Abstract
The objective of this study was to develop an oral transmucosal formulation of an antiemetic drug that can not only serve in the active form but also provide a controlled release profile. In this study, sublingual films based on the biodegradable and water-soluble polymers, that is HPMCK-4M and PVPK-30, were developed by the solvent casting method, and were loaded with the antiemetic drug granisetron hydrochloride (granisetron HCl). The entrapment efficiency of the developed formulation was found to be 86%. The in vitro profile showed an instant release of the drug from the sublingual film, in a pattern following the first order kinetics array. The in vivo studies showed that granisetron HCl was delivered in its active state and showed effective results, as compared to its activity in the marketed formulation.
Introduction
In recent years, various methods have been developed to deliver therapeutic agents through various transmucosal routes, to release a therapeutic amount of drug to a specific site (CitationMarwah et al. 2014), and to maintain the desired therapeutic concentration (CitationSingh et al. 2014c, CitationMalik et al. 2014). The conventional dosage forms, such as tablets (CitationKaur et al. 2014g) or injections (CitationKaur et al. 2014d), have many drawbacks such as poor bioavailability (CitationSingh et al. 2014b), poor solubility (CitationSingh et al. 2014a), quick breakdown of the drug in vivo (CitationSharma et al. 2014c), and rapid loss of drug activity (CitationChaudhary et al. 214). These drawbacks can be overcome through a suitable delivery system, depending on the medical condition (CitationSharma et al. 2014b, CitationKaur et al. 2014a). The concept of fast-dissolving films has become popular as a new delivery system (CitationGagandeep et al. 2014). This delivery system will provide the maximum therapeutic efficacy (CitationGarg 2014) and increase the bioavailability of poorly soluble drugs (CitationSharma et al. 2014a) with minimum side effects (CitationGarg and Goyal 2014b) and maximum stability (CitationGarg and Goyal 2014a), improving patient compliance (CitationRohilla et al. 2014) by reducing the frequency of dosage (CitationGarg and Goyal 2014c), and will also enable the drug to avoid the first pass metabolism, which is a major issue (CitationKataria et al. 2014).
Nausea and vomiting are common problems that occur in disease and non-disease conditions, and after chemotherapy. These result from the activation of protective physiological mechanisms in order to eliminate the toxins from the body. Granisetron HCl is well-recommended for the treatment of nausea and vomiting following cancer chemotherapy. It is a potent serotonin 5-HT3 receptor antagonist. Its prominent action is to reduce the activity of the vagus nerve, which activates the vomiting center in the medulla oblongata (CitationNibha and Pancholi 2012, CitationJoshi et al. 2014, CitationJohal et al. 2014).
Several approaches have been developed and implemented, to improve the therapeutic outcomes of granisetron HCl. A transdermal patch has been prepared to control the release (CitationMorie et al. 2014, CitationGoyal et al. 2014b) and provide better therapeutic outcomes for the prevention of chemotherapy-induced vomiting (CitationGarg et al. 2012b, CitationGoyal et al. 2014a). Recently, transdermal patches based on iontophoresis have also been utilized, to increase the bioavailability of granisetron. Studies have demonstrated the feasibility of transdermal transport of granisetron using lutrol-F127 gel, by iontophoresis (CitationPanzade et al. 2012, CitationGarg and Goyal 2012). A provesicular formulation of granisetron HCl has been developed for buccal delivery (CitationGoyal et al. 2013, CitationGarg et al. 2013). Studies have shown that bioadhesive buccal polymers play an important role in the delivery of granisetron (CitationAhmed et al. 2014).
In the present study, HPMC K-4 and PVPK-30 were selected as polymeric constituents, of which HPMC K-4 acts as a film-forming agent (CitationNyamweya and Hoag 2000), and PVPK-30 acts as a penetration enhancer (CitationPrajapati et al. 2012). The objective of this study was to formulate and evaluate granisetron HCl-loaded HPMCK-4M and PVPK-30 films for transmucosal delivery via the sublingual route, and to improve therapeutic efficacy and bioavailability by avoiding the first pass metabolism.
