Abstract
The molybdenum blue method was used to determine the content of reduced vitamin C (Vc) in a solution of polymerized hemoglobin-based oxygen carriers (HBOCs) of the human placenta. The conditions of absorption wavelength, HCl addition, and reaction time, were investigated. The results of validation experiments showed that under the optimized conditions, a standard curve was confirmed with good linearity of 0.9985, for the Vc amount ranging from 0–200 μg. The values for relative standard deviation (RSD) of the precision and repeatability were both below 5%. Vc recovery was in the range of 97–102%. The conclusion could be made that a reduction in Vc content could be tested effectively by the molybdenum blue method.
Introduction
Hemoglobin-based oxygen carriers (HBOCs) have been paid much attention during the past decades due to their potential applications in blood substitutes and oxygen therapeutics (CitationSakai et al. 2013, CitationSilverman and Weiskopf 2009). To reduce the side effects such as vasoconstriction and renal toxicity, HBOCs were prepared through hemoglobin polymerization or modification to form hemoglobin (Hb) complexes with higher molecular weight. Due to the absence of enzymatic protective systems in red blood cells, HBOCs are subjected to oxidation during the spontaneous and uncontrolled oxidation process of ferrous Hb (Fe2+), leading to the formation of methemoglobin (MetHb) and free radicals (CitationChang 2010), which was considered to be one of main reasons for the adverse reactions of HBOCs in clinical trials (CitationLinberg et al. 1998). It was believed that only HBOCs with a MetHb content less than 10% can effectively deliver oxygen to hypoxic tissues (CitationBuehler et al. 2010). Thus, to prevent the formation of MetHb and free radicals, certain antioxidants were administered for attenuation of oxidative reactions.
As a typical non-enzymatic antioxidant with lower molecular weight in human plasma, Vc attracts much attention because of its powerful antioxidative properties (CitationHarrington et al. 2010, CitationDorman et al. 2002). The results of our previous research work showed that polymerized human placental HBOCs could be protected against antioxidative stress by Vc, indicating its potential applications in the development of HBOCs (CitationChen et al. 2013). During the pharmaceutical development and production process, it is necessary to ensure the safe dose of any additives. Therefore, building a method for determination of the Vc content has a significant role in the development of HBOCs.
Until now, Vc content was usually tested through the fluorescence method (CitationMatsuoka et al. 2012), phenanthroline monohydrate method (CitationZenki et al. 2004), and iodimetry method (CitationPaixao et al. 2006), which were all used for non- protein samples. As derivatives of hemoglobin, it was necessary to isolate the polymerized hemoglobin from solutions before the detection of Vc. In this work, as solutions of polymerized human placental hemoglobin were being chosen as samples, the HBOCs were isolated through the precipitation method in solutions using HCl at certain concentrations. Then, the Vc content of the supernatant was tested through the molybdenum blue method, which is considered to be fast, effective and low-cost.
Materials and methods
Chemicals and reagents
Human placental blood was provided by Sichuan New Life Stem Cell Polytron Technologies Inc. (Chengdu, China). The standard Vc was purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). The other chemicals used were of analytical grade. The water used in this research was obtained from the RO/BIO Water System (18.2 MΩ cm, PALL Corporation, USA).
Instruments
UV/Visible Spectrophotometer (Ultrospec 6300TM, Amersham Biosciences, America); Experimental pH meter (FE20, Mettler-Toledo Instruments, China); Electronic Balance (AL204, Mettler-Toledo Instruments, China); Blood Cell Analyzer (BC-3200, Mindray, China); Electric Thermostatic Water Bath (DK-8AD, Hengyi Technology, China); RO/BIO Water System (CascadaTM, PALL, America).
Experimental methods
Preparation of HBOCs
The polymerized human placental hemoglobin was prepared as described previously (CitationLi et al. 2006).
Preparation of the coloring reaction solution
1. Oxalic Acid-Ethylene Diamine Tetraacetic Acid (OA-EDTA) solution
The OA-EDTA solution was prepared as follows: 6.3000 g OA (with crystal water) and 0.0584 g EDTA were dissolved in pure water and introduced into a 1000 ml flask. The OA-EDTA solution obtained contained 0.05 mol/L OA and 0.2 mmol/L EDTA.
