Abstract
The extracellular environment is a complex network of functional and structural components that impart chemical and mechanical stimuli that affect cellular function and fate. Cell differentiation on three dimensional scaffolds is also determined by the modulus of the substrate. Electrospun PCL nanofibers, which mimic the extra cellular matrix, have been developed with a wide variety of solvents and their combinations. The various studies have revealed that the solvents used influence the physical and mechanical properties, resulting in scaffolds with Young's modulus in the range of 1.8–15.4 MPa, more suitable for engineering of hard tissue like bone. The current study describes the use of benign binary solvent-generated fibrous scaffolds with a Young's modulus of 36.05 ± 13.08 kPa, which is almost 50 times lower than that of scaffolds derived from the commonly used solvents, characterized with myoblast, which can be further explored for applications in muscle and soft tissue engineering.
Introduction
Three dimensional (3D) cell cultures are proving invaluable for several applications, and this approach has given hope for situations that were hitherto impossible, such as for the repair or replacement of irreversibly damaged, fully differentiated, specialized tissue. Several factors are important for 3D cell cultures, which can extend their utility for tissue engineering purposes. Two major parameters are the matrices and the choice of cells that are used. Of these, the matrix is of primary importance, as a suitable and flexible matrix can support a wide variety of cell and tissue lineages. Thus, the biocompatible nature, the physical and mechanical properties, and the ease with which a material can be molded into a 3D cell culture matrix, hold the key for further advancements in this new area of experimental science, with far reaching health care implications. Recent research emphasizes matrix elasticity towards cell differentiation of organ-specific lineage. The behavior of cells on 3D scaffolds is not only dependant on extracellular matrix (ECM) adhesion but also on the stiffness and elasticity of the materials. CitationEngler et al. (2006), confirmed the sensitivity of matrix elasticity to the specificity of stem cell lineage. They observed that when the matrix elasticity mimics the brain, with a modulus of 0.1–1.0 kPa, it is suitable for directing a stem cell population towards neuronal lineage. When the stiffness measures between 8 and 17 kPa, the cells take up a myogenic lineage, while an osteogenic lineage is induced when the modulus measures 25–40 kPa (CitationEngler et al. 2006).
Several other studies also have shown that the modulus of the matrices is also a determining factor in cell migration, proliferation, organization, and differentiation. Fibroblasts displayed significant differences in migration patterns with changes in density and stiffness of the matrices (CitationZaman et al. 2006). Neonatal ventricular cardiomyocytes cultured on substrates of ∼10 kPa developed aligned sarcomeres and produced maximal force, whereas those on stiffer scaffolds had more stress fibres and unaligned sarcomeres (CitationJacot et al. 2008). The morphology and function of neural stem cells is also affected by changes in the elastic modulus of the scaffolds (CitationSaha et al. 2008). Human bone marrow stromal cells cultured on matrices with varying moduli underwent osteogenic differentiation in the absence of osteogenic differentiation supplements, and the effect was more pronounced on stiffer substrates (CitationParekh et al. 2011).
Electrospinning, a technique patented in 1934 by Formhals, is commonly used to create fibrous scaffolds from nanofibers that are synthetic, natural or a combination of both, with a nanotopography that mimics the native environment and promotes cell spreading and proliferation. Nonwoven matrices have been well-studied as promising materials, and have been developed for a wide range of medical applications, such as wound dressing, artificial skin, and heart valves (CitationPham et al. 2006). Polycaprolactone (PCL) is a synthetic polymer that has been approved by the FDA; its properties of solubility, elasticity, inertness, and slow rate of degradation into non toxic products make it favorable for use in regenerative medicine (CitationSun et al. 2006, CitationCiardelli et al. 2005). In general, electrospun PCL matrices tend to have increased stiffness and more rigidity (CitationGunatillake and Adhikari 2003), and are more favorable for hard tissue engineering applications (CitationMiddleton and Tipton 2000). The choice of solvent is essential for nanofiber generation, as the solvent influences the physical and mechanical properties of the electrospun PCL (CitationMondal 2014, CitationAsran et al. 2010). Some commonly used solvents for electrospinning PCL are acetone, dichloromethane, methanol, chloroform, dimethylformamide, and acetic acid (CitationJohnson et al. 2009). Fluoroalcohols, such as Hexafluoroisopropanol (HFIP) and Trifluoroethanol (TFE), have also been used to generate nanofiber scaffolds with natural polymers (CitationThakkar et al. 2013, CitationZhang et al. 2005). Min Sup Kim et al. have also reported electrospinning of PCL composites using HFIP and Trifluoroacetic acid (TFA) (CitationKim et al. 2012a). Both HFIP and TFE are expensive solvents, and along with TFA, are also corrosive and not environment-friendly. There is also evidence to suggest that the integrity of the natural polymer might be affected during the electrospinning process when using fluoroalcohols as solvents (CitationZeugolis et al. 2008). The blending of natural polymers with PCL is primarily to provide bioactive surfaces for improved adhesion. However, the cost, source, batch to batch variability, and shelf life of the biopolymers are also factors to be considered.
