Abstract
Research studies in recent years have found that isoquercetin has an inhibiting effect on multiple carcinogens, but research studies filed on isoquercetin in bladder cancer are quite few. This paper observed the influence of isoquercetin on biological activity of the EJ cell of bladder cancer through HC dyeing and trypan blue counting, studied the EJ cell cycle by flow cytometry (FCM), and then analyzed the influence of isoquercetin and its effect on the protein expression of STAT3 and STAT3-inhibiting factors (PIAS3) in EJ cells. Research has shown that isoquercetin has an inhibitory effect on the EJ cells of bladder cancer, but it is not obvious.
Introduction
In China, bladder cancer is the most commonly occurring malignant tumor of the urinary system, with about 0.356 million new cases annually (about 0.274 million are men and 0.082 million are women). At present, treatment of bladder cancer mainly depends on excision and chemotherapy combined with Chinese herbal medicine. If patients are treated with surgery only and without any drug therapy, then the post-surgical recurrence of bladder cancer is relatively high (CitationWang et al. 2011b). Due to characteristics of easy relapse, it is necessary to fill the bladder with medicine after surgery, so as to eliminate the remnant tumor, prevent relapse, and delay progress. Therefore, tumor researchers attach much attention to finding an effective drug that can strengthen immunity and reduce recurrence, but has less adverse side effect.
Marestail is a kind of ancient herb, with functions of protecting the liver, reducing blood pressure and blood fat, promoting diuresis, resisting inflammation, and relieving pain. Moreover, it can cure urinary system infections and bladder stone. Isoquercetin, extracted from marestail, is the main effective constituent of its medicinal value. Isoquercetin is a kind of flavonoid compound which is a natural antioxidant, existing in plants, vegetables, fruits, and Chinese herbal medicine, for example in onion, apple, grape, ginkgo, tea, etc. In recent years, research studies on isoquercetin by medical workers at home and abroad have been increasing day by day, and it has been found that isoquercetin has many biological activities, such as eliminating phlegm, relieving cough, reducing blood pressure, protecting against myocardial ischemia, easing pain, and resisting oxidation, as well as anti-tumor, anti-fibrosis and anti-inflammatory effects, etc. What's more, it is nontoxic and safe for humans (CitationLiu and Wei 2010, CitationQu et al. 2010, CitationChen et al. 2010).
Since it is well tolerated, isoquercetin is safe to be served as a food additive. Clinical tests abroad have proved that isoquercetin could inhibit the proliferation and induce the apoptosis of various tumor cells, including cells of leukemia, gastric cancer, breast cancer, colon cancer, lung cancer, glioma, pancreatic cancer, etc. However, its disadvantages like insolubility in water, poor lipid solubility, and lower oral bioavailability have greatly limited the clinical application of quercetin. Therefore, quercetin was transformed by increasing water soluble groups or creating various dosage forms, which have been used as medicine in European countries and administered as one of the preferred anticancer agents (CitationWang et al. 2011a, CitationLin et al. 2010b). Through in vitro experiments, researchers have proved that isoquercetin can inhibit bladder cancer (CitationChen et al. 2011). Based on the primary work, this research carried on the study on the effect of isoquercetin on the bioactivity of bladder cancer EJ cells, so as to complete the original literature.
Materials and methods
Source of materials
Human carcinoma of urinary bladder cell line in this experiment was provided by the cell bank of the Archives Center of a certain university research laboratory. The main reagents and equipment in this experiment are shown in .
Table I. Main equipments and reagents.
Cell culture
The cell line of bladder cancer was cultured in DMEM medium containing 10% fetal calf serum under 37°, then the medium was put into the incubator at a constant temperature, with 5% CO2. The culture solution was changed every 2 to 3 days. The medium was divided into 1/3 for passage. After trypsinization, the solution was placed in a refrigerator at − 20° for 1 h, and then stored in a cryogenic refrigerator at − 80° for a long time.
Experimental methods
Conventional observation: A sterilization cover glass was placed under the vessel, the concentration of carcinoma of urinary bladder EJ cells was adjusted to 2.0 × 104/ml, and inoculated into the culture dish. The culture solution was abandoned after cell stabilization, and then 100 μM of fresh culture solution of isoquercetin was added into the culture dish. A normal culture group was established for contrast. The change of cellular morphology was observed and photographed every 12 h, and the observation was continued for 48 h. Then, the cover glass was taken down and immersed into 1 × PBS (pH 7.4) and rinsed thrice, for 3–5 min each time. For fixation, 100% cold acetone was used, under − 20° for 20 min. It was then kept aside for HE dyeing and ICC analysis in the later part of the experiment, after natural airing.
