Abstract
The aim of the current investigation is to evaluate the potential of capsaicin (CAP)-containing liposomes, niosomes and emulsomes in providing localized and controlled delivery, to improve the topical delivery of drug. CAP-bearing systems were prepared by the film hydration method and compared through various in vitro and in vivo parameters. The TEM photographs suggested that the carrier systems were spherical in shape and nanometric in size range. Skin retention studies of CAP from in vitro and in vivo experiments revealed significantly higher accumulation of drug in the case of the emul-gel formulation. Based on the results, we concluded that the emul-gel may be a potential approach for the topical delivery of CAP, for an effective therapy for psoriasis.
Introduction
Psoriasis is a T-lymphocyte-mediated autoimmune skin disorder affecting the epidermis and dermis, characterized by epidermal hyperproliferation, hyperplasia of keratinocytes, and leukocyte infiltration, which lead to the formation of psoriatic plaques. It commonly causes red patches with silvery white scales (CitationRaza et al. 2011, CitationTraub and Marshall 2007). Management of the disease is executed on the basis of occurrence, symptoms, and factors affecting and aggravating the disease (CitationAgrawal et al. 2010).
Since it is only the skin tissue that is affected by this disorder, it has been rationally recommended that the afflicted site be targeted locally, without harming other tissues (CitationRaza et al. 2011, CitationAgrawal et al. 2010). The current treatment options for psoriasis are poorly efficacious, associated with undesirable side effects, and are time-consuming. However, topical agents, viz. corticosteroids, coal tar, and anthralin, are highly successful in the treatment of mild disease. The stratum corneum (SC) represents a barrier for the topical delivery of drugs. Investigations have demonstrated the advantage of the follicular route as an efficient pathway of penetration into or through the skin (CitationLademann et al. 2008).
Capsaicin (CAP), a TNF-α inhibitor, is used in the treatment of psoriasis. It blocks TNF-α by inhibiting the formation of NF-kB, the mediator of psoriasis, in psoriatic epidermal cells (CitationHarada and Okajima 2007). Moreover, CAP is also known to inhibit cutaneous vasodilatation, and blocks the axon reflex vasodilatation produced by a variety of erythematogenic chemicals. It also has a depletory effect on substance P, which is an important parameter in the pathophysiology of psoriasis (CitationChhabra et al. 2012). CAP is also used in topical therapy for various disorders including rheumatism, lumbago, and sciatica. It is also found to be effective against some food-borne pathogenic bacteria, such as Staphylococcus aureus and Salmonella typhimurium, and against microbes and insects. The limited potential for systemically-administered CAP has led clinicians to use capsaicin topically. Researchers have reported topical application of a 1% w/v solution of capsaicin (CitationKenins 1982, CitationCarter and Francis 1991).
Topical application of CAP is highly useful in psoriatic conditions, but use of the drug is limited due to its irritating, burning effects on the skin (CitationCarter 1991). To reduce such effects, it would be preferable to incorporate the drug into a suitable carrier system.
The present work focuses on the development of liposomes, niosomes and emulsomes, in order to increase the therapeutic indices and to overcome the undesirable effects of the drug, for the treatment of psoriasis. CAP-containing liposomes, niosomes, and emulsomes were prepared and then incorporated in gel, and compared for drug localization through different vesicles in the gel form, in the skin layers. The comparative study between them was done through an evaluation of in vitro parameters, viz. degree of entrapment, percentage of drug released, their ability to permeate the drug through skin (skin permeation study), and in vivo parameters, viz. the Draize patch test for determining skin irritation, confocal laser scanning microscopy, and quantification of amount of drug in the skin.
Material and methods
Materials
CAP was obtained as a gift from M/s Chillis Export House Pvt. Ltd, India. Soya phosphatidylcholine (PC), Span 80, cholesterol (CH), tristearin, dialysis membrane, Sephadex G-50, and Triton X- 100 were purchased from Sigma Chemicals Co. (St. Louis, Missouri, USA). Carbopol 934 was procured from Loba Chemicals (Mumbai, India). All other reagents and solvents were either of analytical or high-performance liquid chromatography (HPLC) grades.
Preparation of liposomes and niosomes
Liposomes containing CAP were prepared by the thin-film hydration method described previously, with slight modification (CitationGupta et al. 2010). Briefly, PC and CH were dissolved in the minimum volume of chloroform. The organic solvent was removed by using a rotary flash evaporator (Strike-102; Steroglass S.r.l., Perugia, Italy) under reduced pressure. The lipid film was then hydrated with phosphate-buffered saline (PBS; pH 7.4), and subsequently left at room temperature for 6 h until completely hydrated. The liposomes were sonicated to reduce vesicle size, using a probe sonicator (Soniweld, Mumbai, India) under an ice bath for 3 min, and were extruded 10 times through a 0.45-μm polycarbonate membrane filter. CAP-loaded niosomes were prepared similarly, using Span 80 in place of PC, following the above-described procedure.
Preparation of emulsomes
CAP-loaded emulsomes were prepared using the film hydration method, as previously described, with slight modification (CitationGupta et al. 2007). Accurately weighed quantities of PC, CH, and tristearin were dissolved in the minimum volume of chloroform, and the organic solvent was removed by using a rotary flash evaporator (Strike-102; Steroglass S.r.l., Perugia, Italy) under reduced pressure. The resultant dried film was hydrated with 5 ml of PBS (pH 7.4) for about 1 h. The dispersion was homogenized by ultrasonication (Soniweld, India) for 15 min at 40% frequency, to obtain emulsomes of a nanosize range, and then kept overnight at 4°C before use.