Materials and methods
Materials
Granisetron HCl was obtained from Adley Pharmaceuticals (Solan, India). Water-soluble polymers, that is HPMCK-4M and PVPK-30, were procured from HiMedia Labs, Mumbai, India. All the other chemicals used were of analytical grade.
Preparation of the sublingual film
The sublingual film of granisetron HCl was prepared by the solvent casting method. Aqueous solution 1 was prepared by dissolving the polymer (100/22) in 10 ml of distilled water, and stirred to produce a clear solution, which was kept for 1 h to remove all air bubbles. Then, aqueous solution 2 was prepared by adding the drug, plasticizer, and sweetener, in a specific proportion, (50 mg/20 mg/1.5 ml) to distilled water. Subsequently, solution 1 was added to solution 2, under mixing, and left to be for stirred for 1 h. Then, the solution was cast in a petri dish of 9 cm in diameter, and was dried in oven at 45°C for 24 h. The film was then carefully removed from the petri dish and checked for any imperfection, and cut according to the size required for testing (a square film of 2 cm length, 2 cm width). The samples were stored in a glass container maintained at a temperature of 30°C and a relative humidity of 60% ± 5%, until further analysis (CitationNafee et al. 2004).
Characterization and evaluation
Measurement of tensile strength
The mechanical property was evaluated using the model TexturePro CT V1.3 Build 15. Film strips of a special dimension and free from air bubbles or physical imperfections were held between two clamps which were positioned at a distance of 3 cm. During measurement, the strips were pulled by the top clamp at a rate of 100 mm/min, and the force was measured when the film broke (CitationGarg et al. 2012a).
Tensile strength is calculated by the following equation:
Drug entrapment efficiency
The drug entrapment efficiency (DEE) of the film was calculated by dissolving the prepared film of the dimension of 2 cm × 2 cm in 20 ml of simulated salivary fluid of (pH 6.8), with continuous shaking for 30 min. The amount of drug in the solution was determined by UV spectroscopy at 301 nm (CitationKaur et al. 2014e, Citation2014f). The percentage of entrapment was calculated using the following formula:
In vitro dissolution study
The in vitro dissolution profile of the sublingual film of granisetron HCl was conducted in a USP type 2 (basket apparatus) with 900 ml of simulated salivary fluid of pH 6.8 as the dissolution medium (stirred at 50 rpm), maintained at 37 ± 0.5°C, for 8 h. Samples were withdrawn every 30 min and replaced with the same amount of fresh medium. Absorbance was determined by a UV spectrophotometer at the λmax of 301 nm. The prepared formulation was also compared with granisetron tablets (CitationMashru et al. 2005, CitationKaur et al. 2014c, CitationKaur et al. 2014b).
Ex vivo permeability study
Drug permeability studies were carried out using freshly excised goat tissue. The receptor compartment of the Franz diffusion cell was filled with freshly prepared simulated salivary solution (pH 6.8). The freshly excised tissue was fixed by clamps between the donor and the receptor compartments in such a way that its epithelial surface was oriented towards the donor compartment. The release studies were carried out at 37°C. Samples were withdrawn every 30 min. After each sampling, the volume removed was replaced with fresh medium. The amount of drug present in the release medium was determined by UV spectroscopy at a λmax of 301 nm. The release experiments of each sample were performed in triplicate, and average values were reported. The percent drug release was calculated using the calibration curve of the drug in the simulated salivary solution (pH 6.8) (CitationSemalty et al. 2008, CitationModgill et al. 2014).
In vivo study
The studies were carried out according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India (ISF/CPCSEA/IAEC/2014). The blood samples were taken from the ear vein route through a syringe (CitationSatishbabu and Srinivasan 2008, CitationPv et al. 2011, CitationPabreja et al. 2014). The study was performed in four different groups, each group being administered a different formulation, as follows:
F1: Nothing is put just below the tongue of the rabbit.
F2: A blank film is applied under the tongue of the rabbit.
F3: 200 mcg of granisetron HCl loaded in a film is applied below the/under the tongue of the rabbit.