Trypan blue counting: Another EJ cell culture fluid of bladder cancer at the concentration of 2.0 × 104/ml was obtained and inoculated into a 96-well culture plate; after cell adherence, fresh culture solution with 100 μM of isoquercetin was added so as to obtain the mixed cell culture fluid; cells were collected and counted every 12 h; the liquid was shaken within the hole of the 96-well plate so as to suspend cells; then, 40 μl of mixed cell culture fluid was immediately taken and intensively mixed with the isometric trypan blue dye liquor of same volume, and injected into a sanitized blood counting chamber; the number of dead and live cells were counted under a 40 × light microscope; the above operation was repeated thrice. The control group was simultaneously set up. Live cells were transparent, round and non-staining, while dead cells were mazarine and relatively small.
Flow cytometer (FCM) analysis: Bladder cancer EJ cells were cultured normally and treated with 100 μM of isoquercetin for 48 h; after that, the supernatant was abandoned and 250 μl of Solution A (trypsin buffer) was added; after shaking up the liquid, it was placed at room temperature for a 10 min reaction; next, 200 μl of Solution B (trypsin inhibitor and RNase buffer) was added, and the above process was repeated. In the next step, 200 μl of cold solution C (propidium iodide stain solution) was added and the whole culture was kept away from light, at a temperature of 4° in a refrigerator for 10 min; these samples were filtered into new tubes by a 50 μm nylon net. Finally, the cell cycle was analyzed.
HE dyeing: The cells were digested by pancreatin to a concentration of 1 × 105/ml; then the cells were rinsed thrice. Cold acetone was used for fixation under 20° for 20 min, and rinsed thrice using PBS; hematoxylin dyeing was performed, rinsed with water, and observed; next, eosin dyeing was performed and rinsed with water; neutral gum was used for mounting after air drying.
Immunocytochemistry analysis (ICC): The cells were incubated in 3% H2O2 and rinsed thrice with PBS. Then non-immune animal serum was added and incubated for 10–15 min. Primary antibodies (STAT3, PIAS3, 1:100) were added and incubated overnight under 4°. The cells were rinsed thrice with PBS, and then a second marked antibody was added and incubated for 30 min under 7°. After rinsing with PBS thrice, and adding horseradish peroxidase to label streptavidin, the cells were incubated for 10–15 min under 37°. DAB dyeing was performed after rinsing them with PBS thrice. After that, the cells were rinsed and dyed again. Finally, they were dehydrated, mounted, and observed.
Statistical approaches
Computer statistical software SPSS19.0 was adopted for statistical analysis of the distribution of STAT3 in bladder cancer and pericarcinomatous tissue. A value of p < 0.05 was considered as significant difference.
Results
Influence of isoquercetin on bladder cancer cell
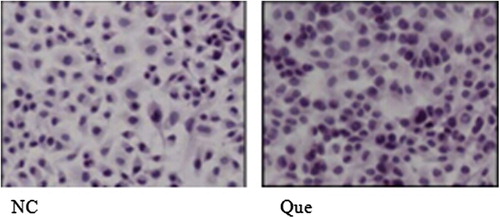
Observation results of HE dyeing morphology after isoquercetin treatment: Carcinoma of urinary bladder EJ cell of normal culture was satiated and intensive; after the cells were treated for 48 h with 100 μM of isoquercetin, no obvious phenomenon of apoptosis appeared (). Here, NC refers to the 48-h normal culture group while Que refers to the group treated with 100 μM of isoquercetin for 48 h.
Trypan blue counting analysis
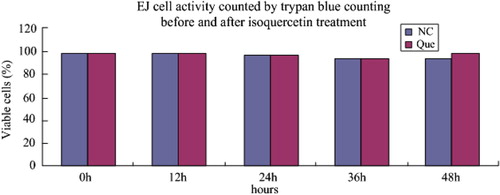
After the bladder cancer EJ cells were treated with 100 μM isoquercetin for 0, 12, 24, 36 and 48 h respectively, trypan blue was used for dyeing. Cell counting plate was used to collect live cells and dead cells at different time points. Cell activity was counted by the following formula: live cell ratio (%) = total cellular score/(total live cellular score + total dead cellular score) × 100%. At the same time, the control group of normal culture was established. The results are shown in and .
Table II. Cell activity after isoquercetin treatment at different time points (%).
From and , it is clear that the cell activity, both after treatment with isoquercetin and under normal cultivation, declined with the extension of culture time. The descent velocity of cell activity under normal cultivation was no different from that after treatment with isoquercetin, which indicates that isoquercetin has no obvious effect on cell apoptosis.
