 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The synthesis of different kinds of magnetic nanoparticles (MNPs) has attracted much attention. During the last few years, a large portion of the articles published about MNPs have described efficient routes to attain shape-controlled and highly stable MNPs with narrow size distribution. In this review, we have reported several popular methods including co-precipitation, microemulsion, thermal decomposition, solvothermal, sonochemical, microwave-assisted, chemical vapor deposition, combustion, carbon arc, and laser pyrolysis, for the synthesis of magnetic nanoparticles.
Introduction
Nanoscience is one of the most important fields of research in modern science. Nanotechnology is beginning to allow researchers to work at the molecular and cellular levels, to achieve important developments in life sciences and healthcare (CitationAkbarzadeh et al. 2012). Nanotechnology has been successfully applied for in vivo molecular imaging, disease diagnosis, and as an improved therapeutic platform (CitationYang et al. 2012).
NPs are submicron moieties with diameters ranging from 1 to 100 nm, made of inorganic or organic materials, which have many novel properties when compared to the bulk materials (CitationLaConte et al. 2005, CitationWu et al. 2008).The use of nanoparticle (NP) materials offers major advantages, due to their unique size and physicochemical properties (CitationAkbarzadeh et al. 2012). Nanomaterials have been attracting great attention owing to their excellent optical, electrical, magnetic, and catalytic properties. It is well known that the phases, sizes, and morphologies of nanomaterials have great impact on their properties and potential applications. In this context, the controlled synthesis of nanostructured materials with new morphologies has recently received much attention (CitationGeng et al. 2006, CitationHonig and Spalek 1998, CitationRao et al. 2007, CitationLiu et al. 2013).
Currently, real uses of nanostructured materials in life sciences are unusual. However, the excellent properties of these materials provide a promising future for their use in this field (CitationAkbarzadeh et al. 2012, CitationDavaran and Entezami 1996, CitationSpanhel et al. 1987, CitationSteigerwald and Brus 1989, CitationSteigerwald and Brus 1990). NPs have been used to deliver drugs to target tissues and to enhance stability against degradation by enzymes, such as superparamagnetic NP, which can be used by an external magnetic field to lead it to the target tissue (CitationMahdavi et al. 2013, CitationPourhassan-Moghaddam et al. 2013). With the recent progress of nanobiotechnology, magnetic nanoparticles (MNPs) have gained more attention for use in biomedical applications (CitationYang et al. 2012). MNPs are the group of engineered and specific materials of sizes less than 100 nm, that can be manipulated under the effect of an external magnetic field (CitationIndira and Lakshmi 2010).
MNPs have attracted researchers from different fields such as biology, medicine, and physics, due to their multifunctional properties such as small size, superparamagnetism and low toxicity, etc. (CitationGu et al. 2006, CitationAhmadi et al. 2014, CitationRoger et al. 1999, CitationWunderbaldinger et al. 2002). For biological and biomedical applications, magnetic iron oxide NPs are the best choice, for their biocompatibility, superparamagnetic actions, and chemical stability (CitationCabrera et al. 2008). Magnetic iron oxide NPs have been considered the best choice, and the application of small iron oxide NPs in in vitro diagnostics has been practiced, for almost half a century (CitationWu et al. 2008, CitationGupta and Gupta 2005).
As a kind of practical magnetic material, Fe3O4 nanomaterials have been used in many fields, because of their unique electric and magnetic properties (CitationLiu et al. 2013, CitationZhu and Diao 2011, CitationHou et al. 2003). Magnetite (Fe3O4) NPs have attracted much interest, not only in the field of magnetic recording media, but also in the areas of medical care, such as medical applications, including magnetic resonance imaging (MRI), drug delivery systems, medical diagnostics, cancer therapy, microwave devices, magneto-optic devices, etc. (CitationSilva et al. 2004, CitationSun et al. 2000, CitationSun 2006, CitationPankhurst et al. 2003, CitationDavaran et al. 2013, CitationPortet et al. 2001, CitationIto et al. 2005, CitationMeng et al. 2009, CitationZi et al. 2009, CitationGhasemali et al. 2013, CitationKashevsky et al. 2008, CitationEl Ghandoor et al. 2012). The use of NPs with sizes smaller than 100 nm has some advantages, such as their higher effective surface areas, lower sedimentation rates, and better tissular diffusion (CitationAkbarzadeh et al. 2012, CitationPuntes et al. 2001, CitationPark et al. 2005, CitationSadat Tabatabaei Mirakabad et al. 2014).
MNPs can bind to drugs, proteins, enzymes, antibodies, or nucleotides, and can be absorbed to an organ, tissue, or tumor using an external magnetic field, or can be heated in alternating magnetic fields for use in hyperthermia (CitationMahdavi et al. 2013).
Targeting of drugs by NPs is intended to decrease drug wastage, lessen the need for regular drug administration, and reduce side effects, providing prolonged and sustained drug delivery to the chosen target organ (CitationIndira and Lakshmi 2010, CitationSimsek and Akif Kilic 2005).
Magnetic iron oxide NPs have a large surface-to-volume ratio and consequently possesses high surface energy. Therefore, they tend aggregate, so as to minimize the surface energy. Moreover, the naked iron oxide NPs have high chemical activity, and are easily oxidized in air, generally resulting in the loss of magnetism and dispersibility. Therefore, it is important to provide proper surface coating and improve some effective protective approaches to retain the stability of magnetic iron oxide NPs. Particularly, for in vivo applications, the MNPs must be encapsulated with a biocompatible polymer during or after the preparation process, to avoid changes from the original structure, the formation of large aggregates, and biodegradation when exposed to the biological system (CitationAkbarzadeh et al. 2012, CitationMuldoon et al. 2005, CitationMoghimi et al. 2001, CitationSosnovik et al. 2007).
Due to the extensive applications of MNPs in biotechnology, biomedicine, material science, engineering, and environmental areas, the synthesis of different kinds of MNPs has attracted much attention (CitationTartaj et al. 2003, CitationFaraji et al. 2010, CitationNiemeyer 2001). Recently, much research has been in progress on the synthesis of iron oxide NPs, and many reports have clarified efficient approaches of synthesis to produce shape-controlled, stable, biocompatible, and monodisperse iron oxide NPs (CitationWu et al. 2008). In the last decade, many efforts have been made to develop techniques and processes that would yield ‘mono-disperse colloids’ containing NPs that are uniform, both in size and shape (CitationBao et al. 2006, CitationKim and Park 2005, CitationKotitz et al. 1999). The synthesis of mono-dispersible nanocrystals with controllable sizes is very important, because the properties of these nanocrystals rely strongly on their dimensions, and it is also important to characterize the size-dependent physico-chemical properties of nanocrystals (CitationAkbarzadeh et al. 2012, CitationPortet et al. 2001, CitationKwon et al. 1997, CitationKwon et al. 1995, CitationDenizot et al. 1999, CitationWormuth 2001). MNPs have been prepared with a number of different compositions and phases, involving pure metals, such as Fe, Co, and Ni (CitationPuntes et al. 2001, CitationPark et al. 2000, CitationSun et al. 2000); metal oxides, such as Fe3O4 and γ-Fe2O3 (CitationNeveu et al. 2002, CitationDavaran et al. 2014, CitationSun and Zeng 2002); ferrites, such as MFe2O4 (M = Cu, Ni, Mn, Mg, etc.) (CitationHu et al. 2007, CitationPark et al. 2004); and metal alloys, such as FePt and CoPt (CitationSun et al. 2000, CitationFaraji et al. 2010, CitationShevchenko et al. 2002). Highly magnetic materials, such as cobalt and nickel, are toxic and are liable to oxidation; hence, they are of little interest (CitationAkbarzadeh et al. 2012, CitationChoi et al. 2007, CitationMurray et al. 1993, CitationPeng et al. 2000).
Several methods have been developed to synthesize Fe3O4 particles with sizes in the nanometer range (CitationThapa et al. 2004, CitationBerger et al. 1999). In nearly all uses, the method of synthesizing nanomaterials represents one of the most important challenges that will determine the shape, size distribution, particle size, and surface chemistry of the particles, and therefore their magnetic properties (CitationLopez Perez et al. 1997, CitationKouhi et al. 2014, CitationSjogren et al. 1997). In addition, the preparation method expresses to a great extent the degree of structural imperfections or impurities in the particle, as well as the distribution of such defects within the particle, hence defining its magnetic behavior (CitationAkbarzadeh et al. 2012, CitationGrossman et al. 2004, CitationChung et al. 2004).
In this review, we focus mainly on methods of preparation of MNPs for biomedical and biological applications. We want to modernize currently available methods for the synthesis of Fe3O4 nanomaterials with several morphologies; in addition, some important and novel findings reported earlier are also involved.
Synthesis of MNPs
There are several methods to synthesize MNPs, which have been reported in several papers (CitationSun et al. 2007). To date, various popular methods comprising co-precipitation, microemulsion, thermal decomposition, solvothermal, sonochemical, microwave-assisted, chemical vapor deposition, combustion, carbon arc, and laser pyrolysis, have been reported for the preparation of MNPs (CitationAkbarzadeh et al. 2012). In addition, these NPs can also be synthesized by other methods such as electrochemical synthesis (CitationCabrera et al. 2008, CitationPascal et al. 1999), laser pyrolysis techniques (CitationBomati-Miguel et al. 2008), microorganism or bacterial synthesis (especially the magnetotactic bacteria and iron reducing bacteria) (CitationBharde et al. 2008, CitationRoh et al. 2006), etc. (CitationWu et al. 2008). Several novel and effective methods have been developed to synthesize Fe3O4 nanomaterials with different shapes, such as nanorods, nanotubes, and hierarchical superstructures (CitationLiu et al. 2013, CitationLi et al. 2011, CitationBarth et al. 2008, CitationAbbasi et al. 2014, CitationLiu et al. 2005, CitationGong et al. 2010).
Green synthesis of MNPs
Green nanotechnology has attracted a lot of attention and includes various processes which decrease or eliminate toxic substances to restore the environment. The biosynthesis of metal nanoparticles by plants is currently under development. The synthesis of metal NPs using inactivated plant tissue (CitationPadil and Cernik 2013), plant extracts (CitationShameli et al. 2012), exudates (CitationLukman et al. 2011), and other parts of living plants (CitationPourhassan-Moghaddam et al. 2014), is a modern option for their production. Green synthesis of NPs makes use of environmentally friendly, non-toxic and safe components. The development of reliable, nontoxic, and eco-friendly methods for the synthesis of NPs is of extreme importance to develop their biomedical applications (CitationSalam et al. 2012, CitationShankar et al. 2004, CitationMahdavi et al. 2013).