F4: 200 mcg of granisetron H film-coated tablets are given orally to the rabbit.
Results and discussion
Transmucosal sublingual patches were successfully prepared by the solvent casting method. During the formulation, PVP K-30 was used, in association with HPMC K-4M. PVP K-30 provided a better dissolution profile; while addition of HPMC K-4M in the developed film may improve the mucoadhesivity, along with modulating the release of the drug. The solvent casting method selected is very simple and effective, to prepare uniform sublingual patches.
Optimization of the drug-loaded film
shows the influence of the polymer ratio on film uniformity. Based on the data, it can be seen that the formulation code GSF4 shows the best results, as compared to other formulations.
Table I. Influence of polymer ratio on film uniformity.
Characterization of drug-loaded film
shows the physical characterization (weight variation, thickness, drug content, folding endurance and tensile strength) of the optimized drug-loaded film. It was observed from the data that the uniformity of weight, thickness, folding endurance, and tensile strength were satisfactory with respect to variation of these parameters between films of same formulation. In the present study, 1.5 ml of PEG was found to be optimized, with respect to the polymer ratio selected for the formulation code GSF 4. As the concentration of HPMC increases beyond 100, there will be a significant increase in viscosity of the resultant solution, which in turn limits the plasticizing effect of PEG, which is also supported by the results of tensile strength and folding endurance, where the respective values were found to be decreased with increasing concentration of HPMC (CitationGarg et al. 2014).
Table II. Physical characterization of the optimized drug-loaded film.
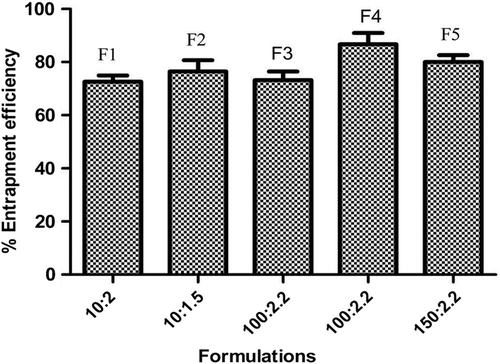
Drug entrapment efficiency
The entrapment efficiency of the sublingual film of granisetron HCl was estimated (). All the formulations showed good reproducible results. There are no significant changes observed in the DEE among different formulations; however a relatively higher entrapment efficacy in GSF 4 was found to be 86.73 ± 4.13, which could be associated with the better solubilizing potential of the formulation.
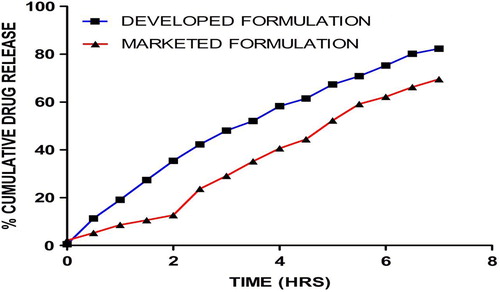
In vitro dissolution study
The data obtained from the in-vitro dissolution study of the developed formulations and the marketed formulation, are represented in . From the in-vitro drug release studies, it was found that of all the three sets of formulations (F1, F2, and F3), the developed formulation showed a high percentage of release of 82% in 7 h, followed by the plain formulation that showed 65% in 7 h, and the marketed formulation that showed 69% in 7 h. In vitro drug release studies clearly demonstrated a steady state release from the developed formulation under the simulated conditions, whereas an initial dump occurred in the marketed formulation, which could be associated to tablet disintegration. The drug release behavior, conceding an initial burst followed by controlled release, was found to be appropriate in the management of anti-emetic therapy. Further, the drug release profile follows first order kinetics (R2 = 0.9901), and shows that the rate of drug release depends on the concentration of drug.