2. Metaphosphoric Acid-Acetic Acid (MA-AA) solution
The MA-AA solution was prepared by introducing 15.0000 g MA and 40 ml AA into a 500 ml volumetric flask and diluted with pure water to 500 ml. The solution could be obtained after filtration.
3. Ammonium Molybdate (AM) solution
The AM solution, at a concentration of 0.037 mol/L, was prepared by dissolving 25.0000 g AM with pure water, in a 500 ml volumetric flask.
Pretreatment of polymerized human placental HBOC solution
A certain amount of hydrochloric acid solution with the concentration of 12 mol/L, 2.0 ml of HBOC solution, and 2.0 ml of OA-EDTA solution, were introduced into a centrifuge tube, and then the mixed solution was diluted to a final volume of 8 ml. After being agitated for 1 min and centrifuged at 7500 rpm for 15 min, the polymerized hemoglobin was precipitated, and the supernatant could be used as the sample solution for Vc detection.
Standard curve of Vc
In each Brown volumetric flask with a volume of 10 ml, the coloring reaction solutions were added, including 3 ml of OA-EDTA solution, 1 ml of MA-AA solution, 2 ml of ammonium molybdate solution, and certain amounts of HCl, followed by the addition of 10, 40, 80, 120, 160, and 200 μl respectively of the standard Vc solution, with a concentration of 1 mg/ml (equal to 10, 40, 80, 120, 160, and 200 μg of Vc quantity). After the coloring reaction solution was diluted with pure water to the required volume and the color reaction was completed, the absorbance of the coloring solutions at certain wavelengths were tested, with pure water as the reference. The standard curve could be obtained by the linear relationship between the absorbance value and the amounts of Vc added.
Detection and calculation of Vc in sample solutions
Sample solutions containing unknown amounts of Vc were added to the coloring reaction solution in a flask, to trigger the color reaction. After the reaction was completed, the absorbance of the resultant color solution was tested, with pure water as the reference. Each sample was tested three times, for calculation of the average value.
The content of Vc in the sample solutions after pretreatment could be calculated from the absorbance value by a standard curve, and then the reduced Vc amounts in the HBOC solutions could be calculated as below:
m: Vc content in coloring reaction solution in the volumetric flask (μg)
V1: Volume of sample solution added into coloring reaction solution after pretreatment (ml)
V2: Total volume of sample solution after pretreatment (ml)
V3: Volume of HBOC solution for pretreatment (ml)
Results
Effects of HCl concentration on the precipitation of HBOCs
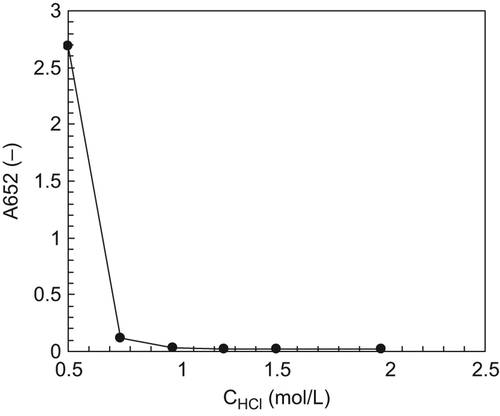
Two milliliters of HBOC solution was added into OA-EDTA solutions with HCl concentrations of 0.50, 0.75, 1.00, 1.25, 1.50 and 2.00 mol/L, to investigate the effect of HCl concentration on the precipitation of HBOCs. After centrifugation, the absorbance of supernatants at the wavelength of 652 nm (A652) was tested, and a lower A652 value indicated more effective precipitation. The results of the precipitation experiment are shown in . It can be seen that the A652 decreased with the increase in HCl concentration, which indicates that the OA-EDTA solution with higher HCl concentration shows more effective precipitation. When the concentration of HCl increased to above 1.00 mol/L, the A652 values of the supernatants were constant, and showed nearly no significant differences compared to the reference, indicating the complete precipitation of HBOCs. Therefore, the concentration of HCl should be above 1.00 mol/L, to ensure the complete precipitation of HBOCs.