Biological methods of nanoparticle preparation using microorganisms (CitationKlaus et al. 1999, CitationNair and Pradeep 2002, CitationAbbasi et al. 2014), enzymes (CitationWillner et al. 2006), fungi (CitationVigneshwaran et al. 2007), and plants or plant extracts (CitationChandran et al. 2006, CitationSong and Kim 2009), have been recommended as possible eco-friendly substitutes to chemical and physical methods. Sometimes, the nanoparticle preparation using plants or parts of plants can prove advantageous over other biological processes, by eliminating the elaborate work involved in maintaining microbial cultures (CitationForough and Fahadi 2011).
The reason for selecting plants for biosynthesis is due to their reducing agents such as citric acid, ascorbic acids, flavonoids, reductases, dehydrogenases and extracellular electron shuttles, that may play an important role in the biosynthesis of MNPs (CitationPandey et al. 2012).
Awwad A. M. and Salem N.M. (CitationAwwad and Salem 2012) recommended a rapid, non-toxic, facile and green synthesis method to prepare magnetite NPs in a single step reaction. Ferric chloride hexahydrate and ferrous chloride tetra hydrate, carob leaf extract, and sodium hydroxide, were the substances used in the synthesis experiments. Magnetite NPs can be obtained in a relatively low temperature range of 80–85°C. Magnetite NPs (Fe3O4) were prepared by a simple, rapid and green method, in a single vessel reaction. The average diameter of magnetite NPs is 4–8 nm, and they have good monodispersible properties. The magnetite NPs were coated by the carboxylic groups of amide I and amide II chains of the protein in carob leaf extract ().
Chin et al. (CitationEatemadi et al. 2014) attempted to prepare Fe3O4 NPs by the thermal decomposition method, without using toxic organic surfactants and solvents. Poly (ethylene glycol), PEO, was being used as both solvent and surfactant simultaneously, to prepare Fe3O4 NPs of controllable particle size and narrow size distribution. PEO has been widely used as a green solvent for several organic syntheses due to its low toxicity and high boiling point (CitationSmith et al. 2005, CitationKidwai et al. 2010, CitationHosseininasab et al. 2014). The approach for MNP synthesis employs an environmentally friendly solvent, PEO, as an alternative to organic solvent. PEO has been applied both as a solvent and as a surfactant which inhibits the agglomeration of Fe3O4 NPs formed during synthesis (CitationEatemadi et al. 2014).
The green synthesis has many advantageous features for the synthesis of magnetite NPs; it is economical, environmentally friendly, non-toxic, and the treatment and size of the product, the magnetite NPs, can be controlled in a single-vessel reaction at mild conditions (CitationAwwad and Salem 2012).
Precipitation from solution
One of the oldest techniques for the preparation of NPs is the precipitation of products from solutions. In precipitation reactions, the metal precursors are dissolved in an ordinary solvent, such as water, and a precipitating agent is added to generate an insoluble solid. The main advantage of precipitation reactions is that large quantities of particles can be obtained (CitationWillard et al. 2004). Uniform particles are usually synthesized by a homogeneous precipitation reaction, a process that includes the separation of the nucleation and growth of the nuclei (CitationIndira and Lakshmi 2010, CitationSugimoto 2000).
Co-precipitation
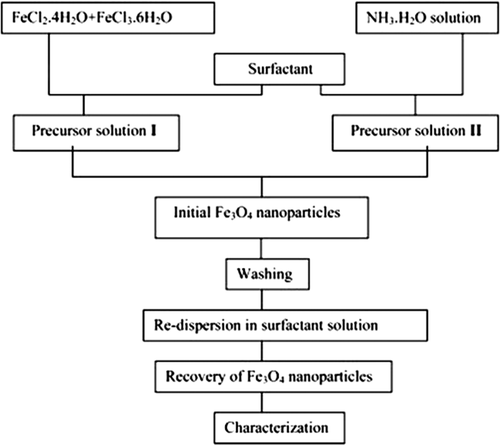
Co-precipitation is the most widely used and most proper method for the synthesis of MNPs of controlled sizes and magnetic properties (CitationSandeep Kumar 2013). It is extensively used for biomedical applications, because of the ease of application and less need for harmful materials and procedures (CitationIndira and Lakshmi 2010). In this method, MNPs are prepared from aqueous salt solutions, by the addition of a base under an inert atmosphere at room temperatures or at high temperature (CitationFaraji et al. 2010). The co-precipitation process is shown in the following , and the reaction is simply as follows (CitationIndira and Lakshmi 2010):
There are two main approaches for the synthesis spherical MNPs in solution: partially oxidizing ferrous hydroxide suspensions with different oxidizing agents (CitationSugimoto and Matijevic 1980), and aging stoichiometric mixtures of ferrous and ferric hydroxides in aqueous media, which yield spherical magnetite particles that are homogeneous in size (CitationIndira and Lakshmi 2010, CitationMassart and Cabuil 1987). The size and shape of the iron oxide NPs depend on the type of salts used, such as chlorides, sulfates, nitrates, perchlorates, etc., the ratio of ferric and ferrous ions, the PH value, the reaction temperature, the ionic strength of the media, and the other reaction parameters (such as stirring rate, and dropping speed of basic solution) (CitationWu et al. 2008).
The pH ranging between 8 and 14 is the expected range for complete precipitation with a stoichiometric ratio of 2/1 (Fe3+/F2+) in a non-oxidizing oxygen environment (CitationFaraji et al. 2010, CitationIida et al. 2007). In addition, it has been demonstrated that by adjusting the pH and the ionic strength of the precipitation medium, it is possible to control the mean size of the particles over one order of magnitude, in the range between 2 and 15 nm (CitationTartaj et al. 2003, CitationJolivet et al. 2000).
This method produces particles with extensive distribution of particle size, which sometimes requires secondary size selection. Kang et al. (CitationKang et al. 1996) reported a synthesis of uniform, monodisperse, Fe3O4 NPs with narrow size distribution, with the diameter of 8.5 ± 1.3 nm, by co-precipitation without surfactants; the reaction takes place in an aqueous solution with a molar ratio of Fe2+/Fe3+ equal to 0.5 and a pH of 11–12.The colloidal suspensions of the magnetite can be then directly oxidized by aeration to form colloidal suspensions of γ-Fe2O3 (CitationWu et al. 2008).
Iida et al. (CitationDavoudi et al. 2014) prepared Fe3O4 NPs by hydrolysis in an aqueous solution, including ferrous and ferric salts at various ratios, with 1,6-hexanediamine as the base. According to this study, when the ratio of Fe2+ to Fe3+ ions was increased, the formation of large hydroxide particles as precursors of Fe3O4 was promoted, which resulted in an increase in the size of Fe3O4 NPs from ∼9 to ∼37 nm. Furthermore, the saturation magnetization values of the samples prepared with both ferrous and ferric salts were 46.7 and 55.4 emu. g − 1 for sulfate and chloride, respectively. These results are proved by the data in literature (CitationFaraji et al. 2010, CitationBabes et al. 1999, CitationTronc et al. 1992).
The size of MNPs decreased with increasing pH value and ionic strength in the medium (CitationJolivet et al. 2000). Both parameters affect the chemical structure of the surface, and consequently, the electrostatic surface charge of the particles (CitationTartaj et al. 2003).
In the preparation of Fe3O4, precipitation at temperatures below 60°C produces an amorphous hydrated oxyhydroxide that can be simply converted to Fe2O3, while higher reaction temperatures (> 80°C) favor the formation of Fe3O4 (CitationFaraji et al. 2010, CitationZiolo et al. 1992, CitationGovan and Gun'ko 2014).
Nitrogen gases bubbling through the solution help to protect magnetite NPs against critical oxidation. Moreover, N2 reduces the particle size, in comparison with methods without oxygen removal (CitationFaraji et al. 2010, CitationGupta and Wells 2004, CitationKim et al. 2001). Hong et al. (CitationHou et al. 2005) used N2H4 .H2O as an oxidation-resistant reagent. According to their results, hydrazine can react with the dissolved oxygen to form [NH3OH] +, as in the following equation:
The cationic [NH3OH] + can also react with Fe2+ to form Fe3O4, as follows:
Fe3O4 MNPs with narrow size distribution can be synthesized by controlling the titration rate of ammonium hydroxide (CitationIndira and Lakshmi 2010, CitationZhao et al. 2008). The smallest particles can also be obtained after adding polyvinylalcohol (PVA) to the iron salts (CitationTartaj et al. 2003, CitationAlimirzalu et al. 2014).
The trouble with the synthesis of Fe3O4 MNPs by chemical co-precipitation is the tendency of the particles to agglomerate because of extremely small particle size, leading to greater specific surface area and high surface energy; we must also consider the influence of alkali, emulsifier, and reaction temperature, which are the determining factors of the final product (CitationIndira and Lakshmi 2010, CitationZhao et al. 2008). Moreover, the drawback in the synthesis of these aqueous solutions is that the high pH value of the reaction mixture has to be adjusted in both the synthesis and purification steps, yielding only very limited success the formation of uniform and monodisperse NPs (CitationWu et al. 2008). The common microstructure of MNPs prepared by this method is shown in .
Microemulsion
Microemulsion is the thermodynamically stable isotropic dispersal of two immiscible water and oil phases in the presence of a surfactant. The surfactant molecules can form a monolayer at the interface between the oil and water, with the hydrophilic head groups in the aqueous phase and the hydrophobic tails of the surfactant molecules dissolved in the oil phase (CitationWu et al. 2008, CitationSolans et al. 2005).
This method has a series of advantages in comparison with other methods, namely, the use of simple equipment, the possibility of synthesizing a great variety of materials with a high degree of control over particle size and composition, the preparation of NPs with crystalline structure and high specific surface area, and the use of simple conditions of synthesis, and near ambient temperature and pressure (CitationWoo et al. 2004). Particles produced by the microemulsion method are smaller in size and are higher in saturation magnetization (CitationWu et al. 2008, CitationChin and Yaacob 2007).