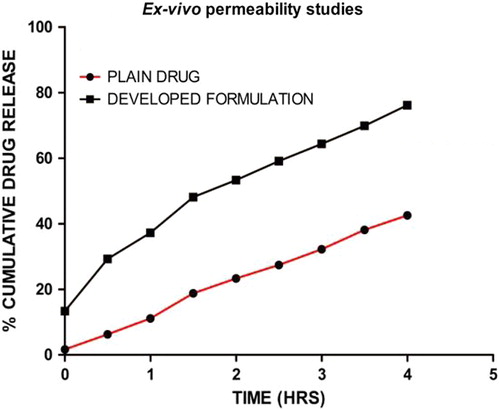
Ex vivo permeability study
The data obtained from the ex vivo study of permeability through the mucosal site are shown in . Studies of permeation through the oral mucosa indicated that the extent of permeation of the developed formulation showed the highest percentage of drug permeated, which was found to be 76.08% in 4 h, as compared to the corresponding value for plain drug, which was found to be 42% in 4 h. The higher drug permeability in the developed formulation could be related to PEG, because PEG, owing to its solubilizing properties, enlarges the aqueous channel which facilitates para-cellular transport of the drug. Further, the mucoadhesive nature of the film helps to maintain a concentration gradient of the drug at the absorption site for a sufficient time, necessary for drug absorption via the passive diffusion process.
Comparative study of in vivo bioavailability
A comparative study of in vivo bioavailability was done using New Zealand white rabbits (n = 6). The graphical representation of plasma concentration of granisetron HCl vs time, relating to the developed and marketed formulation, and a comparison of different pharmacokinetic parameters, are shown in and , respectively.
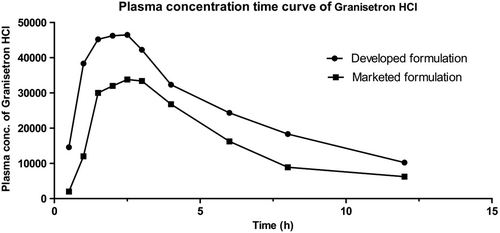
Table III. Comparison of pharmacokinetic parameters.
The oral bioavailability of the sublingual film of granisetron HCl was found to be 1.04-fold greater than that of the marketed formulation (film-coated tablets), which indicates that the bioavailability of the drug in sublingual film is maximum, when compared to that of the marketed formulation. The higher bioavailability of the drug in the developed formulation could be related to better drug absorption and the escape of first pass metabolism. Further, a higher Cmax indicated a higher fraction of drug available in the systemic circulation for a longer time, to produce the therapeutic activity. A study of the pharmacokinetics clearly demonstrated a better therapeutic management by granisetron HCl in the developed formulation.
Conclusions
It has been concluded that the granisetron HCl-loaded sublingual film was successfully formulated. The sublingual carrier system will help in achieving improved bioavailability by avoiding first pass metabolism, and resulting in an improvement in patient compliance. The incorporation of the sweetening agent, mannitol, helped to mask the taste.
Acknowledgements
The author, Dr. Amit K Goyal, is thankful to the Department of Biotechnology (DBT), New Delhi, India.
Declaration of interest
The authors confirm that the contents of this article do not have any conflicts of interest.
References
- Ahmed S, El-Setouhy DA, El-Latif Badawi AA, El-Nabarawi MA. 2014. Provesicular granisetron hydrochloride buccal formulations: In vitro evaluation and preliminary investigation of in vivo performance. Eur J Pharm Sci. 60:10–23.
- Chaudhary S, Garg T, Murthy RS, Rath G, Goyal AK. 2014. Recent approaches of lipid-based delivery system for lymphatic targeting via oral route. J Drug Target. 1–12.
- Gagandeep, Garg T, Malik B, Rath G, Goyal AK. 2014. Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur J Pharm Sci. 53:10–16.
- Garg T. 2014. Current nanotechnological approaches for an effective delivery of bio-active drug molecules in the treatment of acne. Artif Cells Nanomed Biotechnol.1–8.
- Garg T, Goyal AK. 2012. Iontophoresis: Drug delivery system by applying an electrical potential across the skin. Drug Deliv Lett. 2:270–280.
- Garg T, Goyal AK. 2014a. Biomaterial-based scaffolds–current status and future directions. Expert Opin Drug Deliv. 11:767–789.