Characterization of carrier systems
The carrier systems prepared were characterized for morphology under an optical microscope (Motic, USA), by taking a thin film of dispersion, and then spreading their gel on a slide to take a photomicrograph. The vesicles prepared were characterized for shape by transmission electron microscopy (TEM) (Hitachi, Japan), using a copper grid coated with carbon film, with phosphotungstic acid (1% w/v) as a negative stain. Vesicle size was determined by photon correlation spectroscopy, using the Zetasizer Nano ZS90 (Malvern Instruments, Ltd., Malvern, UK). The entrapment efficiency (EE %) was determined using the method reported earlier (CitationFry et al. 1978). Briefly, about 0.2 ml of dispersion was eluted with PBS (pH 7.4) through a Sephadex G-50 column, to separate unentrapped drug. The amount of entrapped drug was determined by disrupting the vesicles, using Triton X-100. The absorbance at 279.8 nm was measured by an ultraviolet (UV) spectrophotometer (Cintra 10, GBC Scientific, Tokyo, Japan).
Preparation of carbopol gel enriched with vesicular dispersion
The gel (1% w/w) was prepared by following the procedure adopted by (CitationPavelic et al. 2001). Carbopol resin (1 g) was dispersed in distilled water (88 g), in which glycerol (10 g) had been previously added. The mixture was stirred until it thickened, and then neutralized by the drop-wise addition of 50% (w/w) triethanolamine, to achieve a transparent gel with a pH of 5.5. The vesicular dispersions were incorporated into the Carbopol gel, following the procedure. Briefly, vesicular dispersions (free from unencapsulated drug) were mixed into the vehicles using a mechanical stirrer (Remi Laboratory Instruments, Mumbai, India) for 5 min. Similarly, plain gel was prepared by adding CAP in Carbopol gel, using a concentration similar to that of the vesicular-dispersion–enriched gel.
In vitro drug release
The in vitro release studies were performed with a vesicular dispersion-based gel, using the dialysis tube method as reported earlier, with slight modification (CitationShah et al. 2007). Briefly, 1 ml of the sample was taken into a dialysis tube having the dialysis membrane MWCO 10,000 (Sigma, USA), and placed in a beaker containing 50 ml of a mixture of PBS pH 7.4 and ethanol solution (1/1, v/v), for a period of 24 h. The beaker was placed over a magnetic stirrer, and the temperature of the assembly was maintained at 37 ± 1°C throughout the study. Samples were withdrawn at predetermined time intervals and replaced with the same volume of release media. The withdrawn samples were assayed for drug content, by measuring the absorbance at 279.8 nm against a reagent blank, using the UV spectrophotometer (Shimadzu, Japan). Sink conditions were maintained throughout the experiment. All the experiments were done in triplicate.
In vitro skin permeation and retention studies
After cervical dislocation of hairless rat skin, the abdominal and dorsal skin was excised surgically. The skin was used at its full thickness, after removing underlying fat and subcutaneous tissues. In vitro skin permeation studies were carried out using a Franz diffusion cell, as described previously, with slight modification (CitationQiu et al. 2008). The skin was mounted between the donor and receptor compartments of the diffusion cell, with the SC (stratum corneum) facing upward (donor compartments). Each diffusion cell, with a diffusion area of 3.14 cm2 and the receptor medium, was filled with mixture of 25 ml of PBS (pH 7.4)/ethanol solution (1/1 v/v), thermostated at 37 ± 1°C, and continuously stirred at 100 rpm throughout the experiment. One ml of the formulation was placed in the donor compartment. At appropriate time intervals of up to 24 h, samples of 200 μl were withdrawn from the receptor compartment and replaced by an equal volume of fresh medium, to keep a stable receiver volume. The experiments were repeated in triplicate. The cumulative amount of drug permeated per unit area was plotted as a function of time. The skin permeation rate (Jss) was calculated from the slope of the linear portion. The samples from the receptor compartment were analyzed by HPLC, as reported earlier (CitationAgarwal et al. 2001). The mobile phase used was a mixture of methanol and water (3/2, v/v); it was used at a flow rate of 0.6 ml/min. The detection was done at a wavelength of 280 nm. Excess formulation was removed after 24 h, by wiping the test area with a cotton swab, washed 3 times with a PBS/ethanol mixture (1/1, v/v), and then dried using a filter paper.
To determine the drug concentration in the SC, 20 tapes (Transpore™, VWR International, Herlev, Denmark) were applied with constant pressure for 2 min, and then gradually and carefully removed, with constant force applied at a 45° angle to the skin, using clean forceps (CitationRaza et al. 2012). The first two strips were discarded due to the potential amount of drug remaining on the skin's surface, and strips 3–20 were collected. The tapes were pooled in a tube containing a PBS (pH 7.4)/ethanol mixture (1.1, v/v). Then, the tubes were vortexed for 20 min, sonicated for 30 min, and vortexed again for 1 h, to extract CAP from the tapes. To determine the amount of drug in the viable skin (epidermis and dermis), the remaining skin was cut into small pieces and homogenized (York, Mumbai, India) in the PBS (pH 7.4)/ethanol (1/1, v/v) mixture, after vortexing for 20 min. The resulting solution was sonicated for 30 min, vortexed again for 30 min, and centrifuged at 10,000 × g (Hitachi CPMax-100, Japan) for 30 min. The amount of CAP extracted from the tapes, and the amount remaining in the skin samples, were determined using HPLC.