Results of FCM diagnosis
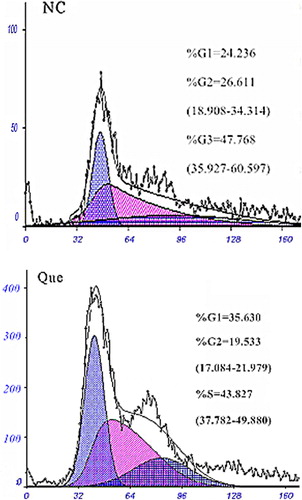
The results of FCM detection are shown as follows (). The proportions of bladder cancer EJ cells in normal culture in the G1, G2, and S phases were respectively 24.236%, 26.611%, and 47.768%; after treatment with 100 μM isoquercetin for 48 h, the proportions were 35.63%, 19.533%, and 43.827%. The cell proliferation index (PI)% = (S + G2/M)(G0/G1 + G2/M) × 100%. The PI of the two groups after normal culture and after treatment with 100 μM isoquercetin were respectively 74.3% and 63.4%. NC refers to the 48-h normal culture group, while Que refers to the group treated with 100 μM isoquercetin for 48 h.
Function of STAT3 signal path in bladder cancer
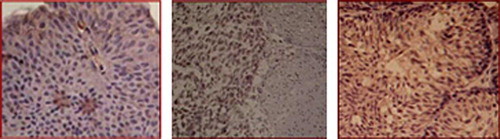
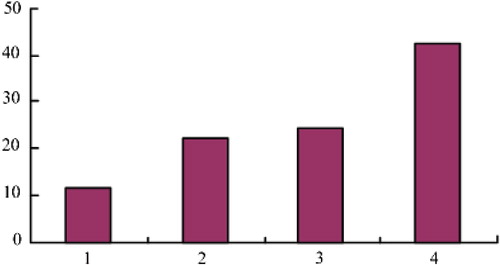
The results of dyeing by the immunohistochemistry method showed that STAT3 was mostly positively expressed in carcinoma of urinary bladder cytoplasm and karyon. The appearance of claybank to sepia particles in the karyon demonstrated positive staining, as shown in . In bladder cancer tissue, the positive expression rate of STAT3 was 11.6%. In , ,,, and 4 refer to negative (-), weak positive (±), positive (+) and strong positive (≧++), respectively.
ICC detection of STAT3 in EJ cell
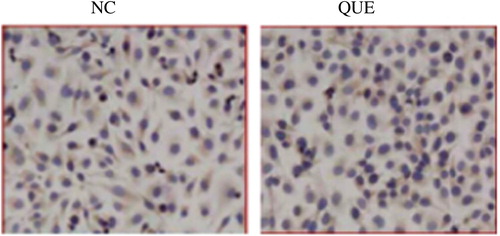
The immunity test showed that under normal culture, STAT3 presented positive expression in EJ cell cytoplasm and had slight expression in the karyon. After isoquercetin treatment, STAT3 expression was mainly in the cytoplasm (). Here, NC refers to the 48-h normal culture group, while Que refers to the group treated with 100 μM isoquercetin for 48 h.
ICC detection of PIAS3 in EJ cell
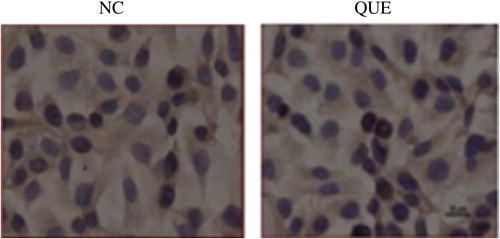
The results showed that under normal circumstances, PIAS3 presented positive in cell cytoplasm, while negative in karyon. The treatment with 100 μM isoquercetin was mainly performed in cytoplasm, and no karyon translocation phenomenon appeared (CitationSun and Yu 2011, ). Here NC refers to the 48 h normal culture group while Que refers to the group treated with 100 μM isoquercetin for 48 h.
Discussion
Isoquercetin widely exists in various plants, and can inhibit the growth of various malignant tumors in in vitro experiments, such as breast cancer, gastric cancer, leukemia, etc. Its mechanism of action might be associated with the following factors: detention of cell cycle, reduction of cancer cell apoptosis and differentiation, inhibition of cancer cell signaling, etc. The previous research studies on isoquercetin mainly focused on the diseases related to estrogen, such as breast cancer, cervical cancer, etc. However, fewer research studies and reports were about the effect of isoquercetin on human bladder cancer. This paper found that isoquercetin has the property of tolerance to bladder cancer EJ cells, which also hinted that the effect of isoquercetin on bladder cancer cell might be influenced by many factors.