The properties of NPs prepared by the microemulsion method depend on the type and structure of the surfactant (CitationSanchez-Dominguez et al. 2012). The surfactant is an amphiphilic molecule which lowers the interfacial tension between water and oil, resulting in the formation of a transparent solution (CitationFaraji et al. 2010). Quintela M. A. and Rivas J. (CitationLopez-Quintela and Rivas 1993) reported that magnetite NPs around 4 nm in diameter have been synthesized by the controlled hydrolysis of FeCl2 with ammonium hydroxide and FeCl3 aqueous solutions, within the inverse micelle nanocavities formed by using AOT (Sodium 2-ethylhexyl sulfosuccinate) as surfactant and heptane as the continuous oil phase (CitationTartaj et al. 2003). AOT-based systems are amongst the best characterized systems, and it has been found that the size of the inverse microemulsion droplets generated by this type of system increases linearly with the amount of water added to the system (CitationPileni 1998). It can increase from 4 nm to 18 nm with 0.1 M of sodium AOT surfactant (water/AOT/isooctane). The use of AOT-based systems is probably the best method for the synthesis of inorganic NPs in W/O microemulsions, for two reasons: good control of droplet size, and the large microemulsion regions found in the water/AOT/alkane systems, which give rise to a great deal of compositions available for NP preparation (CitationSanchez-Dominguez et al. 2012).
The nanodroplets of water containing reagents as nanoreactors, endure rapid coalescence allowing for mixing, a precipitation reaction, and an aggregation process, for the synthesis of MNPs (). By mixing two identical water-in-oil microemulsions consisting of the chosen reactants, the microdroplets will continuously collide, coalesce and break again, and finally a precipitate forms in the micelles (CitationFaraji et al. 2010). Pileni and co-workers (CitationJalil et al. 2014) synthesized MNPs with average sizes ranging from 4 to 12 nm, and standard deviation ranging from 0.2 to 0.3, using microemulsions. A ferrous dodecyl sulfate (Fe(DS)2)) micellar solution was used to generate nanosized magnetic particles whose size could be controlled by the concentration and temperature of the surfactant.
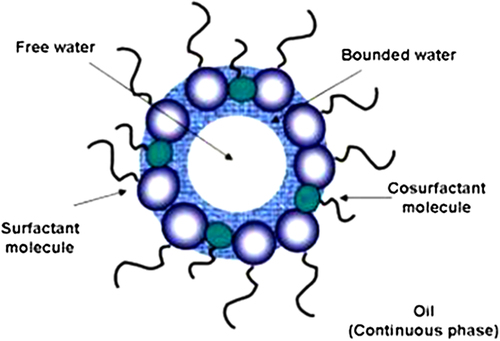
As in the binary systems (water/surfactant or oil/surfactant), self-assembled structures of various types can be generated, ranging, for example, from spherical and cylindrical micelles to lamellar phases and bicontinuous microemulsions, which can coexist with predominantly oil or aqueous phases (CitationSolans et al. 2005). In this sense, the use of microemulsions and inverse micelles are routes that can be used to achieve the shape- and size-controlled iron oxide NPs (CitationWu et al. 2008).
The sequential preparation obtainable with reverse micelles is employed to first prepare an iron core, by the reduction of ferrous sulfate by sodium borohydride. After the reaction is completed, the micelles within the reaction mixture are expanded to accommodate the shell, using a larger micelle including additional sodium borohydride (CitationTartaj et al. 2003).
Vidal-Vidal et al. (CitationVidal-Vidal et al. 2006) have reported the preparation of monodisperse maghemite NPs by the one- vessel microemulsion method. The spherical-shaped particles, covered with a monolayer coating of oleylamine (or oleic acid), demonstrate a narrow size distribution of 3.5 ± 0.6 nm, are well crystallized, and have high saturation magnetization values (76.3 Am2/kg for uncoated NPs, 35.2 Am2/kg for oleic acid-coated NPs, and 33.2 Am2/kg for oleylamine-coated NPs) (CitationWu et al. 2008). The oil and water phases often comprise several dissolved components, and consequently, the selection of the surfactant depends upon the physicochemical characteristics of the system. The challenges in their scale-up procedures, and the adverse effects of the remaining surfactants on the properties of the particles, are the main disadvantages of the microemulsion method (CitationHasany et al. 2012). A common microstructure of MNPs prepared by the microemulsion method is shown in .

Polyol method
A very promising method for the synthesis of uniform NPs that could be used in biomedical applications such as magnetic resonance imaging, is the polyol technique. Fine metallic particles can be produced by reducing dissolved metallic salts and directly precipitating metals from a solution including a polyol (CitationTartaj et al. 2003, CitationSugimoto 2000, CitationNejati-Koshki et al. 2014, CitationMatijevic 1993).
Fe3O4 MNPs have been synthesized by the co-precipitation method, combining a surface decoration process and the polyol process, and the factors affecting the adsorption of metal ions, such as pH, temperature, amount of adsorbent, and contact time, have been reported (CitationIndira and Lakshmi 2010, CitationShen et al. 2009). The polyol method has also been a useful preparative technique for the synthesis of nanocrystalline alloys and bimetallic clusters (CitationWillard et al. 2004). In the polyol method, the liquid polyol acts as a solvent for the metallic precursor, a reducing agent, and in some cases, as a complexing agent for the metallic cations. The solution is stirred and heated to a certain temperature, reaching the boiling point of the polyol, for less reducible metals. A better control of the mean size of the metal particles can be achieved by seeding the reactive medium with foreign particles (heterogeneous nucleation). In this way, the steps of nucleation and growth can be entirely separated, and uniform particles prepared (CitationTartaj et al. 2003).
By this method, precursor compounds such as oxides, acetates, and nitrates, are either dissolved or suspended in a diol, such as ethylene glycol or diethylene glycol. The reaction mixture is then heated to reflux between 180°C and 199°C. During the reaction, the metal precursors become solubilized in the diol and form an intermediate, and are then reduced to form metal nuclei, which form metal particles. Submicrometer-sized particles can be obtained by increasing the reaction temperature or inducing heterogeneous nucleation, by adding or forming foreign nuclei in situ. This method was also used to prepare nano-crystalline powders, such as Fe, Co, Ni, Cu, Ru, Rh, Pd, Ag, Sn, Re, W, Pt, Au, (Fe,Cu), (Co,Cu), (Co,Ni), and (Ni,Cu), using different salt precursors. (CitationChow et al. 1995, CitationEbrahimi et al. 2014, CitationViau et al. 2001, CitationGiri et al. 2000, CitationSaravanan et al. 2001, CitationToneguzzo et al. 2000, CitationPoul et al. 2000).
Iron particles with a size of around 100 nm can be obtained by disproportionation of ferrous hydroxide in organic media (CitationGhalhar et al. 2014). Fe(II) chloride and sodium hydroxide react with ethylene glycol (EG) or polyethylene glycol (PEG), and the precipitation occurs at a temperature ranging between 80 ˚C–100 ˚C. Additionally, iron alloys can be synthesized by co-precipitation of Fe, Ni, and/or Co, in EG and PEG. Monodisperse, quasi-spherical, and non-agglomerated metallic particles, with an average size of around 100 nm, have been prepared without seeding (homogeneous nucleation), while particles of a size between 50 and 100 nm have been prepared using Pt as the nucleating agent (heterogeneous nucleation) (CitationTartaj et al. 2003, CitationViau et al. 1996).
Oxides can be prepared by modifying the polyol method with the addition of water, to act more like a sol-gel reaction (forced hydrolysis) (CitationJungk and Feldmann 2000, CitationFeldmann and Jungk 2001, CitationFeldmann 2001, CitationTabatabaei Mirakabad et al. 2014). For example, 6 nm of CoFe2O4 was synthesized by the reaction of ferric chloride and cobalt acetate in 1,2-propanediol, with the addition of water and sodium acetate (CitationWillard et al. 2004, CitationRajamathi et al. 2002). A common structure of MNPs prepared by this method is shown in .
Compared to aqueous methods, the polyol method was found to result in the synthesis of metallic NPs protected by surface-adsorbed glycol, thus minimizing the oxidation. The use of a non-aqueous solvent such as polyol also reduced the problem of hydrolysis of fine metal particles, a phenomenon that often occurs in the aqueous situation (CitationWillard et al. 2004).
Thermal decomposition of organic precursors
The decomposition of iron precursors in the presence of hot organic surfactants has yielded improved samples with good size control, narrow size distribution, good crystallinity of individual and dispersible magnetic iron oxide NPs (CitationIndira and Lakshmi 2010).
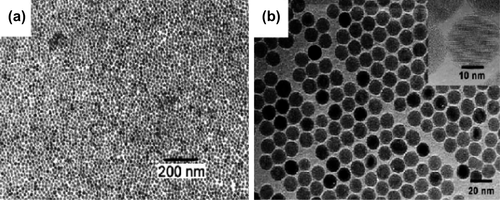
Nanoparticles with a high level of monodispersity and size control can be achieved by high-temperature decomposition of organometallic precursors, such as [Mn+(acac)n], (M = Fe, Mn, Co, Ni, Cr; n = 2 or 3, acac = acetylacetonate), Mx (cup)x (cup = N-nitrosophenyl hydroxylamine) or carbonyls (such as Fe(CO)5), using organic solvents and surfactants such as fatty acids, oleic acid, and hexadecylamine (CitationFaraji et al. 2010). Alivisatos and co-workers (CitationRockenberger et al. 1999) have demonstrated that injecting solutions of FeCup3 in octylamine into long-chain amines at 250˚C –300˚C resulted in the synthesis of nanocrystals of maghemite. These nanocrystals are crystalline, and are dispersible in organic solvents, and their sizes range from 4 to 10 nm in diameter () (CitationTartaj et al. 2003).
Biomedical applications like MRI are extremely dependent on particle size, and thus MNP synthesis by this method could be potentially used for these applications (CitationTartaj et al. 2003). The temperature and time of the reaction, as well as the aging period, may also be vital for the precise control of size and morphology (CitationLu et al. 2007). The prepared NPs annealed at 300°C, 700°C and 900°C, and the annealing temperature allowed the control of size and size distribution of the particles, as well as their structure and magnetic properties (CitationIndira and Lakshmi 2010, CitationAmara et al. 2009).