In vivo studies
The entire study was carried out under the guidelines compiled by the CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals), Ministry of Culture, Government of India, and the local institutional Animal Ethics Committee had approved all the study protocols. All the experimental rats were housed in cages, with access to food and water ad libitum until use.
Drug localization in the different skin layers by in vivo tape stripping
The dorsal skin of the albino rat was washed with physiological saline solution after the removal of hair. Drug localization in different skin layers was determined by the method reported (CitationKnudsen et al. 2012). The animals were anesthetized with an intraperitoneal injection of ketamine hydrochloride (100 mg/kg). Various formulations were applied topically onto the well-marked test area (2 × 3 cm2) on the dorsal skin with an approximate CAP amount (CitationMagnusson and Koskinen 2000). After 24 h, the rats were sacrificed by cervical dislocation, and the skin was stripped, as mentioned in the above section. Excess formulation was wiped off with a cotton swab, washed 3 times with a mixture of PBS 7.4/ethanol (1/1, v/v), and then dried with a filter paper. To determine the drug concentration in the SC and other layers of viable skin, a similar procedure was followed or adopted, as described in the previous section.
Skin irritation study
The irritation potential of the different carriers incorporated in gel, in comparison to that of the plain gel, was evaluated by carrying out the Draize patch test on rabbits (CitationGupta et al. 2013). The backs of the animals were clipped free of hair, 24 h prior to the application of the formulations. A quantity of 0.5 ml of the formulation was applied on the hair-free skin by uniform spreading within an area of 4 cm2. The skin was observed for any visible change such as erythema (redness) at 24, 48 and 72 h after the application of various formulations. The mean erythemal scores were recorded (ranging from 0 to 4), depending on the degree of erythema, as follows: no erythema = 0, slight erythema (barely perceptible-light pink) = 1, moderate erythema (dark pink) = 2, moderate to severe erythema (light red) = 3, and severe erythema (extreme redness) = 4.
Confocal laser scanning microscopy
Depth of skin penetration and fluorescence intensity of rhodamine-B dye-loaded optimized liposomes/niosomes and capsaicin-loaded emulsomal gel were determined by CLSM, as reported, with slight modification (CitationDubey et al. 2007). Quantities of 0.5 ml of the dye-loaded gel formulations were applied on the skin. After 4 h, the rats were sacrificed by cervical dislocation. The excess of formulation was removed with a cotton swab and then the piece of skin including the application zone was excised, placed on aluminum foil, and the adhering fat was removed. The skin was washed with distilled water and preserved in 10% v/v formalin in PBS (pH 7.4). The formulation-treated skin was cross-sectioned mechanically and scanned at different increments through the z-axis of a CLSM.
In vitro skin permeation through hyperproliferative skin
The tape-stripping technique was used to produce hyperproliferative skin that mimics psoriasis-affected skin (CitationAgrawal et al. 2013). The hair-free dorsal skin was stripped using a Transpore™ tape (VWR International, Herlev, Denmark) twice daily for 5 days, and the stripping was repeated 10 times for each process. Hyperproliferation of the skin was verified by histological observation. Each specimen was dehydrated using ethanol, embedded in paraffin wax, and stained with hematoxylin and eosin. For each skin sample, three different sites were examined and evaluated under light microscopy (Eclipse 4000, Nikon, Tokyo, Japan).
Statistical analysis
All the results were expressed as mean ± standard deviation. The treated groups were compared with control by employing the Student t-test and the analysis of variance (ANOVA) test, using the Graph Pad INSTAT software, version 3.00 (Graph Pad Software, San Diego, CA). A value of p < 0.05 was considered statistically significant.
Results and discussion
Preparation and characterization of the carrier systems
The present investigation was aimed at developing liposomes, niosomes and emulsomes for topical/localized delivery of drug with greater skin accumulation. The delivery, penetration, and localization of the drug in the different skin layers are greatly affected by the composition of the nanocarrier system.
