA, Brown K, Ellington A, Frazier B, Garcia-Manero G, Gau V, et al. 2006. Point-of-care biosensor systems for cancer diagnostics/prognostics. Biosens Bioelectron. 21:1932–1942.
- Steichen M, Decrem Y, Godfroid E, Buess-Herman C. 2007. Electrochemical DNA hybridization detection using peptide nucleic acids and [Ru (NH3)6]3+ on gold electrodes. Biosens Bioelectron. 22:2237–2243.
- Su X, Wu YJ, Knoll W. 2005. Comparison of surface plasmon resonance spectroscopy and quartz crystal microbalance techniques for studying DNA assembly and hybridization. Biosens Bioelectron. 21:719–726.
- Suparpprom C, Srisuwannaket C, Sangvanich P, Vilaivan T. 2005. Synthesis and oligodeoxynucleotide binding properties of pyrrolidinyl peptide nucleic acids bearing prolyl-2-aminocyclopentanecarboxylic acid (ACPC) backbones. Tetrahedron Lett. 46:2833–2837.
- Suzuki H. 2000. Microfabrication of chemical sensors and biosensors for environmental monitoring. Mater Sci Eng C. 12:55–61.
- Syvanen AC. 2001. Accessing genetic variation: genotyping single nucleotide polymorphisms. Nat Rev Genet. 2:930–942.
- Tan TH, Hickman DT, Morral J, Beadham IG, Micklefield J. 2004. Nucleic acid binding properties of thyminyl and adeninyl pyrrolidine-amide oligonucleotide mimics (POM). Chem Commun (Camb). 7:516–517.
- Thévenot DR, Toth K, Durst RA, Wilson GS. 2001. Electrochemical biosensors: recommended definitions and classification. Biosens Bioelectron 16:121–131.
- Tombelli S, Minunni M, Mascini M. 2005. Piezoelectric biosensors: Strategies for coupling nucleic acids to piezoelectric devices. Methods. 37:48–56.
- Tost J, Gut IG. 2005. Genotyping single nucleotide polymorphisms by MALDI mass spectrometry in clinical applications. Clin Biochem. 38:335.
- Tothill IE. 2009. Biosensors for cancer markers diagnosis. Semin Cell Dev Biol. 20:55–62.
- Turner APF. 1989. Current trends in biosensor research and development. Sensor Actuat. 17:433–450.
- Uhlmann E, Peyman A, Breipohl G, Will DW. 1998. PNA: synthetic polyamide nucleic acids with unusual binding properties. Angewandte Chemie International Edition. 37:2796–2823.
- Valizadeh A, Mikaeili H, Samiei M, Farkhani SM, Zarghami N, Kouhi M, et al. 2012. Quantum dots: synthesis, bioapplications,and toxicity. Nanoscale Res Lett. 7:480.
- Vercoutere W, Akeson M. 2002. Biosensors for DNA sequence detection. Curr Opin Chem Biol. 6:816–822.
- Vikholm-Lundin I, Piskonen R, Albers WM. 2007. Hybridisation of surface-immobilised single-stranded oligonucleotides and polymer monitored by surface plasmon resonance. Biosens Bioelectron. 22:1323–1329.
- Vilaivan T, Srisuwannaket C. 2006. Hybridization of pyrrolidinyl peptide nucleic acids and DNA: Selectivity, base-pairing specificity, and direction of binding. Org Lett. 8:1897–1900.
- Vo-Dinh T, Cullum B. 2000. Biosensors and biochips: advances in biological and medical diagnostics. Fresenius J Anal Chem. 366: 540–551.
- Vo-Dinh T, Cullum BM, Stokes DL. 2001. Nanosensors and biochips: frontiers in biomolecular diagnostics. Sensor Actuat B Chem. 74: 2–11.
- Vo-Dinh T. 2007. Biosensors and biochips. BioMEMS and Biomedical Nanotechnology. Springer; pp. 1–20.
- Wang DG, Fan JB, Siao CJ, Berno A, Young P, Sapolsky R, et al. 1998. Large-scale identification, mapping, and genotyping of single- nucleotide polymorphisms in the human genome. Science. 280: 1077–1082.
- Wang J, Liu G, Jan MR. 2004. Ultrasensitive electrical biosensing of proteins and DNA: carbon-nanotube derived amplification of the recognition and transduction events. J Am Chem Soc. 126: 3010–3011.
- Wang J, Rivas G, Cai X, Chicharro M, Parrado C, Dontha N, et al. 1997. Detection of point mutation in the p53 gene using a peptide nucleic acid biosensor. Anal Chim Acta. 344:111–118.
- Wang J. 1998. DNA biosensors based on peptide nucleic acid (PNA) recognition layers. A review. Biosens Bioelectron. 13:757–762.
- Wang J. 1999. PNA biosensors for nucleic acid detection. Curr Issues Mol Biol. 1:117–122.
- Wang J. 2000. Survey and summary from DNA biosensors to gene chips. Nucleic Acids Res. 28:3011–3016.
- Wang J. 2002. Electrochemical nucleic acid biosensors. Anal Chim Acta. 469:63–71.
- Wang J. 2006. Electrochemical biosensors: towards point-of-care cancer diagnostics. Biosens Bioelectron. 21:1887–1892.
- Wooster R, Neuhausen SL, Mangion J, Quirk Y, Ford D, Collins N, et al. 1994. localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12–13. Science. 265:2088.
- Wu SH, Ramonell K, Gollub J, Somerville S. 2001. Plant gene expression profiling with DNA microarrays. Plant Physiol Biochem. 39:917–926.