It has been reported that monodisperse magnetite NPs with sizes from 3 to 20 nm, could be prepared by the high-temperature (265˚C) reaction of iron (III) acetylacetonate in phenyl ether in the presence of alcohol, oleic acid, and oleylamine. Success in accurate control of particle size of Fe3O4 NPs has only been achieved through thermal decomposition, using large quantities of toxic and expensive precursors and surfactants in organic solvent. Thermal decomposition of organometallic precursors, in which the metal is zero-valent in the composition (such as Fe(CO)5), initially leads to a generation of metal NPs, but if followed by oxidation, can lead to high quality monodisperse metal oxides. In contrast, the decomposition of precursors with cationic metal centers (such as Fe(acac)3) leads directly to metal oxides NPs (CitationLu et al. 2007).
Laborious purification steps are essential before the end product can be used in biomedical applications (CitationEatemadi et al. 2014). The other disadvantage of this method is the production of organic-soluble NPs, which limit the range of applications for their use in biological fields. In addition, surface treatment is needed after synthesis (CitationFaraji et al. 2010); moreover, the resulting NPs are generally only dissolved in nonpolar solvents (CitationWu et al. 2008).
Hydrothermal method
The hydrothermal method, also called the solvothermal method, is a preparation method for the synthesis of MNPs and ultrafine powders, as described in literature (CitationButter et al. 2005, CitationMao et al. 2006, CitationZhu et al. 2007, CitationFekri Aval et al. 2014, CitationGozuak et al. 2009, CitationWang et al. 2009). This technique is one of the most successful ways to grow crystals of many different materials (CitationFaraji et al. 2010). As an alternative, the hydrothermal method contains various wet-chemical technologies of crystallizing material in a sealed container, from aqueous solution at the high temperature range of 130°C to 250°C, and at high vapor pressure, generally in the range of 0.3 to 4 MPa (CitationWu et al. 2008).
However, despite several studies to find proper ligands to synthesize monodisperse nanocrystals in a hydrophilic environment (CitationXu and Wang 2012), hydrothermal approaches still fail to obtain quality nanocrystals smaller than 10 nm with hydrophilic surface properties (CitationStojanovic et al. 2013). For example, Zheng et al. (CitationZheng et al. 2006) have prepared Fe3O4 NPs with a diameter of 27 nm using the hydrothermal method in the presence of a surfactant, sodium bis (2-ethylhexyl) sulfosuccinate. Wang et al. (CitationZohre et al. 2014) reported that the nanoscale Fe3O4 powder with a diameter of 40 nm, can be prepared by using the hydrothermal method at 140°C for 6 hours, having a saturation magnetization of 85.8 emu. g− 1, which is lower than that of the corresponding bulk Fe3O4 (92 emu. g− 1) (CitationWunderbaldinger et al. 2002).
The particle size and size distribution increased with precursor concentration. However, the residence time had a more significant impact on the average particle size than the feed concentration. Monodisperse particles were produced at short residence times (CitationXu et al. 2008, CitationOsuna et al. 1996). The study of changing the precursor (ferric nitrate) concentration from 0.03 to 0.06 M, when all other variables were kept constant, showed that at the precursor concentration of 0.03 M, spherical particles with an average particle radius of 15.6 ± 4.0 nm were obtained (CitationHasany et al. 2012). One of the main problems of the conventional hydrothermal method is the slow reaction kinetics at any given temperature. Using microwave heating can increase the kinetics of crystallization during the hydrothermal synthesis (CitationFaraji et al. 2010, CitationKomarneni and Katsuki 2002).
Chemical vapor deposition (CVD)
In vapor-phase preparation of NPs, conditions are created where the vapor phase mixture is thermodynamically unstable relative to generation of the solid material to be prepared in nanoparticulate form. This method has wonderful flexibility in producing a wide range of materials, and can take advantage of the enormous database of precursor chemistries that have been developed for CVD processes. The precursors can be solid, liquid, or gas at ambient conditions, but are delivered to the reactor as a vapor (CitationSwihart 2003). Wegner et al. (CitationWegner et al. 2002) presented a detailed, systematic modeling and experimental study of this method, as applied to the synthesis of bismuth NPs. They reported that they could control the particle size distribution by controlling the flow field and the mixing of the cold gas with the hot gas carrying the evaporated metal.
Gas phase methods for preparing nanomaterials depend on thermal decomposition (pyrolysis), reduction, hydrolysis, disproportionation, oxidation, or other reactions, to cause precipitation of solid products from the gas phase (CitationFaraji et al. 2010, CitationPierson 1999). Spray and laser pyrolysis have been shown to be excellent techniques for the direct and continuous production of well-defined MNPs (CitationIndira and Lakshmi 2010).
Spray pyrolysis
Spray pyrolysis is a method in which a solid is prepared by spraying a solution into a series of reactors where the aerosol droplets endure evaporation of the solvent, with condensation of the solute within the droplet, followed by drying and thermolysis of the precipitated particle at a higher temperature (CitationMessing et al. 1993). Recently, this method has been used to synthesize colloidal aggregates of superparamagnetic maghemite NPs in the form of hollow or dense spheres, with the possibility of having a surface enriched in silica (CitationTartaj et al. 2003, CitationTartaj et al. 2001, CitationTartaj and Serna 2002, CitationTartaj et al. 2004). Their high rate of production can indicate a promising future for the synthesis of MNPs useful in in vivo and in vitro applications (CitationTartaj et al. 2003). Most of the pyrolysis-based processes used to generate maghemite NPs start with an Fe3+ salt and some organic compound that acts as the reducing agent. In this procedure, Fe3+ is partially reduced to a mixture of Fe2+ and Fe3+ by organic compounds, with the formation of magnetite, which is finally oxidized to maghemite (CitationPecharroman et al. 1995). In alcoholic solutions, uniform γ-Fe2O3 particles can be obtained with an extensive variety of particle morphologies and sizes in the range from 5 to 60 nm, depending on the nature of the iron precursor salt (CitationGonzalez-Carreno et al. 1993). Dense aggregates with a spherical shape composed of γ-Fe2O3 subunits, with an average diameter of 6 and 60 nm, have been generated using Fe(III) nitrate and Fe(III) chloride solutions, respectively (CitationTartaj et al. 2003).
Laser pyrolysis
An alternate means of heating the precursors to persuade reaction and homogeneous nucleation is the absorption of laser energy. This method allows highly localized heating and rapid cooling in comparison with heating the gases in a furnace.
The laser pyrolysis method includes heating a flowing mixture of gases with a continuous wave CO2 laser, which initiates and sustains a chemical reaction (CitationVeintemillas-Verdaguer et al. 2002). Biocompatible magnetic dispersions have been synthesized from γ-Fe2O3 NPs (5 nm) by continuous laser pyrolysis of Fe(CO)5 vapors (CitationVeintemillas-Verdaguer et al. 2004). Nanoparticles of many materials have been obtained by this method, such as the synthesis of Si NPs synthesized by Ledoux et al. (CitationLedoux et al. 2002, CitationLedoux et al. 2002). They used a pulsed CO2 laser, thereby shortening the reaction time and allowing the synthesis of even smaller particles (CitationSwihart 2003).
Sonochemical reactions
The sonochemical method has been widely used as a competitive alternative to synthesize novel materials with unusual properties (CitationWu et al. 2008, CitationFaraji et al. 2010). The chemical effects of ultrasound arise from acoustic cavitation, that is, the generation, growth, and implosive collapse of bubbles in liquid. The conditions designed in the hotspots which are formed by the implosive collapse of the bubble, show temporary temperatures of 5000 K, pressures of 1800 atm, and cooling rates in excess of 1010 K/s(CitationWu et al. 2008, CitationSuslick 1990). illustrates the common steps of iron oxide synthesis by the sonolysis method.
Commonly, volatile precursors in solvents of low vapor pressure are used to improve the particle yield. Acoustic irradiation is carried out with an ultrasound probe, such as a titanium horn, operating at 20 kHz (CitationWillard et al. 2004). This method has been applied for the synthesis of several nanocomposites, and its versatility has been successfully confirmed in iron oxide NP synthesis (CitationBang and Suslick 2007). Vijayakumar et al. (CitationVijayakumar et al. 2000) reported a sonochemical synthetic route for producing pure nanometer-sized Fe3O4 powder with a particle size of 10 nm. The Fe3O4 NPs obtained are superparamagnetic, and their magnetization is very low (< 1.25 emu g− 1) at room temperature (CitationRoger et al. 1999). The powders prepared by this method are usually porous, amorphous, and agglomerated. For example, an amorphous iron powder was synthesized by the sonication of iron carbonyl in decalin (CitationSuslick et al. 1991, CitationSuslick et al. 1996, CitationSuslick et al. 1996), producing a powder with a surface area of 120 m2. g− 1. Annealing this powder at 350°C under nitrogen resulted in α-Fe with a diameter of 50 nm (CitationWillard et al. 2004). The addition of stabilizers or polymers added during or after sonication, resulted in metal colloids (CitationSuslick et al. 1996, CitationSuslick et al. 1995, CitationKataby et al. 1998).
Kim et al. (CitationHee Kim et al. 2005) prepared Fe3O4 NPs using the sonochemical and co-precipitation methods. The crystallinity and magnetic properties of the products generated using these two methods were compared, and the results achieved indicated that the Fe3O4 NPs prepared by the sonochemical method had a higher crystallinity and saturation magnetization than those achieved in the NPs prepared by the co-precipitation method ().
Sol-gel reaction
The sol-gel method is a proper wet route for the preparation of nanostructured metal oxides. This method is based on the hydroxylation and condensation of molecular precursors in solution, initiating a “sol” of nanometric particles. Additional condensation and inorganic polymerization lead to a three-dimensional metal oxide network, denominated as wet gel. Extra heat treatments are needed to obtain the final crystalline state (CitationLaurent et al. 2008, CitationSafarik et al. 2011), because these reactions are performed at room temperature.