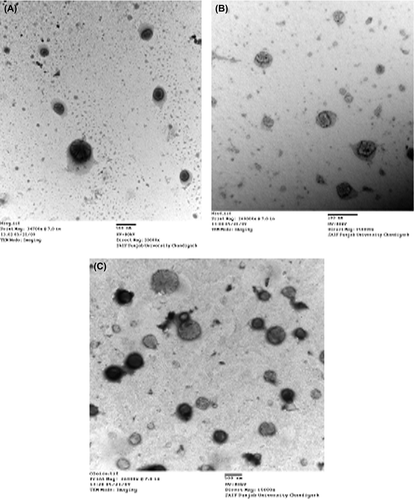
The various types of vesicles were prepared by the conventional film-hydration method, which produced a stable system (composition of liposomes, PC/CH = 7/3, niosomes, Span 80/CH = 8/2, and emulsomes, tristearin/PC/CH = 1/1/0.05). The size and polydispersity index (PDI) of the liposomes were found to be 368.5 ± 43 nm and 0.136 ± 0.024 μm, respectively, while in the case of niosomes, they were recorded to be 342.7 ± 35 nm and 0.167 ± 0.045 μm, respectively. On the other hand, the size of emulsomes was recorded to be 172.8 ± 10 nm and the PDI was 0.116 ± 0.019 μm. The data showed that all carrier systems were of a size range suitable for dermal delivery (). The size of liposomes was slightly greater than that of niosomes. CitationLawrence et al. 1996 and CitationManosroi et al. 2003, reported that CH is an important constituent of liposomes and niosomes for the stabilization of the vesicular bilayer membrane and to achieve suitable molecular geometry and hydrophobicity, which are desirable for the formation of closed bilayer vesicles. The shape and morphology of the various vesicles were determined by transmission and scanning electron microscopy (TEM). The TEM photograph suggested that the vesicles are spherical in shape and nano-metric in size (). Particle sizes and surface charges of the developed systems are shown in . The values of EE% of CAP in the liposomal, niosomal, and emulsomal formulations were found to be 70.98 ± 2.36%, 54.30 ± 2.16%, and 83.79 ± 3.58%, respectively. Due to the lipophilic nature of the drug, it gets intercalated into the lipid (liposomes), surfactant (niosomes) and solid lipid core, as well as into the PC lipid layers (emulsomes). The maximum entrapment was observed in the case of emulsomes, due to higher lipid composition. To facilitate dermal application, fluid dispersions were incorporated into a gel base. However, the dispersion incorporated in the gel matrix revealed that there was marginal increase in the size after gelling, as shown: 382.4 ± 39 nm, 374.7 ± 33 nm and 198.2 ± 17 nm in the liposome-gel (lipo-gel), niosome-gel (nio-gel) and emulsome-gel (emul-gel), respectively. The values for drug encapsulation efficiency of dispersion within the gel matrix were found to be 68.58 ± 3.41%, 50.77 ± 2.32%, and 81.12 ± 3.19%, for lipo-gel, nio-gel and emul-gel respectively, and demonstrated slight decrease in entrapment efficiency ().
Table I. The characterization of the formulations by particle size, PI and EE %.
In vitro release
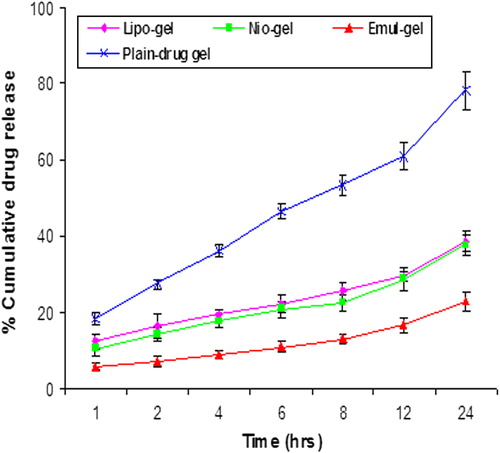
The in vitro release profile of CAP from different formulations was determined in PBS (pH 7.4) for a period of 24 h. The percentage of cumulative drug release after 24 h from different formulations, namely the lipo-gel, nio-gel and emul-gel, was found to be 38.80 ± 2.57%, 37.66 ± 2.86%, and 22.93 ± 1.35%, respectively, at the end of 24 h (). The data obtained clearly show that drug release from lipo-gel was slightly higher than that from nio-gel. The lower drug release from niosomes may be ascribed to the presence of Span 80, which has been reported to interact with the amino group present on the surface of the CAP. This interaction might hold a substantial amount of drug and release the drug molecules slowly from the niosomal formulations. A similar trend was observed in the case of liposomal and niosomal formulations of acyclovir, in which niosomes containing Span-20 were reported to interact with amino groups and were ultimately responsible for slow and sustained drug release from niosomal formulations (CitationMukherjee et al. 2007). In the emul-gel, the cholesterol provides rigidity to the formulation. The drug release from emul-gel is minimal due to presence of drug moiety in lipid core as well as in surrounding PC layers. The prolonged release could be attributed to the embedding of drug in the solid lipid matrix. The release of the drug from the formulation was found to be controlled, sustained, and consistent after 24 h, but this sustained effect was more pronounced in the case of emul-gel. The release profile can be followed by the sequence emul-gel > lipo-gel > nio-gel, as their sustainment potential.
In vitro skin permeation study
To assess the skin targeting potential of the developed systems and their permeation ability, the hairless rat skin was examined by conducting in vitro skin permeation and retention studies. Compounds that are essentially insoluble in water may not partition freely from excised skin into an aqueous receptor fluid at the in vitro level. CAP is essentially insoluble in water, hence a mixture of PBS/ethanol (1/1) was chosen as solubilizing agent and added into the receptor compartment of the Franz cell (CitationTavano et al. 2011).