- Garg T, Goyal AK. 2014b. Liposomes: targeted and controlled delivery system. Drug Deliv Lett. 4:62–71.
- Garg T, Goyal AK. 2014c. Medicated chewing gum: patient compliance oral drug delivery system. Drug Deliv Lett. 4:72–78.
- Garg T, Goyal AK, Arora S, Murthy R. 2012a. Development, optimization & evaluation of porous chitosan scaffold formulation of gliclazide for the treatment of type-2 diabetes mellitus. Drug Deliv Lett. 2:251–261.
- Garg T, Kumar A, Rath G, Goyal AK. 2014. Gastroretentive drug delivery systems for therapeutic management of peptic ulcer. Crit Rev Ther Drug Carrier Syst. 31:531–557.
- Garg T, Singh O, Arora S, Murthy R. 2012b. Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst. 29:1–63.
- Garg T, Singh S, Goyal AK. 2013. Stimuli-sensitive hydrogels: an excellent carrier for drug and cell delivery. Crit Rev Ther Drug Carrier Syst. 30:369–409.
- Goyal AK, Rath G, Garg T. 2013. Nanotechnological approaches for genetic immunization. In: Erdmann VA, Barciszewski J, Eds. DNA and RNA nanobiotechnologies in medicine: Diagnosis and Treatment of Diseases Berlin: Springer, pp. 67–120.
- Goyal G, Garg T, Rath G, Goyal AK. 2014a. Current nanotechnological strategies for an effective delivery of drugs in treatment of periodontal disease. Crit Rev Ther Drug Carrier Syst. 31:89–119.
- Goyal G, Garg T, Rath G, Goyal AK. 2014b. Current nanotechnological strategies for treating glaucoma. Crit Rev Ther Drug Carrier Syst. 31:365–405.
- Johal HS, Garg T, Rath G, Goyal AK. 2014. Advanced topical drug delivery system for the management of vaginal candidiasis. Drug Deliv. 1–14.
- Joshi D, Garg T, Goyal AK, Rath G. 2014. Advanced drug delivery approaches against periodontitis. Drug Deliv. 1–15.
- Kataria K, Sharma A, Garg T, Goyal AK, Rath G. 2014. Novel technology to improve drug loading in polymeric nanofibers. Drug Deliv Lett. 4:79–86.
- Kaur M, Garg T, Rath G, Goyal AK. 2014a. Current nanotechnological strategies for effective delivery of bioactive drug molecules in the treatment of tuberculosis. Crit Rev Ther Drug Carrier Syst. 31:49–88.
- Kaur M, Malik B, Garg T, Rath G, Goyal AK. 2014b. Development and characterization of guar gum nanoparticles for oral immunization against tuberculosis. Drug Deliv.
- Kaur N, Garg T, Goyal AK, Rath G. 2014c. Formulation, optimization and evaluation of curcumin-beta-cyclodextrin-loaded sponge for effective drug delivery in thermal burns chemotherapy. Drug Deliv. 1–10.
- Kaur P, Garg T, Rath G, Murthy RS, Goyal AK. 2014d. Surfactant-based drug delivery systems for treating drug-resistant lung cancer. Drug Deliv.1–12.
- Kaur R, Garg T, Das Gupta U., Gupta P, Rath G, Goyal AK. 2014e. Preparation and characterization of spray-dried inhalable powders containing nanoaggregates for pulmonary delivery of anti-tubercular drugs. Artif Cells Nanomed Biotechnol. 1–6.
- Kaur R, Garg T, Malik B, Gupta UD, Gupta P, Rath G, Goyal AK. 2014f. Development and characterization of spray-dried porous nanoaggregates for pulmonary delivery of anti-tubercular drugs. Drug Deliv. 1–6.
- Kaur R, Garg T, Rath G, Goyal AK. 2014g. Advanced aerosol delivery devices for potential cure of acute and chronic diseases. Crit Rev Ther Drug Carrier Syst. 31:495–530.
- Malik R, Garg T, Goyal AK, Rath G. 2014. Polymeric nanofibers: targeted gastro-retentive drug delivery systems. J Drug Target.1–16.