The sol-gel process includes hydrolysis and condensation of metal alkoxides. Metal alkoxides are good precursors, due to their endurance in the face of hydrolysis, i.e. the hydrolysis step replaces an alkoxide with a hydroxide group from water and a free alcohol is generated. Factors that need to be considered in a sol-gel method are the solvent type, temperature, precursors, catalysts, pH, additives and mechanical agitation. These factors can affect the kinetics, growth, and hydrolysis and condensation reactions (CitationWillard et al. 2004, CitationScherrer and Brinker 1990). For metal oxides, the sol-gel process offers some advantages compared to other methods, including good homogeneity, low cost, and high purity. Moreover, the sol-gel method has been developed for the synthesis of magnetite NPs using metallo-organic precursors (CitationShaker et al. 2013). Shakeel Akbar et al. (CitationAkbar et al. 2004) have successfully synthesized NPs, primarily of α-Fe2O3, by the sol-gel process, and studied their magnetic characterization. They reported that by using a modified sol-gel method, they achieved best results for obtaining alpha phase particles in two conditions. Particles were synthesized at a citric acid concentration of 0.2 M and 0.1 M of iron nitrate, with aging of the dry precursors (gel) at 90°C for about 16 hours in an open atmosphere. For samples annealed at high temperatures, pure alpha phase particles were achieved for an annealing temperature of 180°C, with a concentration of 0.1 M of both iron nitrate and citric acid. Sara Shaker et al. (CitationShaker et al. 2013) reported that magnetite NPs (Fe3O4) were successfully prepared the via sol-gel technique combined with annealing using inexpensive, nontoxic ferric nitrate and ethylene glycol, at temperatures of 200°C, 300°C, and 400°C. The characterization results indicated that the size of Fe3O4 NPs could be changed by changing the annealing temperature.
The sol-gel method has some advantages. In this method, it is possible to prepare pure amorphous phases, with monodispersity and good control of the particle size, and also to obtain materials with a predetermined structure based on experimental conditions. Moreover, the microstructure and the homogeneity of the reaction products are controllable. The sol-gel method includes pollution from by-products of reactions, as well as the need for post-treatment of the products. The disadvantage of this method is that it produces 3D oxide networks, and hence, it is limited in its efficiency (CitationHasany et al. 2012).
Conclusion
The investigation for novel routes of synthesis or the improvement of established ones which are able to fabricate MNPs with the proper characteristics of improved colloidal stability and biocompatibility, is continuously developing. Therefore, we focus mainly on routes of synthesis of magnetic nanoparticles for application in nanobiomedicine. We would like to modernize currently available methods for the preparation of Fe3O4 NPs with several morphologies; however, some major and new findings reported earlier are also involved.
Authors’ contributions
AA and MSG conceived of the study and participated in its design and coordination. SM, FZS, and SMF participated in the sequence alignment and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors thank the Department of Medical Nanotechnology, Faculty of Advanced Medical Science of Tabriz University for all support provided. This work is funded by the 2014 Drug Applied Research Center Tabriz University of Medical Sciences Grant.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abbasi E, Aval SF, Akbarzadeh A, Milani M, Nasrabadi HT, Hanifepour Y, et al. 2014. Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett 9 :247
- Abbasi E, Milani M, Fekri Aval S, Kouhi M, Akbarzadeh A, Tayefi Nasrabadi H. 2014. Silver nanoparticles: synthesis, properties, bio-applications and limitations. Crit Rev Microbiol 1.
- Ahmadi A, Shirazi H, Pourbagher N, Akbarzadeh A, Omidfar K. 2014. An electrochemical immunosensor for digoxin using core-shell gold coated magnetic nanoparticles as labels. Mol Biol Rep. 41 :1659–1668.
- Akbar S, Hasanain SK, Azmat N, Nadeem M. 2004. Synthesis of Fe2O3 nanoparticles by new Sol-Gel method and their structural and magnetic characterizations. arXiv preprint cond-mat/0408480.
- Akbarzadeh A, Samiei M, Davaran S. 2012. Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res Lett. 7:144.
- Alimirzalu S, Akbarzadeh A, Abbasian M, Alimohammadi S, Davaran S, Hanifehpour Y, et al. 2014. Synthesis and study of physicochemical characteristics of Fe3O4 magnetic nanocomposites based on poly(Nisopropylacrylamide)for anti-cancer drugs delivery. Asian Pac J Cancer Prev, 15 :049–054
- Amara D, Felner I, Nowik I, Margel S. 2009. Synthesis and characterization of Fe and Fe3O4 nanoparticles by thermal decomposition of triiron dodecacarbonyl. Colloids Surf A Physicochem Eng Aspects. 339:106–110.
- Ashokkumar M, Lee J, Kentish S, Grieser F. 2007. Bubbles in an acoustic field: an overview. Ultrason Sonochem. 14:470–475.
- Awwad AM, Salem NM. 2012. A green and facile approach for synthesis of magnetite nanoparticles. Nanosci Nanotechnol. 2:208–213.
- Babes L, Denizot B, Tanguy G, Le Jeune JJ, Jallet P. 1999. Synthesis of iron oxide nanoparticles used as MRI contrast agents: a parametric study. J Colloid Interface Sci. 212:474–482.
- Bang JH, Suslick KS. 2007. Sonochemical synthesis of nanosized hollow hematite. J Am Chem Soc. 129:2242–2243.
- Bao Y, Pakhomov AB, Krishnan KM. 2006. Brownian magnetic relaxation of water-based cobalt nanoparticle ferrofluids. J Appl Phys. 99:08H107.
- Barth S, Estrade S, Hernandez-Ramirez F, Peiro F, Arbiol J, Romano-Rodriguez A, et al. 2008. Studies on surface facets and chemical composition of vapor grown one-dimensional magnetite nanostructures. Crystal Growth and Design. 9:1077–1081.
- Berger P, Adelman NB, Beckman KJ, Campbell DJ, Ellis AB, Lisensky GC. 1999. Preparation and properties of an aqueous ferrofluid. J Chem Educ. 76:943.
- Bharde AA, Parikh RY, Baidakova M, Jouen S, Hannoyer B, Enoki T, et al. 2008. Bacteria-mediated precursor-dependent biosynthesis of superparamagnetic iron oxide and iron sulfide nanoparticles. Langmuir. 24:5787–5794.
- Bomati-Miguel O, Mazeina L, Navrotsky A, Veintemillas-Verdaguer S. 2008. Calorimetric study of maghemite nanoparticles synthesized by laser-induced pyrolysis. Chem Mater. 20:591–598.
- Butter K, Kassapidou K, Vroege GJ, Philipse AP. 2005. Preparation and properties of colloidal iron dispersions. J Colloid Interface Sci. 287:485–495.
- Cabrera L, Gutierrez S, Menendez N, Morales MP, Herrasti P. 2008. Magnetite nanoparticles: electrochemical synthesis and characterization. Electrochim Acta. 53:3436–3441.
- Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M. 2006. Synthesis of gold nanotriangles and silver nanoparticles using Aloevera plant extract. Biotechnol Progr. 22:577–583.
- Chin AB, Yaacob II. 2007. Synthesis and characterization of magnetic iron oxide nanoparticles via w/o microemulsion and Massart's procedure. J Mater Process Technol. 191:235–237.
- Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, et al. 2007. Renal clearance of quantum dots. Nat Biotechnol. 25: 1165–1170.
- Chow GM, Kurihara LK, Kemner KM, Schoen PE, Elam WT, Ervin A, Keller S, et al. 1995. Structural, morphological, and magnetic study of nanocrystalline cobalt-copper powders synthesized by the polyol process. J Mater Res. 10:1546–1554.
- Chung SH, Hoffmann A, Bader SD. 2004. Biological sensors based on Brownian relaxation of magnetic nanoparticles. Appl Phy Lett. 85:2971–2973.
- Davaran S, Akbarzadeh A, Nejati-Koshki K, Alimohammadi S, Ghamari MF, Soghrati MM, et al. 2013. In vitro studies of NIPAAM-MAA-VP copolymer-coated magnetic nanoparticles for controlled anticancer drug release. J Encapsul Adsorpt Sci, 3:108–115
- Davaran S, Entezami AA. 1996. Synthesis and hydrolysis of modified poly vinyl alcohols containing Ibuprofen pendent groups. Iran Polym J. 5:188–191.
- Davaran S, Rezaei A, Alimohammadi S, Ahmad Khandaghi A, Nejati-Koshki K, Nasrabadi HT, Akbarzadeh A. 2014. Synthesis and Physicochemical Characterization of Biodegradable star-shaped poly lactide-co-glycolide– β -cyclodextrin copolymer Nanoparticles Containing Albumin. Adv Nanopart, 3:14–22
- Davoudi Z, Akbarzadeh A, Rahmatiyamchi M, Movassaghpour AA, Alipour M, Nejati-Koshki K, et al. 2014. Molecular target therapy of AKT and NF-kB signaling pathways and multidrug resistance by specific cell penetrating inhibitor peptides in HL-60 cells. Asian Pac J Cancer Prev 15 :4353.
- Denizot B, Tanguy G, Hindre F, Rump E, Lejeune JJ, Jallet P. 1999. The preparation of magnetite nanoparicles for biomedical. J Colloid Interface Sci. 209:66.
- Eatemadi A, Daraee H, Zarghami N, Yar HM, Akbarzadeh A, Hanifehpour Y. 2014. Nanofiber: synthesis and biomedical applications. Artif Cells Nanomed Biotechnol, 1–11.
- Ebrahimi E, Abbasi E, Akbarzadeh A, Khandaghi AA, Davaran S. 2014. Novel drug delivery system based on doxorubicin-encapsulated magnetic nanoparticles modified with PLGA-PEG1000 copolymer. Artif Cells Nanomed Biotechnol, 1–8
- El Ghandoor H, Zidan HM, Khalil MMH, Ismail MIM. 2012. Synthesis and some physical properties of magnetite (Fe 3 O 4) nanoparticles. Int J Electrochem Sci 7:5734–5745.
- Faraji M, Yamini Y, Rezaee M. 2010. Magnetic nanoparticles: synthesis, stabilization, functionalization, characterization, and applications. J Iran Chem Soc. 7:1–37.
- Fekri Aval S, Akbarzadeh A, Yamchi MR, Zarghami F, Nejati-Koshki K, Zarghami N. 2014. Gene silencing effect of SiRNA-magnetic modified with biodegradable copolymer nanoparticles on hTERT gene expression in lung cancer cell line. Artif Cells Nanomed Biotechnol, 1–6
- Feldmann C, Jungk H. 2001. Polyol-mediated preparation of nanoscale oxide particles. Angew Chem Int Ed Engl. 40:359–362.
- Feldmann C. 2001. Preparation of nanoscale pigment particles. Adv Mater. 13:1301–1303.
- Forough M, Fahadi K. 2011. Biological and green synthesis of silver nanoparticles. Turkish J Eng Environ Sci. 34:281–287.