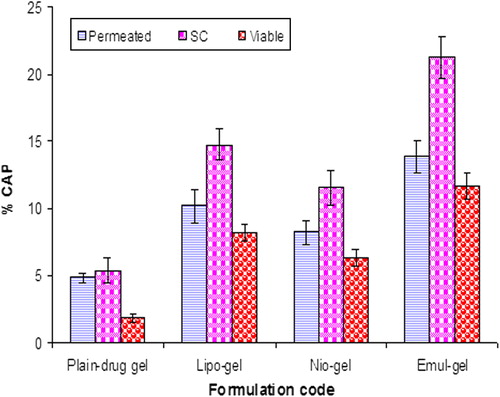
The amounts of CAP that permeated from the emul-gel, lipo-gel, nio-gel, and plain-gel, after 24 h, were 32.78 ± 2.84 μg/cm2, 19.67 ± 1.65 μg/cm2, 16.34 ± 1.02 μg/cm2and 7.34 ± 1.04 μg/cm2, respectively (). The flux value of the CAP from the emul-gel, lipo-gel, nio-gel and plain CAP-gel permeation rate constants, which were 2.6 ± 0.06 μg/cm2/hr, 1.19 ± 0.01 μg/cm2/hr, 0.92 ± 0.03 μg/cm2/hr and 0.65 ± 0.01 μg/cm2/hr, respectively, clearly indicate the enhancing effect of vesiculation on drug permeation and penetration on the skin. The data depicts that emulsome-based gel showed maximum permeation profile, whereas nio-gel and lipo-gel showed relatively low permeation characteristics, because cholesterol profoundly affects their membrane properties. The improved skin permeation of drug and the consequently enhanced drug-transport abilities through the nanosystem can be explained on the basis of the presence of drug molecules in a solubilized state (CitationAgarwal et al. 2001). The highest permeation was obtained for emul-gel, which can be attributed to its high flux value (). It may be attributed to the bio-compatible nature of phospholipids, as these are known to interact with the biological membranes efficiently. Earlier, it has been reported by other groups that phospholipids are able to interact with the skin cells and are able to form micro-reservoirs efficiently (CitationRaza et al. 2012). summarizes the transdermal flux from the gel-incorporated dispersion. Among all the formulations, emul-gel showed highest flux value as compared to other vesicular dispersion-based gels. The values for permeation flux from emul-gel showed about 2.19 and 2.83 times higher flux as compared to the values for lipo-gel and nio-gel respectively. Topically applied lipid vesicles affect characteristics and integrity of the skin permeability barrier. In addition, they may extract the lipid from the skin or disrupt the order within and between the corneocytes, upon binding to the keratin filament (CitationGupta et al. 2005). These findings confirm the assumption that emul-gel provokes the accumulation of the embedded moiety into the skin layers and creates a reservoir able to prolong the skin residence time. This evidence may be due to the fact that the drug has to cross two diffusional barriers in all types of vesicular systems. However, in the case of emul-gel, this barrier becomes complex, due to the active moiety present in the surrounding PC layers, along with being embedded in the inner solid lipid core.
Figure 3. In vitro skin permeation of CAP for emul-gel, lipogel, nio-gel and plain drug- gel. Values are expressed as mean ± standard deviation (n = 3).
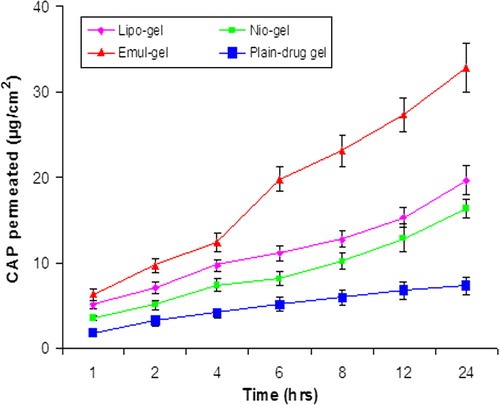
Table II. Results of in vitro permeation and skin retention studies from vesicular-gel systems and plain-drug gel: amount permeated through the skin after 24 h, flux and percentage of CAP accumulated into the skin at the end of the permeation experiments (24 h).
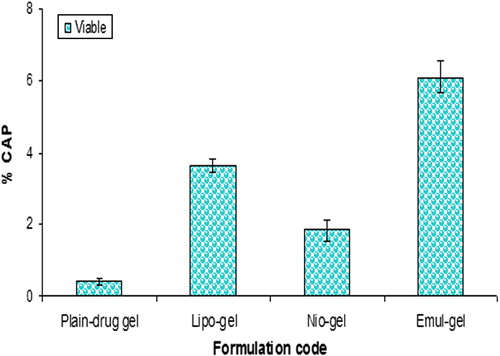
The percentage values of CAP permeated through the skin and accumulated into the SC, as well as viable skin for all formulations at the end of the experiments, have been compiled in . Invariably, the drug retention was found to be maximal in SC for all the formulations. A significantly higher SC retention of drug was obtained for emul-gel (3.95 times), lipo-gel (2.75 times) and nio-gel (2.16 times), as compared to plain-gel. However, there was no significant difference in drug concentration observed for liposomal and niosomal gels in the SC layer. The data predicted that in viable skin layers, emulsome-based gel also provided a significantly higher drug accumulation compared with lipo-gel and nio-gel or plain CAP-gel ( and ). Compared with the plain CAP-gel, the accumulation of drug in viable skin was higher in emul-gel by 6.21-fold, in lipo-gel by 4.37-fold, and in nio-gel by 3.39-fold. The penetration and accumulation into the skin layers can be illustrated on the basis of different lipidic composition of vesicles, small size, penetration enhancer effect of surfactants, and also the interaction between emulsomes, liposomes and niosomes with the SC lipids, providing a deposit effect of drug in the skin. Lipidic composition of emulsomes and liposomes are more similar to SC lipids, in contrast to plain solution and niosomes, which may promote the accumulation of the encapsulated CAP moiety into the upper skin layers, thus creating a reservoir which may prolong the skin residence time. Therefore, these findings confirm the assumption that the type of lipids in emulsomes promotes the accumulation of the embedded drug moiety into the upper skin layers, thus creating a reservoir which also prolongs the skin residence time of the drug (CitationGupta et al. 2013). Moreover, PC present in the emulsomes acts as surfactant, and the lipidic nature may be considered for the higher drug retention and penetration effect which may also integrate as well as mix with skin lipids to loosen their structure, by disturbing the lamellar arrangement of the lipids (CitationSouto et al. 2004, CitationEl Maghraby et al. 2008, CitationQingzhi et al. 2009). However, lipo-gel also showed a significant increase in the drug accumulation in SC and viable skin, compared to nio-gel or plain gel. This behavior can be explained as a probable consequence of liposome-skin interaction, providing a deposit effect for drug in the skin. The liposomes have been referred to as skin “drug localizers” (CitationSchmid and Korting 1996).