- Marwah H, Garg T, Goyal AK, Rath G. 2014. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 1–15.
- Mashru R, Sutariya V, Sankalia M, Parikh P. 2005. Development and evaluation of fast-dissolving film of salbutamol sulphate. Drug Dev Ind Pharm. 31:25–34.
- Modgill V, Garg T, Goyal AK, Rath G. 2014. Permeability study of ciprofloxacin from ultra-thin nanofibrous film through various mucosal membranes. Artif Cells Nanomed Biotechnol. 1–6.
- Morie A, Garg T, Goyal AK, Rath G. 2014. Nanofibers as novel drug carrier – An overview. Artif Cells Nanomed Biotechnol. 1–9.
- Nafee NA, Ismail FA, Boraie NA, Mortada LM. 2004. Mucoadhesive delivery systems. I. Evaluation of mucoadhesive polymers for buccal tablet formulation. Drug Dev Ind Pharm. 30:985–993.
- Nibha KP, Pancholi S. 2012. An overview on: sublingual route for systemic drug delivery. Int J Res Pharmaceut Biomed Sci. 3: 913–923.
- Nyamweya N, Hoag SW. 2000. Assessment of polymer-polymer interactions in blends of HPMC and film forming polymers by modulated temperature differential scanning calorimetry. Pharm Res. 17:625–631.
- Pabreja S, Garg T, Rath G, Goyal AK. 2014. Mucosal vaccination against tuberculosis using Ag85A-loaded immunostimulating complexes. Artif Cells Nanomed Biotechnol. 1–8.
- Panzade P, Heda A, Puranik P, Patni M, Mogal V. 2012. Enhanced transdermal delivery of granisetron by using iontophoresis. Iran J Pharm Res. 11:503.
- Prajapati V, Bansal M, Sharma PK. 2012. Mucoadhesive buccal patches and use of natural polymer in its preparation. Int J Pharm Tech Res. 4:582–589.
- Pv S, Kinagi M, Biradar S, Gada S, Shilpa H. 2011. Formulation design and evaluation of bilayer buccal tablets of granisetron hydrochloride. Ind J Pharm Edu Res. 45:242.
- Rohilla R, Garg T, Goyal AK, Rath G. 2014. Herbal and polymeric approaches for liver-targeting drug delivery: novel strategies and their significance. Drug Deliv.1–17.
- Satishbabu B, Srinivasan B. 2008. Preparation and evaluation of buccoadhesive films of atenolol. Indian J Pharm Sci. 70:175.
- Semalty M, Semalty A, Kumar G. 2008. Formulation and characterization of mucoadhesive buccal films of glipizide. Indian J Pharm Sci. 70:43.
- Sharma A, Garg T, Aman A, Panchal K, Sharma R, Kumar S, Markandeywar T. 2014a. Nanogel-an advanced drug delivery tool: Current and future. Artif Cells Nanomed Biotechnol. 1–13.
- Sharma R, Garg T, Goyal AK, Rath G. 2014b. Development, optimization and evaluation of polymeric electrospun nanofiber: A tool for local delivery of fluconazole for management of vaginal candidiasis. Artif Cells Nanomed Biotechnol.1–8.
- Sharma R, Singh H, Joshi M, Sharma A, Garg T, Goyal AK, Rath G. 2014c. Recent advances in polymeric electrospun nanofibers for drug delivery. Crit Rev Ther Drug Carrier Syst. 31:187–217.
- Singh B, Garg T, Goyal AK, Rath G. 2014a. Recent advancements in the cardiovascular drug carriers. Artif Cells Nanomed Biotechnol. 1–10.
- Singh H, Sharma R, Joshi M, Garg T, Goyal AK, Rath G. 2014b. Transmucosal delivery of Docetaxel by mucoadhesive polymeric nanofibers. Artif Cells Nanomed Biotechnol.
- Singh O, Garg T, Rath G, Goyal AK. 2014c. Microbicides for the treatment of sexually transmitted HIV infections. J Pharm.1–18.