- Geng J, Lu D, Zhu JJ, Chen HY. 2006. Antimony(III)-doped PbWO4 crystals with enhanced photoluminescence via a shape-controlled sonochemical route. J Phys Chem. 110:13777–13785.
- Ghalhar MG, Akbarzadeh A, Rahmati M, Mellatyar H, Dariushnejad H, Zarghami N, Barkhordari A. 2014. Comparison of inhibitory effects of 17-AAG nanoparticles and free 17-AAG on HSP90 gene expression in breast cancer. Asian Pac J Cancer Prev 15 :7113
- Ghasemali S, Nejati-Koshki K, Akbarzadeh A, Tafsiri E, Zarghami N, Rahmati-Yamchi M, et al. 2013. Study of Inhibitory Effect of β-Cyclodextrin-HelenalinComplex on HTERT Gene Expression in T47D Breast Cancer Cell Line by Real TimeQuantitative PCR (q-PCR), Asian Pac J Cancer Prev, 14 :6949–6953
- Giri AK, Chowdary KM, Majetich S. 2000. AC magnetic properties of compacted FeCo nanocomposites. Mater Phys Mech (Russia). 1:1–10.
- Gong J, Li S, Zhang D, Zhang X, Liu C, Tong Z. 2010. High quality self-assembly magnetite (Fe3O4) chain-like core-shell nanowires with luminescence synthesized by a facile one-pot hydrothermal process. Chem Commun. 46:3514–3516.
- Gonzalez-Carreno T, Morales MP, Gracia M, Serna CJ. 1993. Preparation of uniform γ-Fe2O3 particles with nanometer size by spray pyrolysis. Mater Lett. 18:151–155.
- Govan J, Gun'ko YK. 2014. Recent Advances in the Application of Magnetic Nanoparticles as a Support for Homogeneous Catalysts. Nanomaterials. 4:222–241.
- Gozuak F, Y Koseoglu A Baykal H Kavas. 2009. Synthesis and characterization of CoxZn1 − xFe2O4 magnetic nanoparticles via a PEG-assisted route. J Magn Magn Mater. 321:2170–2177.
- Grossman HL, Myers WR, Vreeland VJ, Bruehl R, Alper MD, Bertozzi CR, Clarke J. 2004. Detection of bacteria in suspension by using a superconducting quantum interference device. Proc Natl Acad Sci. 101:129–134.
- Gu H, Xu K, Xu C, Xu B. 2006. Biofunctional magnetic nanoparticles for protein separation and pathogen detection. Chem Commun, :941–949.
- Gupta AK, Gupta M. 2005. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 26:3995–4021.
- Gupta AK, Wells S. 2004. Surface-modified superparamagnetic nanoparticles for drug delivery: preparation, characterization, and cytotoxicity studies. IEEE Trans Nanobioscience. 3:66–73.
- Hasany SF, Ahmed I, Rajan J, Rehman A. 2012. Systematic review of the preparation techniques of iron oxide magnetic nanoparticles. Nanosci Nanotechnol. 2:148–158.
- Hee Kim E, Lee HS, Kwak BK, Kim BK. 2005. Synthesis of ferrofluid with magnetic nanoparticles by sonochemical method for MRI contrast agent. J Magn Magn Mater. 289:328–330.
- Honig JM, Spalek J. 1998. Electronic Properties of NiS2-x Se x Single Crystals: From Magnetic Mott-Hubbard Insulators to Normal Metals. Chem Mater. 10:2910–2929.
- Hosseininasab S, Pashaei-Asl R, Khandaghi AA, Nasrabadi HT, Nejati-Koshki K, Akbarzadeh A, et al. 2014. Synthesis, characterization, and In vitro studies of PLGA-PEG nanoparticles for oral Insulin delivery. Chem Biol Drug Des.84 307–315.
- Hou Y, Kondoh H, Shimojo M, Sako EO, Ozaki N, Kogure T, Ohta T. 2005. Inorganic nanocrystal self-assembly via the inclusion interaction of β-cyclodextrins: toward 3D spherical magnetite. J Phys Chem B. 109:4845–4852.
- Hou YL, Yu JF, Gao S. 2003. Solvothermal reduction synthesis and characterization of superparamagnetic magnetite nanoparticles. J Mater Chem. 13:1983–1987.
- Hu J, Lo I, Chen G. 2007. Performance and mechanism of chromate (VI) adsorption by γ-FeOOH-coated maghemite γ-Fe 2 O3) nanoparticles. Sep Purif Technol. 58:76–82.
- Hyeon T, Lee SS, Park J, Chung Y, Na HB. 2001. Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection process. J Am Chem Soc. 123:12798–12801.
- Iida H, Takayanagi K, Nakanishi T, Osaka T. 2007. Synthesis of Fe3O4 nanoparticles with various sizes and magnetic properties by controlled hydrolysis. J Colloid Interface Sci. 314:274–280.
- Indira TK, Lakshmi PK. 2010. Magnetic nanoparticles - A review. Int J Pharm Sci Nanotechnol. 3:1035–1042.
- Ito A, Shinkai M, Honda H, Kobayashi T. 2005. Medical application of functionalized magnetic nanoparticles. J Biosci Bioeng. 100:1–11.
- Jalil KY, Majid M, Abolfazl A, Soodabeh D, Ali Reza OR, Hamed H, et al. 2014. Preparation of pH sensitive insulin-loaded Nano hydrogels and evaluation of insulin releasing in different pH conditions. Mol Biol Rep 41 :6705–6712
- Jolivet J-P, Henry M, Livage J. 2000. Metal oxide chemistry and synthesis: from solution to solid state. New York: Wiley-Blackwell.
- Jungk HO, Feldmann C. 2000. Nonagglomerated, submicron α-Fe2O3 particles: Preparation and application. J Mater Res. 15:2244–2248.
- Kang YS, Risbud S, Rabolt JF, Stroeve P. 1996. Synthesis and characterization of nanometer-size Fe3O4 and γ-Fe2O3 particles. Chem Mater. 8:2209–2211.
- Kashevsky BE, Agabekov VE, Kashevsky SB, Kekalo KA, Manina EY, Prokhorov IV, Ulashchik VS. 2008. Study of cobalt ferrite nanosuspensions for low-frequency ferromagnetic hyperthermia. Particuology. 6:322–333.
- Kataby G, Koltypin Yu, Rothe J, Hormes J, Felner I, Cao X, Gedanke A. 1998. The adsorption of monolayer coatings on iron nanoparticles: Mossbauer spectroscopy and XANES results. Thin Solid Films. 333:41–49.
- Kidwai M, Bhatnagar D, Mishra NK. 2010. Polyethylene glycol (PEG) mediated green synthesis of 2, 5-disubstituted 1, 3, 4-oxadiazoles catalyzed by ceric ammonium nitrate (CAN). Green Chem Lett Rev. 3:55–59.
- Kim DK, Zhang Y, Voit W, Rao KV, Muhammed M. 2001. Synthesis and characterization of surfactant-coated superparamagnetic monodispersed iron oxide nanoparticles. J Magn Magn Mater. 225:30–36.
- Kim KS, Park J-K. 2005. Magnetic force-based multiplexed immunoassay using superparamagnetic nanoparticles in microfluidic channel. Lab Chip. 5:657–664.
- Klaus T, Joerger R, Olsson E, Granqvist CG. 1999. Silver-based crystalline nanoparticles, microbially fabricated. Proc Natl Acad Sci. 96:13611–13614.
- Komarneni S, Katsuki H. 2002. Nanophase materials by a novel microwave-hydrothermal process. Pure Appl Chem. 74:1537–1543.
- Kotitz R, Weitschies W, Trahms L, Brewer W, Semmler W. 1999. Determination of the binding reaction between avidin and biotin by relaxation measurements of magnetic nanoparticles. J Magn Magn Mater. 194:62–68.
- Kouhi M, Vahedi A, Akbarzadeh A, Hanifehpour Y, Joo SW. 2014. Investigation of quadratic electro-optic effects and electro absorption process in GaN/AlGaN spherical quantum dot. Nanoscale Res Lett 9:131–136
- Kwon G, M Naito M Yokoyama T Okano Y Sakurai K Kataoka. 1997. Block copolymer micelles for drug delivery: loading and release of doxorubicin. J Control Release. 48:195–201.
- Kwon GS, Naito M, Yokoyama M, Okano T, Sakurai Y, Kataoka K. 1995. Physical entrapment of adriamycin in AB block copolymer micelles. Pharm Res. 12:192–195.
- LaConte L, Nitin N, Bao G. 2005. Magnetic nanoparticle probes. Mater Today. 8:32–38.
- Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller RN. 2008. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev. 108:2064–2110.
- Ledoux G, Amans D, Gong J, Huisken F, Cichos F, Martin J. 2002. Nanostructured films composed of silicon nanocrystals. Mater Sci Eng C. 19:215–218.
- Ledoux G, Gong J, Huisken F, Guillois O, Reynaud C. 2002. Photoluminescence of size-separated silicon nanocrystals: Confirmation of quantum confinement. Appl Phys Lett. 80:4834–4836.
- Li X, Liu D, Song S, Wang X, Gea X, Zhang H. 2011. Rhombic dodecahedral Fe 3 O 4: ionic liquid-modulated and microwave-assisted synthesis and their magnetic properties. CrystEngComm. 13:6017–6020.
- Liu X, Zhong Z, Tang Y, Liang B. 2013. Review on the Synthesis and Applications of Nanomaterials. J Nanomater.
- Liu Z, Zhang D, Han S, Li C, Lei B, Lu W, et al. 2005. Single crystalline magnetite nanotubes. J Am Chem Soc. 127:6–7.
- Lopez Perez JA, Lopez-Quintela MA, Mira J, Rivas J, Charles SW. 1997. Advances in the preparation of magnetic nanoparticles by the microemulsion method. J Phys Chem B. 101:8045–8047.
- Lopez-Quintela MA, Rivas J. 1993. Chemical reactions in microemulsions: a powerful method to obtain ultrafine particles. J Colloid Interface Sci. 158:446–451.
- Lu A-H, Salabas EL, Schuth F. 2007. Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed Engl. 46:1222–1244.
- Lukman AI, Gong B, Marjo CE, Roessner U, Harris AT. 2011. Facile synthesis, stabilization, and anti-bacterial performance of discrete Ag nanoparticles using Medicago sativa seed exudates. J Colloid Interface Sci. 353:433–444.