In vivo drug localization in the skin layers
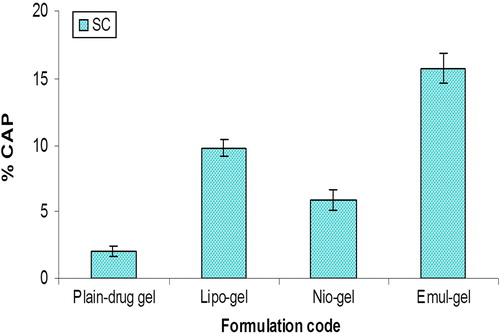
The amounts of CAP accumulated in the various strata of skin, 24 h post application, from different formulations, were as shown in and . The findings suggest that in vivo drug localization may be influenced by the disruption of nanosystems due to interaction with skin components, such as lipase activity and other hydrolyzed products in viable skin, which might affect the low magnitude of skin retention of drug in vivo compared to those recorded in the in vitro studies (CitationBouwstra et al. 2003). The absorption of CAP following an application of different formulations (containing an equivalent of 1% w/v of CAP) through SC () decreased in the following sequence: emul-gel > lipo-gel > nio-gel. The data supported 7.75-fold, 4.8-fold, and 2.9-fold higher SC retention in the case of emulsomal, liposomal and niosomal gel systems, as compared to plain gel. On the other hand, the drug quantity recovered from viable skin following the application of various formulations followed a pattern similar to that recorded in the case of SC; however, the amount accumulated was notably lower, as compared to SC. Compared to the plain gel, the emulsome-gel system showed a 15.6-fold increase in drug retention, while liposome-gel and niosome-gel systems showed 9.31 fold and 4.72-fold higher drug retention in viable skin, respectively (). The data clearly demonstrate that emulsomes in gel form serve as a better reservoir and provide a local depot in viable skin. In addition, they also continuously release the drug in a sustained manner, which is the prime requirement in our study, compensating for the drawback of skin irritation. However, though the liberated drug was transported into the deeper layers of the skin, the extent of reach was too minimal. Thus, the CAP-loaded formulations developed in the present study have the potential to deliver the effective amount of drug in a sustained and controlled fashion, enabling the maintenance of its localized depot and, therefore, a prolonged/greater residence time in viable skin for the treatment of skin diseases.
Skin irritation test
The major disadvantages associated with conventional CAP therapy is the burning sensation and skin irritation, which strongly limit its utility and applicability among the patients (CitationTurini et al. 2006, CitationAgrawal et al. 2013). An ideal delivery system would be required to diminish the drawbacks implicated in CAP therapy. The skin-irritation studies indicated that all types of vesicular system-based gels exhibited minimum to no irritation, as compared with plain gel, even after 72 h of application (). The primary irritation index (PII) was found to be 0.00 for emulsomal, liposomal, and niosomal gels, showing no irritation. Therefore, the lipidic vesicular systems developed resulted in no erythema and are safe for topical application, as compared with the plain solution.
Table III. Mean erythemal scores and PII observed for various CAP formulations obtained at the end of 24, 48, and 72 h (n = 3).
Confocal laser scanning microscopy
Images obtained with confocal laser scanning microscopy (CLSM) () showed that the dye-loaded optimized liposomal, niosomal and emulsomal gel formulations exhibit deposition of dye in the skin layers. The images are taken from the bottom section (parallel to the skin surface), which reveal information about dye distribution in the hair follicles, and the optical section (perpendicular to the skin surface), which provide information on distribution in the stratum corneum and viable skin. The smaller sized vesicles showed a homogenous distribution on the skin surface and maximum penetration of emulsomes, in comparison to liposomal and niosomal gels, which might be due to the rigidity provided by the solid core to the carrier.
Figure 7. CLSM images of a cross-section of rat skin with different dye-loaded formulations. The formulations were applied for 4 h: (a) lipo-gel (b) nio-gel (c) emul-gel. The scale bar represents 200 μm.
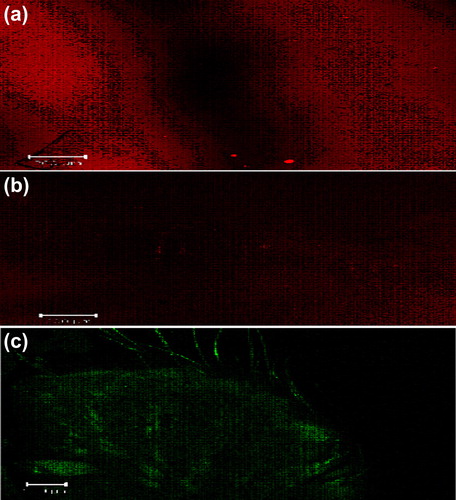
CLSM studies were conducted to measure the extent of penetration and fluorescence intensity of topical delivery of the designed system. shows the blind scoring data from CLSM images. In all the images ( and ), the application of carrier systems resulted in medium, weak and bright fluorescence in viable skin through liposomal, niosomal, and emulsomal gels. respectively. This efficient delivery of dye-loaded carriers suggests their enhanced penetration with maximum fluorescence intensity into viable skin. The systems depicted an increase in the depth (up to 100 μm) of penetration on application of dye-loaded emul-gel as compared to lipo-gel (55 μm) and nio-gel (41.0 μm), that were confined to depths that were smaller in terms of micrometers. This prominently efficient delivery by the emulsomal system suggests enhanced penetration and consequent fusion with the membrane lipids in the depths of the skin, supporting the hypothesis of (CitationTouitou et al. 2000).