Optimization of color reactions
Maximum absorption wavelength
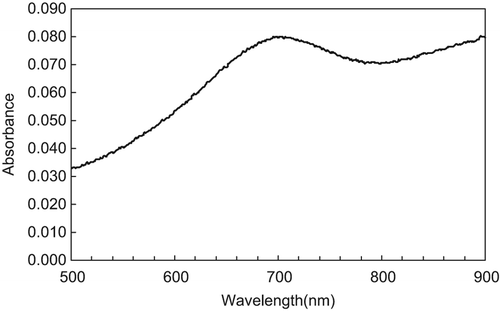
Fifty microliters of Vc solution with a concentration of 0.1% (w/v) were added into a coloring reaction solution; after completion of the reaction, the absorbance values of the coloring reaction solution were measured at different wavelengths in the range of 500–900 nm, and the results are shown in . From the spectrum curve, an absorption peak can be detected in the range of 650–760 nm, and the maximum absorption is seen to be located at 710 nm, which was chosen as the test wavelength in this study.
Additional amount of HCl
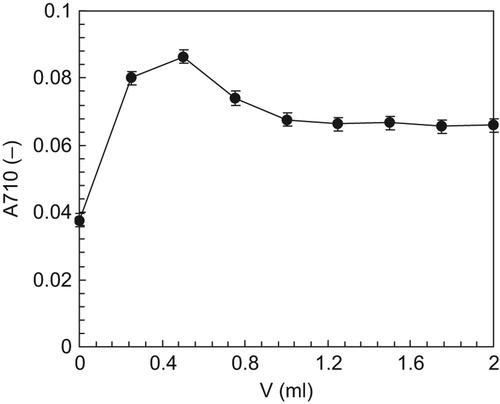
Fifty microliters of Vc solution, with a concentration of 0.1% (w/v) were added into coloring reaction solutions, with different amounts of HCl addition at 0.00, 0.25, 0.50, 0.75, 1.00, 1.25, 1.50, 1.75 and 2.00 ml, respectively. After completion of the reaction, the absorbance of the reaction solutions at the wavelength of 710 nm (A710) was tested, and the results are shown in . It can be seen that when smaller amounts of HCl were added, (≤ 0.5 ml), the A710 values increased with the increase in the amount of HCl added. When the amounts of HCl added were above 0.5 ml, the A710 values gradually decreased, to remain constant with further increase in the amount of HCl added, within the range of experimental study. Therefore, it can be concluded that A710 can remain stable when the amount of HCL added ranges from 1.00 to 2.00 ml.
Colorimetric reaction time
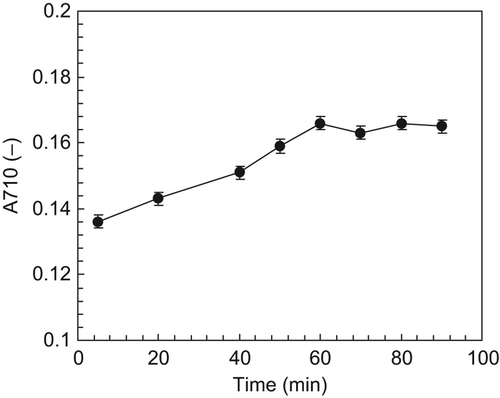
A quantity of thirty μl of Vc solution, with the concentration of 0.1% (w/v), was added into the coloring reaction solutions, and the A710 values were tested at certain time intervals. The data in show that the A710 values gradually increased during the early reaction time. After the reaction time of 60 min, the A710 values remained constant, indicating that the coloring reaction was nearly complete. Thus, it is reasonable to believe that the A710 value can be tested 60 min after the start of the coloring reaction.
Method validation
Standard curve
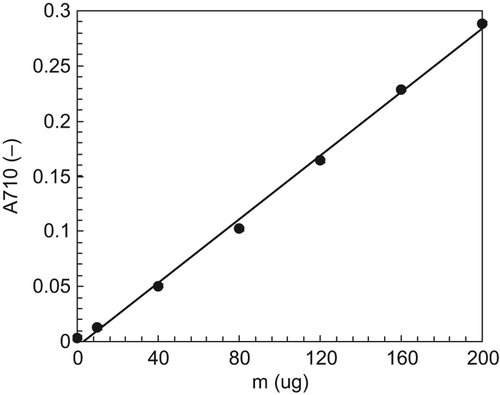
Based on the optimization of the conditions of the coloring reaction, the standard curve could be obtained, and the data are shown in . The results of the standard curve suggest that with the addition of Vc ranging from 0 to 200 μg, the linear coefficient of the standard curve is 0.9985, indicating a good linearity between the A710 and the amounts of Vc added.