- Mahdavi M, Ahmad MB, Haron MJ, Namvar F, Nadi B, Rahman MZ, Amin J. 2013. Synthesis, Surface Modification and Characterisation of Biocompatible Magnetic Iron Oxide Nanoparticles for Biomedical Applications. Molecules 18:7533–7548.
- Mahdavi M, Namvar F, Ahmad MB, Mohamad R. 2013. Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules. 18:5954–5964.
- Mao B, Z Kang E Wang S Lian L Gao C Tian C Wang. 2006. Synthesis of magnetite octahedrons from iron powders through a mild hydrothermal method. Mater Res Bull. 41:2226–2231.
- Massart R, Cabuil V. 1987. Effect of some parameters on the formation of colloidal magnetite in alkaline-medium-yield and particle-size control. J Chim Phy PhysicoChim Biol. 84:967–973.
- Matijevic E. 1993. Preparation and properties of uniform size colloids. Chem Mater. 5:412–426.
- Meng X, Haibo L, Jingyan C, Liu M, Keqiang W, Xiao L. 2009. Mossbauer study of cobalt ferrite nanocrystals substituted with rare-earth Y3 + ions. J Magn Magn Mater. 321:1155–1158.
- Messing GL, Zhang SC, Jayanthi GV. 1993. Ceramic powder synthesis by spray pyrolysis. J Am Ceram Soc. 76:2707–2726.
- Moghimi SM, Hunter AC, Murray JC. 2001. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 53:283–318.
- Moulik SP, Paul BK. 1998. Structure, dynamics and transport properties of microemulsions. Adv Colloid Interface Sci. 78:99–195.
- Muldoon LL, Sàndor M, Pinkston KE, Neuwelt EA. 2005. Imaging, distribution, and toxicity of superparamagnetic iron oxide magnetic resonance nanoparticles in the rat brain and intracerebral tumor. Neurosurgery. 57:785–796.
- Murray CB, Norris DJ, Bawendi MG. 1993. Synthesis and characterization of nearly monodisperse Cde (E = S, Se, Te) semiconductor nanocrystallites. J Am Chem Soc. 115:8706–8715.
- Nair B, Pradeep T. 2002. Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Crystal Growth Des. 2:293–298.
- Nejati-Koshki K, Mesgari M, Ebrahimi E, Abhari A, Aval SF, Khandaghi AA, Akbarzadeh A. 2014. Synthesis and In-vitro study of Cisplatin-loaded Fe3O4 Nanoparticles Modified with PLGA-PEG6000 Copolymers in Treatment of Lung Cancer. J Microencapsul. 1–9
- Neveu S, Bee A, Robineau M, Talbot D. 2002. Size-selective chemical synthesis of tartrate stabilized cobalt ferrite ionic magnetic fluid. J Colloid Interface Sci. 255:293–298.
- Niemeyer CM. 2001. Nanoparticles, proteins, and nucleic acids: biotechnology meets materials science. Angew Chem Int Ed Engl. 40:4128–4158.
- Osuna J, de Caro D, Amiens C, Chaudret B. 1996. Synthesis, characterization, and magnetic properties of cobalt nanoparticles from an organometallic precursor. J Phys Chem. 100:14571–14574.
- Padil VVT, Cernik M. 2013. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int J Nanomed. 8:889.
- Pandey S, Oza G, Mewada A, Sharon M. 2012. Green synthesis of highly stable gold nanoparticles using Momordica charantia as nano fabricator. Arch Appl Sci Res. 4:1135–1141.
- Pankhurst QA, Connolly J, Jones SK, Dobson J. 2003. Applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys. 36:R167.
- Park J, An K, Hwang Y, Park JG, Noh HJ, Kim JY, et al. 2004. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat Mater. 3:891–895.
- Park J, Lee E, Hwang NM, Kang M, Kim SC, Hwang Y, et al. 2005. One-nanometer-scale size-controlled synthesis of monodisperse magnetic Iron oxide nanoparticles. Angew Chem Int Ed Engl. 117:2932–2937.
- Park S-J, Kim S, Lee S, Khim ZG, Char K, Hyeon T. 2000. Synthesis and magnetic studies of uniform iron nanorods and nanospheres. J Am Chem Soc. 122:8581–8582.
- Pascal C, Pascal JL, Favier F, Elidrissi Moubtassim ML, Payen C. 1999. Electrochemical synthesis for the control of γ-Fe2O3 nanoparticle size. Morphology, microstructure, and magnetic behavior. Chem Mater. 11:141–147.
- Pecharroman C, Gonzalez-Carreno T, Iglesias JE. 1995. The infrared dielectric properties of maghemite.γ-Fe2O3, from reflectance measurement on pressed powders. Phys Chem Miner, . 22:21–29.
- Peng XG, Manna L, Yang W, Wickham J, Scher E, Kadavanich A, Alivisatos AP. 2000. Shape control of CdSe nanocrystals. Nature. 404:59–61.
- Pierson HO. 1999. Handbook of Chemical Vapor Deposition: Principles, Technology and Applications. New York: William Andrew.
- Pileni MP. 1998. Fabrication and properties of nanosized material made by using colloidal assemblies as templates. Crys Res Technol. 33:1155–1186.
- Portet D, Denizot B, Rump E, Lejeune JJ, Jallet P. 2001. Nonpolymeric coatings of iron oxide colloids for biological use as magnetic resonance imaging contrast agents. J Colloid Interface Sci. 238:37–42.
- Poul L, Jouini N, Fievet F. 2000. Layered hydroxide metal acetates (metal = zinc, cobalt, and nickel): elaboration via hydrolysis in polyol medium and comparative study. Chem Mater. 12:3123–3132.
- Pourhassan-Moghaddam M, Rahmati-Yamchi M, Akbarzadeh A, Daraee H, Nejati-Koshki K, Hanifehpour Y, Joo SW. 2013. Protein detection through different platforms of immuno-loop-mediated isothermal amplification. Nanoscale Res Lett 8:485
- Pourhassan-Moghaddam M, Zarghami N, Mohsenifar A, Rahmati-Yamchi M, Gholizadeh D, Akbarzadeh A, et al. 2014. Watercress-based gold nanoparticles: biosynthesis, mechanism of formation and study of their biocompatibility in vitro. IET Digital Library, 4:5
- Puntes VF, Krishnan KM, Alivisatos AP. 2001. Colloidal nanocrystal shape and size control: the case of cobalt. Science. 291:2115–2117.
- Rajamathi M, Ghosh M, Seshadri R. 2002. Hydrolysis and amine-capping in a glycol solvent as a route to soluble maghemite α-Fe 2 O 3 nanoparticles. Chem Commun, :1152–1153.
- Rao CN, Vivekchand SR, Biswas K, Govindaraj A. 2007. Synthesis of inorganic nanomaterials. Dalton Trans, :3728–3749.
- Rockenberger JR, Scher EC, Alivisatos AP. 1999. A new nonhydrolytic single-precursor approach to surfactant-capped nanocrystals of transition metal oxides. J Am Chem Soc. 121:11595–11596.
- Roger J, et al. 1999. Some biomedical applications of ferrofluids. Eur Phys J Appl Phys. 5:321–325.
- Roh Y, Vali H, Phelps TJ, Moon JW. 2006. Extracellular synthesis of magnetite and metal-substituted magnetite nanoparticles. J Nanosci Nanotechnol. 6:3517–3520.
- Sadat Tabatabaei Mirakabad F, Akbarzadeh A, Zarghami N, Zeighamian V, Rahimzadeh A, Alimohammadi S. 2014. PLGA-Based Nanoparticles As Cancer Drug Delivery Systems, APJCP. Asian Pac J Cancer Prev.15 :517–535
- Safarik I, Horska K, Safarikova M. 2011. Magnetic Nanoparticles for Biomedicine. In Intracellular Delivery. Berlin: Springer. 363–372.
- Salam HA, Rajiv P, Kamaraj M, Jagadeeswaran P, Gunalan S, Sivaraj R. 2012. Plants: green route for nanoparticle synthesis. Int Res J Biol Sci. 1:85–90.
- Sanchez-Dominguez M, Aubery C, Solans C. 2012. New Trends on the Synthesis of Inorganic Nanoparticles Using Microemulsions as Confined Reaction Media.
- Sandeep Kumar V. 2013. Magnetic nanoparticles-based biomedical and bioanalytical applications. J Nanomed Nanotechol. 4:e130.
- Saravanan P, Jose TA, Thomas PJ, Kulkarni GU. 2001. Submicron particles of Co, Ni and Co-Ni alloys. Bulletin of Materials Science. 24:515–521.
- Scherrer GW, Brinker CJ. 1990. Sol-gel Science: the Physics and Chemistry of Sol-gel. Boston MA: Academic Press.
- Shaker S, Zafarian SH, Chakra SH, Rao KV. 2013. Preparation and characterization of magnetite nanoparticles by sol-gel method for water treatment. Int J Inn Res Sci Eng Technol. 2:2969–2973.
- Shameli K, Ahmad MB, Zamanian A, Sangpour P, Shabanzadeh P, Abdollahi Y, Zargar M. 2012. Green biosynthesis of silver nanoparticles using Curcuma longa tuber powder. Int J Nanomed. 7:5603.
- Shankar SS, Rai A, Ahmad A, Sastry M. 2004. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 275:496–502.
- Shen YF, Tang J, Nie ZH, Wang YD, Renc Y, Zuo L. 2009. Preparation and application of magnetic Fe3O4 nanoparticles for wastewater purification. Sep Purif Technol. 68:312–319.
- Shevchenko EV, Talapin D, Rogach A, Kornowski A, Haase M, Weller H. 2002. Colloidal synthesis and self-assembly of CoPt3 nanocrystals. J Am Chem Soc. 124:11480–11485.
- Silva JB, Brito WD, Mohallem NDS. 2004. Influence of heat treatment on cobalt ferrite ceramic powders. Mater Sci Eng B. 112:182–187.
- Simsek E, Akif Kilic M. 2005. Magic ferritin: A novel chemotherapeutic encapsulation bullet. J Magn Magn Mater. 293:509–513.
- Sjogren CE, Johansson C, Naevestad A, Sontum PC, Briley-Saebo K, Fahlvik AK. 1997. Crystal size and properties of superparamagnetic iron oxide (SPIO) particles. Magn Reson Imaging. 15:55–67.