Figure 8. CLSM images of a cross-section of rat skin with different dye-loaded formulations for determining the depth of penetration into deeper skin. The formulations were applied for 4 h (a) lipo-gel (b) nio-gel (c) emul-gel.
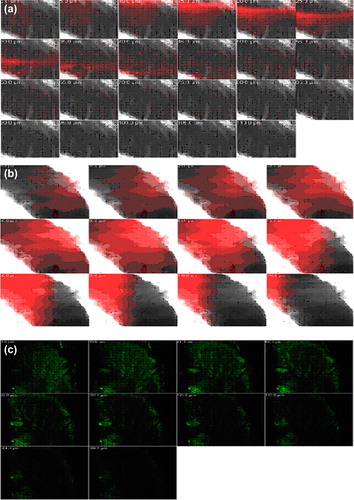
Table IV. Blind summarized scoring data from CLSM images.
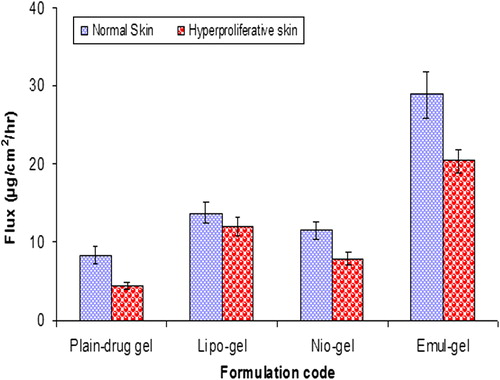
Skin permeation of CAP across hyperproliferative skin
compares CAP fluxes across normal and hyperproliferative skin. Drug permeation through the hyperproliferative skin was comparatively lower than that through the normal skin, with all the formulations. Fluxes obtained from hyperproliferative skin for emul-gel, lipo-gel, nio-gel, and plain gel, were found to be 20.36 ± 1.56 μg/cm2/h, 11.98 ± 1.14 μg/cm2/h, 7.89 ± 0.89 μg/cm2/h and 4.45 ± 0.43 μg/cm2/h, respectively.
Conclusion
The present work describes the potential and applicability of vesicle-based gels loaded with CAP as promising carriers for the treatment of psoriasis. The results showed that incorporation of CAP into various vesicular systems proved useful in reducing the side effects of the drug and increasing the therapeutic efficacy. Emulsomes are therapeutically effective for the delivery of antipsoriatic agents through the topical route, as indicated by the zeta potential value (indicating good physical stability), and a high value of entrapment efficiency. They can be used as effective carriers of lipophilic drugs, depending on the desired drug permeation profile, and demonstrate better skin permeation through hyperproliferative skin. Moreover, the enhanced accumulation of CAP by the emulsomal gel within the skin might help to target localized delivery to the epidermal and dermal sites, and possesses ability to increase drug accumulation in the various skin layers, with creation of a depot effect and maximal therapeutic efficacy. Thus, it can be concluded that emulsomal gels offer a localized effect and may be a potential approach in the treatment of the psoriatic infection, as an alternative to the systemic use of CAP.
Acknowledgements
The authors acknowledge the help of Professor S. C. Lakhotia and Professor J. C. Roy (Department of Zoology, Banaras Hindu University (BHU), Varanasi, India) in the confocal microscopy study. One of the authors, Ruchi Gupta, wants to acknowledge the help of Mr. Praveen Garg, Chairman, ISF College of Pharmacy, Moga, Punjab, for providing excellent research facilities.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Agarwal R, Katare OP, Vyas SP. 2001. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antipsoriatic drug dithranol. Int J Pharm. 228:43–52.
- Agrawal U, Gupta M, Vyas SP. 2013. Capsaicin delivery into the skin with lipidic nanoparticles for the treatment of psoriasis. Artif Cells Nanomed Biotechnol. doi:10.3109/21691401.2013.832683.
- Agrawal Y, Petkar KC, Sawant KK. 2010. Development, evaluation and clinical studies of Acitretin loaded nanostructured lipid carriers for topical treatment of psoriasis. Int J Pharm. 401:93–102.
- Bouwstra JA, Honeywell-Nguyena PL, Goorisa GS. 2003. Structure of the skin barrier and its modulation by vesicular formulations. Prog Lipid Res. 42:1–36.
- Carter RB. 1991. Topical capsaicin in the treatment of cutaneous disorders. Drug Dev Res. 22:109–123.
- Carter RB, Francis WR. 1991. Capsaicin desensitisation to plasma extravasation evoked by antidromic C fiber stimulation is not associated with anti-nociception in the rat. Neurosci Lett. 127:49–52.
- Chhabra N, Aseri ML, Goyal V, Sankhla S. 2012. Capsaicin: a promising therapy – a critical reappraisalInt. J Nutri Pharmacol Neurolog Dis. 2:8–15.