The experimental results showed that in HE dyeing, there were no obvious changes in cell morphology of bladder cancer EJ cells after isoquercetin treatment, and these cells covered the slide. Trypan blue counting showed that there was no obvious death of cells after treatment. All of these suggested that isoquercetin could not inhibit the proliferation of bladder cancer EJ cells. However, it was proved that human bladder cancer BIU-287 cells, after isoquercetin treatment in vitro, had an inhibiting effect on cancer cells (CitationMei et al. 2010). The experimental result showed in this paper, that isoquercetin could not inhibit the proliferation of bladder cancer EJ cells, was quite different from the result of the experiment performed by CitationChen et al. (2011).
In in vitro experiments, the effect of isoquercetin is diverse in the estrogen-related diseases, including inducing cell apoptosis and regulatory metabolism, and regulated by estrogen. Research studies have demonstrated that isoquercetin, in a certain dose, could promote the proliferation of MCF-7 cell in the estrogen receptor positive cell line. However, there was no proliferation of MDA-MB231 cells in the estrogen receptor negative cell line, indicating that isoquercetin has the effect of estrogen and influences the index of cell division growth(CitationYang et al. 2011). Isoquercetin and the relevant portion combined with estrogen receptor could regulate receptor and signal path, but its inhibiting effect on the receptor is influenced by many factors, and the answer to whether it has functions of antagonism or incitation on the receptor is awaiting further research. As for bladder cancer, its ER expression is positively correlated to pathological grading and clinical stages, and closely related to high-grade bladder cancer and infiltrating bladder cancer (CitationLi and Wang 2012). ER is the receptor of bladder cancer RT4 cell line, and its antergic or excited receptor could have the effects of inhibiting and promoting cell growth (CitationLi and Yang 2010). In this experiment, whether the unobvious performance was caused by the balance between the promotion and suppression of cancer has not been detected. However, part of cells were blocked by isoquercetin to G1 phase, and the index of cell proliferation was decreased. It indicates that isoquercetin could affect bladder cancer EJ cells, but is insufficient to inhibit cell proliferation.
In addition, isoquercetin has the effect of oxidation and removal. There were research studies showing that isoquercetin has antagonistic action on the myocardial cell toxin of adriamycin to different extents, thereby increasing cell survival rate, and in addition, 100 μM isoquercetin had no obvious toxic and side effect on normally cultured cardiac myocytes from the neonatal rat (CitationTang et al. 2012). It is clear that 100 μM isoquercetin has a protective effect on cells, which enriches the variety and mechanisms of the antitumor effects of isoquercetin. From now on, we will further analyze the different effects of isoquercetin on human bladder cancer cell line, study the sensitivity and mechanism of tolerance of the cell line to isoquercetin, and discuss the utility level and mechanism of its antioxidation and estrogen receptor-regulating effect in the bladder cancer cell line.
As the intracellular signal of cells, STAT3 regulates and controls the cell proliferation, differentiation, and apoptosis. If the pathway is activated continuously, then STAT3 would be involved in the occurrence, development and evolution of malignant tumor (CitationLin et al. 2010a, CitationLi et al. 2010). The experimental results showed that the positive expression rate of STAT3 was 88.4%, which was basically consistent with the result that the high expression of bladder cancer coordinated with STAT3, as concluded by CitationZeng and Zhang (2010).
CitationSun Ying et al. (2010) adopted siRNA technique to silence the STAT3 gene and found that the growth of the human gastric carcinoma cell was greatly inhibited, and cell apoptosis was promoted. In the experiment, after isoquercetin intervention, the expression of STAT3 in bladder cancer EJ cells was not inhibited, and no obvious inhibition on cell activity appeared. Thus, it was concluded that isoquercetin did not intervene in the STAT3 pathway in the aspect of STAT3 expression. As the profiling of activation of STAT3 protein, its expression rate, and level of PIAS on normal bladder epithelium, was obviously lower than that in bladder cancer tissue, its expression level would increase with the decrease of differentiation degree. Through the observation in this experiment, under normal culture conditions, PIAS3 expressed highly in EJ cell cytoplasm, while it was negative in the karyon. After 100 μM isoquercetin treatment, there was no karyon translocation of PIAS3. PIAS3 was not able to play its inhibitory effect on p-STAT3. Because PIAS3 did not enter into the karyon, isoquercetin could not inhibit the STAT3 signal pathway through the PIAS3 pathway. To sum up, isoquercetin did not inhibit the STAT3 signal pathway of bladder cancer EJ cells, which may be one of the reasons that cause the tolerance of EJ cells to isoquercetin.