- Smith CB, Raston CL, Sobolev AN. 2005. Poly (ethyleneglycol)(PEG): a versatile reaction medium in gaining access to 4’-(pyridyl)-terpyridines. Green Chem. 7:650–654.
- Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ. 2005. Nano-emulsions. Curr Opin Colloid Interface Sci. 10:102–110.
- Song JY, Kim BS. 2009. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng. 32:79–84.
- Sosnovik DE, Nahrendorf M, Weissleder R. 2007. Molecular magnetic resonance imaging in cardiovascular medicine. Circulation. 115:2076–2086.
- Spanhel L, Haase M, Weller H, Henglein A. 1987. Photochemistry of colloidal semiconductors. 20. Surface modification and stability of strong luminescing CdS particles. J Am Chem Soc. 109:5649–5655.
- Steigerwald ML, Brus LE. 1989. Synthesis, stabilization, and electronic structure of quantum semiconductor nanoclusters. Ann Rev Mater Sci. 19:471–495.
- Steigerwald ML, Brus LE. 1990. la3 bination as opposed to improving the minority carrier. Acc Chem Res. 23:183–188.
- Stojanovic Z, Otoničar M, Lee J, Stevanović MM, Hwang MP, Lee KH, et al. 2013. The solvothermal synthesis of magnetic iron oxide nanocrystals and the preparation of hybrid poly (l-lactide)–polyethyleneimine magnetic particles. Colloids Surf B Biointerfaces. 109:236–243.
- Sugimoto T, Matijevic E. 1980. Formation of uniform spherical magnetite particles by crystallization from ferrous hydroxide gels. J Colloid Interface Sci. 74:227–243.
- Sugimoto T. 2000. Fine Particles: Synthesis, Characterization, and Mechanisms of Growth. Marcel Dekker: New York.
- Sun J, Zhou S, Hou P, Yang Y, Weng J, Li X, Li M. 2007. Synthesis and characterization of biocompatible Fe3O4 nanoparticles. J Biomed Mater Res A. 80:333–341.
- Sun S, Murray CB, Weller D, Folks L, Moser A. 2000. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science. 287:1989–1992.
- Sun S, Zeng H. 2002. Size-controlled synthesis of magnetite nanoparticles. J Am Chem Soc. 124:8204–8205.
- Sun S. 2006. Recent advances in chemical synthesis, self-assembly, and applications of FePt nanoparticles. Adv Mater. 18:393–403.
- Sun X, Gutierrez A, Yacaman MJ, Dong X, Jin S. 2000. Investigations on magnetic properties and structure for carbon encapsulated nanoparticles of Fe, Co, Ni. Mater Sci Eng A. 286:157–160.
- Suslick KS, Choe SB, Cichowlas AA, Grinstaff MW. 1991. Sonochemical synthesis of amorphous iron. Nature. 353:414–416.
- Suslick KS, Fang M, Hyeon T. 1996. Sonochemical synthesis of iron colloids. J Am Chem Soc. 118:11960–11961.
- Suslick KS, Hyeon T, Fang M, Cichowlas AA. 1995. Sonochemical synthesis of nanostructured catalysts. Mater Sci Eng A. 204:186–192.
- Suslick KS, Hyeon T, Fang M. 1996. Nanostructured materials generated by high-intensity ultrasound: sonochemical synthesis and catalytic studies. Chem Mater. 8:2172–2179.
- Suslick KS. 1990. Sonochemistry. Science. 247:1439–1445.
- Swihart MT. 2003. Vapor-phase synthesis of nanoparticles. Curr Opin Colloid Interface Sci. 8:127–133.
- Tabatabaei Mirakabad FS, Akbarzadeh A, Milani M, Zarghami N, Taheri-Anganeh M, Zeighamian V, et al. 2014. A comparison between the cytotoxic effects of pure curcumin and curcumin-loaded PLGA-PEG nanoparticles on the MCF-7 human breast cancer cell line. Artif Cells Nanomed Biotechnol. 1–8
- Tartaj P, del Puerto Morales M, Veintemillas-Verdaguer S, González-Carreño T, Serna CJ. 2003. The preparation of magnetic nanoparticles for applications in biomedicine. J Phys Appl Phys. 36:R182.
- Tartaj P, González-Carreño T, Bomatí-Miguel O, Serna CJ, Bonville P. 2004. Magnetic behavior of superparamagnetic Fe nanocrystals confined inside submicron-sized spherical silica particles. Phys Rev B. 69:094401.
- Tartaj P, Gonzalez-Carreno T, Serna CJ. 2001. Single-step nanoengineering of silica coated maghemite hollow spheres with tunable magnetic properties. Adv Mater. 13:1620–1624.
- Tartaj P, Serna CJ. 2002. Microemulsion-assisted synthesis of tunable superparamagnetic composites. Chem Mater. 14:4396–4402.
- Thapa D, Palkar VR, Kurup MB, Malik SK. 2004. Properties of magnetite nanoparticles synthesized through a novel chemical route. Mater Lett. 58:2692–2694.
- Toneguzzo P, Viau G, Acher O, Guillet F, Bruneton E, Fievet-Vincent F, Fievet F. 2000. CoNi and FeCoNi fine particles prepared by the polyol process: Physico-chemical characterization and dynamic magnetic properties. J Mater Sci. 35:3767–3784.
- Tronc E, Belleville P, Jolivet JP, Livage J. 1992. Transformation of ferric hydroxide into spinel by iron (II) adsorption. Langmuir. 8:313–319.
- Veintemillas-Verdaguer S, Bomati-Miguel O, Morales MP. 2002. Effect of the process conditions on the structural and magnetic properties of .γ-Fe2O3 nanoparticles produced by laser pyrolysis. Scr Mater. 47:589–593.
- Veintemillas-Verdaguer S, del Puerto Morales M, Bomati-Miguel O, Bautista C, Zhao X. 2004. Colloidal dispersions of maghemite nanoparticles produced by laser pyrolysis with application as NMR contrast agents. J Phys D Appl Phys. 37:2054.
- Viau G, Fievet-Vincent F, Fievet F. 1996. Nucleation and growth of bimetallic CoNi and FeNi monodisperse particles prepared in polyols. Solid State Ionics. 84:259–270.
- Viau G, Toneguzzo Ph., Pierrard A, Acher O, Fievet-Vincent F, Fievet F. 2001. Heterogeneous nucleation and growth of metal nanoparticles in polyols. Scripta Mater. 44:2263–2267.
- Vidal-Vidal J, Rivas J, Lopez-Quintela MA. 2006. Synthesis of monodisperse maghemite nanoparticles by the microemulsion method. Colloids Surf A Physicochem Eng Aspects. 288:44–51.
- Vigneshwaran N, Ashtaputre NM, Varadarajan PV, Nachane RP, Paralikar KM, Balasubramanya RH. 2007. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater Lett. 61:1413–1418.
- Vijayakumar R, Koltypin Y, Felner I, Gedanken A. 2000. Sonochemical synthesis and characterization of pure nanometer-sized Fe3O4 particles. Mater Sci Eng A. 286:101–105.
- Wang J, Ren F, Yi R, Yan A, Qiu G, Liu X. 2009. Solvothermal synthesis and magnetic properties of size-controlled nickel ferrite nanoparticles. J Alloys Compd. 479:791–796.
- Wegner K, Walker B, Tsantilis S, Pratsinis SE. 2002. Design of metal nanoparticle synthesis by vapor flow condensation. Chem Eng Sci. 57:1753–1762.
- Willard MA, Kurihara LK, Carpenter EE, Calvin S, Harris VG. 2004. Chemically prepared magnetic nanoparticles. Int Mater Rev. 49:125–170.
- Willner I, Baron R, Willner B. 2006. Growing metal nanoparticles by enzymes. Adv Mater. 18:1109–1120.
- Woo K, Hong J, Choi S, Lee HW, Ahn JP, Kim CS, Lee SW. 2004. Easy synthesis and magnetic properties of iron oxide nanoparticles. Chem Mater. 16:2814–2818.
- Wormuth K. 2001. Superparamagnetic latex via inverse emulsion polymerization. J Colloid Interface Sci. 241:366–377.
- Wu W, He Q, Jiang C. 2008. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res Lett. 3:397–415.
- Wunderbaldinger P, Josephson L, Weissleder R. 2002. Tat peptide directs enhanced clearance and hepatic permeability of magnetic nanoparticles. Bioconjug Chem. 13:264–268.
- Xu B, Wang X. 2012. Solvothermal synthesis of monodisperse nanocrystals. Dalton Trans. 41:4719–4725.
- Xu C, Lee J, Teja AS. 2008. Continuous hydrothermal synthesis of lithium iron phosphate particles in subcritical and supercritical water. J Supercrit Fluids. 44:92–97.
- Yang HW, Hua MY, Liu HL, Huang CY, Wei KC. 2012. Potential of magnetic nanoparticles for targeted drug delivery. Nanotechnol Sci Appl. 5:73–86.
- Zhao Y, Qiu Z, Huang J. 2008. Preparation and analysis of Fe3O4 magnetic nanoparticles used as targeted-drug carriers. Chinese J Chem Eng. 16:451–455.
- Zheng Y-H, Cheng Y, F Bao Wang Y. 2006. Synthesis and magnetic properties of Fe3O4 nanoparticles. Mater Res Bull. 41:525–529.
- Zhu H, Yang D, Zhu L. 2007. Hydrothermal growth and characterization of magnetite (Fe3O4) thin films. Surf Coat Technol. 201:5870–5874.
- Zhu M, Diao G. 2011. Synthesis of porous Fe3O4 nanospheres and its application for the catalytic degradation of xylenol orange. J Phys Chem. 115:18923–18934.
- Zi Z, Sun Y, Zhu X, Yang Z, Dai J, Song W. 2009. Synthesis and magnetic properties of CoFe2O4 ferrite nanoparticles. J Magn Magn Mater. 321:1251–1255.
- Ziolo RF, Giannelis EP, Weinstein BA, O’horo MP, Ganguly BN, Mehrotra V, et al. 1992. Matrix-mediated synthesis of nanocrystalline خ³-Fe2O3: a new optically transparent magnetic material. Science. 257:219–223.
- Zohre S, Kazem NK, Abolfazl A, Mohammad RY, Aliakbar M, Effat A, et al. 2014. Trichostatin A-induced apoptosis is mediated by kruppel-like factor 4 in ovarian and lung cancer. Asian Pac J Cancer Prev. 15:6581–6.