- Dubey V, Mishra D, Dutta T, Nahar M, Saraf DK, Jain NK. 2007. Dermal and transdermal delivery of an anti-psoriatic agent via ethanolic liposomes. J Control Release. 123:148–154.
- El Maghraby GM, Barry BW, Williams AC. 2008. Liposomes and skin: from drug delivery to model membranes. Eur J Pharm Sci. 34: 203–222.
- Fry DW, White JC, Goldman ID. 1978. Rapid separation of low molecular weight solutes from liposomes without dilution. J Anal Biochem. 90:809–815.
- Gupta M, Goyal AK, Paliwal S, Paliwal R, Mishra N, Vaidya B, et al. 2010. Development and characterization of effective topical liposomal system for localized treatment of cutaneous candidiasis. J Liposome Res. 20:341–350.
- Gupta M, Tiwari S, Vyas SP. 2013. Influence of various lipid core on characteristics of SLNs designed for topical delivery of fluconazole against cutaneous candidiasis. Pharma Dev Technol. 18:550–559.
- Gupta PN, Mishra V, Rawat A, Dubey P, Mahor S, Jain S, et al. 2005. Non-invasive vaccine delivery in transfersomes, niosomes and and liposomes: a comparative study. Int J Pharm. 293:73–82.
- Gupta S, Dube A, Vyas SP. 2007. Antileishmanial efficacy of amphotericin B bearing emulsomes against experimental visceral leishmaniasis. J Drug Target. 15:437–444.
- Harada N, Okajima K. 2007. Effect of topical application of capsaicin and its related compounds on dermal insulin-like growth factor-I levels in mice and on facial skin elasticity in humans. Growth Hormone IGF Res. 17:171–176.
- Kenins P. 1982. Response of single nerve fibres to capsaicin applied to the skin. Neurosci Lett. 29:83–88.
- Knudsen N, Rønholt S, Salte RD, Jorgensen L, Thormann T, Basse LH. 2012. Calcipotriol delivery into the skin with PEGylated liposomes. Eur J Pharm Biopharm. 81:532–539.
- Lademann J, Knorr F, Richter H, Blume-Peytavi U, Vogt A, Antoniou C, et al. 2008. Hair follicles – an efficient storage and penetration pathway for topically applied substances. Skin Pharmacol Physiol. 21:150–155.
- Lawrence MJ, Chauhan S, Lawrence SM, Barlow DJ. 1996. The formation, characterization, and stability of nonionic surfactant vesicles. STP Pharm Sci. 6:49–60.
- Magnusson BM, Koskinen LD. 2000. In vitro percutaneous penetration of topically applied capsaicin in relation to in vivo sensation responses. Int J Pharm. 195:55–62.
- Manosroi A, Wongtrakul P, Manosroi J, Sakai H, Sugawara F, Yuasa M, Abe M. 2003. Characterization of vesicles prepared with various nonionic surfactants mixed with cholesterol. Coll Sur B Bioint. 30:129–138.
- Mukherjee B, Patra B, Layek B, Mukherjee A. 2007. Sustained release of acyclovir from nano-liposomes and nano-niosomes: An in vitro study. Int J Nanomed. 2:213–225.
- Pavelic Z, Skalko-Basnet N, Schubert R. 2001. Liposomal gels for vaginal drug delivery. Int J Pharm. 219:139–149.
- Qingzhi LV, Aihua Y, Yanwei X, Houli L, Zhimei S, Jing C. 2009. Development and evaluation of penciclovir-loaded solid lipid nanoparticles for topical delivery. Int J Pharm. 372:191–198.
- Qiu Y, Gao Y, Hu K, Li F. 2008. Enhancement of skin permeation of docetaxel: a novel approach combining microneedle and elastic liposomes. J Control Release. 129:144–150.
- Raza K, Katare OP, Setia A, Bhatia A, Singh B. 2012. Improved therapeutic performance of dithranol against psoriasis employing systematically optimized nanoemulsomes. J Microencapsul. doi:10.3109/02652048.2012.717115.
- Raza M, Negi P, Takyar S, Shukla A, Amarji B, Katare OP. 2011. Novel dithranol phospholipid microemulsion for topical application: development, characterization and percutaneous absorption studies. J Microencapsul. 28:190–199.
- Schmid MH, Korting HC. 1996. Therapeutic progress with topical liposome drugs for skin disease. Adv Drug Deliv Rev. 18:335–342.
- Shah AK, Date AA, Joshi MD, Patraval VB. 2007. Solid lipid nanoparticles (SLN) of tretinoin: potential in topical delivery. Int J Pharm. 345:163–171.
- Souto EB, Wissing SA, Barbosa CM, Müller RH. 2004. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int J Pharm. 278:71–77.
- Tavano L, Alfano P, Muzzalupoa R, de Cindio B. 2011. Niosomes vs microemulsions: new carriers for topical delivery of Capsaicin. Colloids Sur B Biointerfaces. 87:333–339.
- Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M. 2000. Ethosomes-novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release. 65:403–418.
- Traub M, Marshall K. 2007. Psoriasis-Pathophysiology, conventional, and alternative approaches to treatment. Alternative Med Rev. 12:319–330.
- Turini MPD, Beneforti P, Spinelli M, Malagutti S, Lazzeri M. 2006. Heat/burning sensation induced by topical application of capsaicin on perineal cutaneous area: new approach in diagnosis and treatment of chronic prostatitis/chronic pelvic pain syndrome. Urology. 67:910–913.