Sensitivity
In sensitivity experiments, different amounts of Vc solution at a concentration of 0.01% (w/v), were added to the coloring reaction solutions. Each sample was tested three times, and the data are shown in . It can be seen that the A710 value with the addition of 12 μg of Vc, was about 3 times more than that on addition of 0 μg Vc, indicating the detection limitation of 12 μg.
Table I. Results of sensitivity experiments.
Repeatability and precision
The repeatability and precision were evaluated using the relative standard deviations (RSD). Three samples with different Vc concentrations were prepared and then analyzed by the established method. Each sample was tested in six replicates and the data are listed in and . The RSD values were 3.24%, 3.76% and 4.54% for repeatability, and 3.26%, 2.30% and 2.81% for precision, indicating good repeatability and precision of the colorimetric method developed.
Table II. Results of repeatability experiments.
Table III. Results of precision experiments.
Recovery
The recovery experiments were carried out through the decrement method, in which a certain amount of Vc was added into the reaction solution for reference, before the addition of a spiked amount of Vc. The detected amount of standard Vc addition could be obtained by subtracting the reference amount from that of the total Vc. In this experiment, three levels of standard samples were tested. The data of results, in , show that the average recoveries were 101.81%, 97.93% and 97.02% respectively, indicating good accuracy of the colorimetric method established.
Table IV. Results of recovery experiments.
Discussion
Metaphosphoric acid and phosphomolybdate can interact with reduced Vc in acidic conditions, leading to the formation of the molybdenum blue complex. The amounts of molybdenum blue complex obtained are proportional to the reduced amounts of Vc in the reaction. Based on this principle, the reduced Vc content could be detected through the molybdenum colorimetric method. In this work, the quantities of the metaphosphoric acid and ammonium molybdate were in excess, relative to that of reduced Vc, which ensured the completion of coloring reaction. For the accuracy of testing, it is necessary to add the OA-EDTA solution into the coloring reaction solution during the process of addition, to avoid the interferential effect of free metal cations by breaking the reaction between the reduced Vc and the oxidation cations, especially by the free Fe3+ cations.
Due to the dark pigment color of the HBOCs, the molybdenum colorimetric method cannot be used directly to detect the Vc content in the HBOC solution. In this work, the HBOCs were isolated through precipitation in acidic solution, to eliminate the interference of HBOCs in Vc detection. In previous studies, sulfuric acid was usually used as the acidic medium in the colorimetric reaction solution (CitationSayed Elnenaey et al. 1979, CitationEldawy et al. 1975). However, the system of conversion of HBOCs usually contained Ca2+ cations, which were subjected to precipitation by the sulfate radicals. Therefore, hydrochloric acid was used as the acidic medium for the HBOC precipitation and coloring reaction in this experiment, because of the negative effects of Cl− on the reaction. Due to the controversial reports about the maximum absorption (CitationSayed Elnenaey et al. 1979, CitationEldawy et al. 1975, CitationTiwari 2010) and the changes in the acidic medium, it is necessary to optimize the reaction conditions, including the maximum absorption wavelength, HCl addition, and reaction time. In this work, the effects of temperature on the reaction were not investigated, because detailed elucidation has been provided in previous work (CitationTiwari 2010).
Vc is a kind of efficient natural antioxidant, and can react with a variety components in the HBOC solution, including free Fe3+, free oxygen, Hb-O2, and MetHb. These reactions lead to the rapid variation in Vc content, which the recovery experiments should take into consideration. In this work, the decrement method was used, based on the assumption of nearly equivalent consumption of Vc by the reference and samples during the testing. The detected amounts of standard Vc addition could be obtained by subtracting the reference amount from that of total Vc. Consequently, the decrement method was proved to be suitable for Vc detection in HBOCs by the recovery experiments, yielding results in the range of 97–102%.