Conclusion
In the treatment of bladder cancer, isoquercetin could change the cell activity through changing the index of EJ cell proliferation and cell cycle. The cell activity after HE dyeing and counting by trypan blue showed no evident changes. Expression of STAT3 in human bladder cancer tissue tends to show high expression. However, isoquercetin showed no translocation to PIAS3 in EJ cells, indicating that isoquercetin has no obvious inhibitory effect on the STAT3 signal pathway. Research studies have shown that isoquercetin had no obvious inhibitory effect in changing cell proliferation, cell cycle and its treatment to PLASS, which might be related to the diversification of isoquercetin in disease. It was found that there was no special effect of isoquercetin in bladder treatment, though there is great potential for quercetin in the treatment of bladder cancer. It is supposed that it might be related to the structure of isoquercetin. The mechanism of action of isoquercetin on the bladder cancer cell line differs from that on other cells, or it might be related to the sensitivity and drug resistance of the cell line to isoquercetin. In short, treatment of bladder cancer with isoquercetin needs further study.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Chen C, Zhou J, Ji CY. 2010. Quercetin: A potential drug to reverse multidrug resistance. Life Sci. 87:333–338.
- Chen JL, Liu JJ, Huang YS, Chen SN, Chen JH. 2011. Research of quercetin and cisplatin on the tumor suppression of human bladder cancer nude mouse transplantation tumor. Guangdong Medical Journal. 32:2500–2504.
- Li F, Rajendran P, Sethi G. 2010. Thymoquinone inhibits proliferation, induces apoptosis and chemosensitizes human multiple myeloma cells through suppression of signal transducer and activator of transcription 3 activation pathway. Br J Pharmacol. 161:541–554.
- Li H, Yang JJ. 2010. Recent progress in research of the relationship between bladder cancer and sexual hormone receptors. Journal of Southeast University 29:352–354.
- Li QH, Wang XH. 2012. Expression of Ang-2 and HIF-1α in transitional cell carcinoma of urinary bladder. China Medicine and Pharmacy. 2:26–28.
- Lin CM, Shyu KG, Wang BW, Chang H, Chen YH, Chiu JH. 2010a. Chrysin suppresses IL-6-induced angiogenesis via down-regulation of JAK1/STAT3 and VEGF: an in vitro and in vivo approach. J Agric Food Chem. 58:7082–7087.
- Lin ZH, Meng Y Ma T. 2010b. Research status of quercetin on tumor. J Pract Med. 26:3446–3447.
- Liu MX, Wei CH. 2010. Pharmacology effect of isoquercetin and its clinical application prospect. Chinese Pharmacy. 21:2581–2583.
- Mei ZQ, Liu XY, Duan C, Xie HF, Li J, Wang L, Yu HQ. 2010. Research of quercetin inhibition on human bladder cancer cell BIU-87 growth and reduction on its apoptosis. Journal of Modern Medicine & Health. 26:1538–1539.
- Qu WT, Zhu W, Zhai GY, Yan ZT. 2010. Research progress of synthesis and biological activity of quercetin derivatives. Chemical Research. 23:101–110.
- Sun J, Yu SC. 2011. Research progress of quercetin. Research and Practice of Modern Medicine. 25:85–88.
- Sun Y, Yu H, Zhang L, Gao LF, Zhao XJ. 2010. Silencing of STAT3 by RNAi inhibits the growth of SGC-7901 cells and induces apoptosis in vitro. Chinese Journal of Pathophysiology. 26:393–395.
- Tang XL, Liu JX, Li P, Li L, Ma YL, Shi Y, Li R. 2012. Protective effects of kaempferol and quercetin on hypoxia/reoxyenation and peroxidation injury in neonatal cardiomyocytes. Pharmacology and Clinics of Chinese Materia Medica. 28:56–59.
- Wang G, Du SM, Yang GY. 2011a. Research progress of the antineoplastic molecular mechanism of quercetin. Chinese Journal of Hospital Pharmacy. 31:322–324.
- Wang JW, Jiang Q, Fan XD, Sun CY. 2011b. Meta analysis on comparison of curative effect and toxicity of prevention after superficial bladder cancer operation of early pirarubicin bladder irrigation and conventional bladder irrigation. Journal of Modern Urology. 2:139–142.
- Yang Y, Cao Y, Zhang TT, Zhu Y, Cao L. 2011. The pharmacologic actions progression of quercetin on estrogen related diseases. Journal of International Reproductive Health/Family Planning. 30:69–72.
- Zeng WL, Zhang DL. 2010. Expression and clinical significance of STAT3 in bladder transitional carcinoma. Journal of Mathematical Medicine. 23:1–313.