HBOCs are usually preserved in the solution system containing additives such as glucose, Ringer's solution, saline, and hydroxyethyl starch, which prove to bear a negative effect on the molybdenum blue method (CitationSayed Elnenaey et al. 1979). In addition, the reagents used in this work are easy to obtain and the operations during the testing are simple, suggesting the simplicity and convenience of the testing process in the molybdenum blue method.
Conclusion
In this work, the molybdenum blue method was used to detect the Vc content in the solution of HBOCs, and the conditions of the colorimetric reaction were optimized. The results show that HBOCs could be isolated effectively by the OA-EDTA solution with HCl as the acidic medium, which has no interferential effects on the reaction. The RSD of the data from method validation were below 5%, and the recoveries were between 97% and 102%. In conclusion, the molybdenum blue method can detect the Vc content effectively in the solution of HBOCs, and thus has potential application in development of HBOCs due to its convenient, low-cost, and simple operation.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
The authors are grateful to the financial support from the National High Technology Research and Development Program (863 Program) of China (No.2012021903-3), the Science and Technology support program of Sichuan Province in China (No.2013SZ0059), and the Innovation project of Sichuan Province in China (No.2013ZZ0006).
References
- Buehler PW, D’Agnillo F, Schaer DJ. 2010. Hemoglobin-based oxygen carriers: from mechanisms of toxicity and clearance to rational drug design. Trends Mol Med 16:447–457.
- Chang TM. 2010. Blood replacement with nanobiotechnologically engineered hemoglobin and hemoglobin nanocapsules. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2:418–430.
- Chen G, Duan Y, Li S, Wang H, Liu J, Yang C . 2013. Antioxidant effects of Vc on humna placenta hemoglobin-based oxygen carriers. The14th International Symposium of Blood Substitutes and Oxygen Therapeutics. Chengdu, China.
- Dorman SC, Kenny CF, Miller L, Hirsch RE, Harrington JP. 2002. Role of redox potential of hemoglobin-based oxygen carriers on methemoglobin reduction by plasma components. Artif Cells Blood Substit Immubil Biotechnol 30:39–51.
- Eldawy MA, Tawfik AS, Elshabouri SR. 1975. Rapid, sensitive spectrophotometric method for the determination of ascorbic acid. Anal Chem 47:461–464.
- Harrington JP, Orlig K, Zito SL, Wollocko J, Wollocko H. 2010. Structural and redox behavior of OxyVitaTM, a zero-linked polymeric hemoglobin: comparison with natural acellular polymeric hemoglobins. Artif Cells Blood Substit Immubil Biotechnol 38: 64–68.
- Li T, Zhang H, Wang H, Yu R, Yang C. 2006. Purification and viral inactivation of hemoglobin from human placenta blood. J Biomed Eng 23:640–644.
- Linberg R, Conover CD, Shum KL, Shorr RG. 1998. Hemoglobin-based oxygen carriers: how much methemoglobin is too much? Artif Cells Blood Substit Immubil Biotechnol 26:133–148.
- Matsuoka Y, Yamato M, Yamasaki T, Mito F, Yamada K. 2012. Rapid and convenient detection of ascorbic acid using a fluorescent nitroxide switch. Free Radic Biol Med 53:2112–2118.
- Paixao TR, Lowinsohn D, Bertotti M. 2006. Use of an electrochemically etched platinum microelectrode for ascorbic acid mapping in oranges. J Agric Food Chem. 54:3072–3077.
- Sakai H, Ng K, Li B, Sugimura N. 2013. Swine hemoglobin as a potential source of artifical oxygen carriers, hemoglobin-vesicles. Artif Cells Nanomed Biotechnol 41:37–41.
- Sayed Elnenaey E, Soliman R. 1979. Asensitive colorimetric method for estimation of ascorbic acid. Talanta 26:1164–1166.
- Silverman TA, Weiskopf RB. 2009. Hemoglobin-based oxygen carriers: current status and future directions. Transfusion 49: 2495–2515.
- Tiwari KK. 2010. A new spectrophotometric method for the determination of ascorbic acid using leuco malachite green. J Chinese Chem Soc 57:105–110.
- Zenki M, Tanishita A, Yokoyama T. 2004. Repetitive determination of ascorbic acid using iron(Ⅲ)-1.10-phenanthroline-peroxodisulfate system in a circulatory flow injection method. Talanta 64:1273–1277.