PCL scaffolds generated using the above mentioned solvents were used in experimental approaches for applications in tissue engineering, such as hyaline cartilage, autologous chondrocytes, and bone replacement (CitationKim et al. 2012b). Mesenchymal stem cells seeded on electrospun PCL scaffolds and cultured in a rotating bioreactor displayed matrix mineralization and deposition of Type I collagen after 4 weeks (CitationYoshimoto et al. 2003). Analysis of similar constructs in vivo in rats revealed osteoblast-like cells in multiple layers, with a woven bone-like structure coupled with the presence of osteocyte-like cells within the mineralized matrix (CitationShin et al. 2004b). Bovine fetal chondrocytes were able to preserve their chondrocytic morphology on PCL nanofibers during 3 weeks in culture, and also showed an upregulated expression of Collagen type II B, a marker for mature chondrocytes (CitationLi et al. 2003). PCL nanofiber scaffolds seeded with bone marrow-derived mesenchymal stem cells differentiated into chondrocytes in the presence of transforming growth factor beta. The constructs also displayed a typical zonal morphology with a cartilaginous matrix of type II collagen, aggrecan, and cartilage proteoglycan link protein (CitationLi et al. 2005). The various studies elucidate that nanofiber scaffolds of PCL have been widely investigated for engineering of tissues where the native elastic modulus is above 1 MPa, such as bone, cartilage, ligament, tendon, trileaflet valve (CitationGaudio et al. 2008), and vascular tissues.
The current study describes the process used to generate electrospun matrices of PCL with a Young's modulus of elasticity 50 times lower than that of scaffolds derived from the commonly used solvents (). The solvent used was a binary solvent comprising acetic acid and dimethyl sulfoxide. Scanning electron microscopy (SEM) of the electrospun matrices revealed an intricate network of random fibers with fiber diameters ranging from 200 to 600 nm. Myoblasts seeded on the PCL nanofibers proliferated well, as ascertained by the viability assay, and were found to maintain their phenotype for longer time points. Fluorescence microscopy analysis of immuno-stained sections revealed that the scaffolds were strongly positive for muscle-specific actin and desmin. The lower modulus of elasticity of the PCL nanofiber scaffolds generated make them interesting for potential applications in muscle, vascular, and soft tissue engineering applications.
Table I. Tensile property of PCL with different solvents.
Materials and methods
Electrospinning of polycaprolactone
Polycaprolactone (PCL) (Mw-80,000) was procured from Sigma Aldrich, and the solvents acetic acid and dimethylsulfoxide (DMSO) were of analytical grade and were obtained from SRL, Mumbai and Merck, USA. PCL was dissolved in a binary solvent of acetic acid and DMSO (93:7), and homogenous solutions of 10, 12 and 15% (weight/volume) were prepared. The electrospinning apparatus was a custom set up consisting of a stabilized high voltage supply, Premier Combines, Chennai, India; a programmable peristaltic pump, Ravels Tech, Chennai, India; silicone tubing, a 26-G needle and a grounded metal plate as a stationary collector. The PCL solutions were maintained at a flow rate of 0.2 ml/min, and nanofibers were generated at 16 KV and collected onto a stationary collector. The distance between the needle and the collector plate was 23 cm. Prepared scaffolds were cut into 1 cm squares, sterilized by ethanol treatment and stored in sterile PBS.
Characterisation of electrospun polycaprolactone membranes
Scanning electron microscopy and measurements. Electrospun PCL scaffolds of 3 different concentrations – 10, 12 and 15%, were morphologically evaluated by SEM (S-3400NSEM). The matrices were fixed and dehydrated by serial dilutions of ethanol. They were sputter-coated with gold before being analyzed. Digital images of 3 different scaffolds were captured, and the average fiber diameters were measured using ImageJ Software, from 30 measurements chosen at random across each image set. The porosity of the scaffolds was analyzed by examining the SEM images, using ImageJ software as previously described. [24] The SEM images with a gray scale level of 256 were converted to binary images by calculating the threshold, and the porosity of the scaffolds was measured using the formula , where n represents the number of white pixels, N is the total number of pixels in the binary image, and P is the percentage of porosity of the binary image. The 3 thresholds that aid in classifying various layers of the nanofiber mat were selected as described by (CitationMobarakeh et al. 2007), and their porosity percentages were measured as P1, P2 and P3. Four SEM images of magnifications of 2000 and 5000 were analyzed for P1, P2 and P3, and the overall percentage of porosity was estimated by calculating the mean and standard deviation ().
Table II. Porosity measurement of binary images with 3 different thresholds.
Tensile property
PCL nanofibers were collected for 4 h and prepared into strips with dimensions of 30 mm in length and 15 mm in width, and stored in PBS. The mechanical properties were measured using a universal tensile tester (INSTRON 3365) with a 10 N load cell, and measured at a speed of 1 mm/min (CitationZorlutuna et al. 2009) (). A total of 4 samples were tested and the mean and standard deviation were calculated.
Table III. Tensile property of the nanofibrous matrices collected for 4 h.
MTT assay
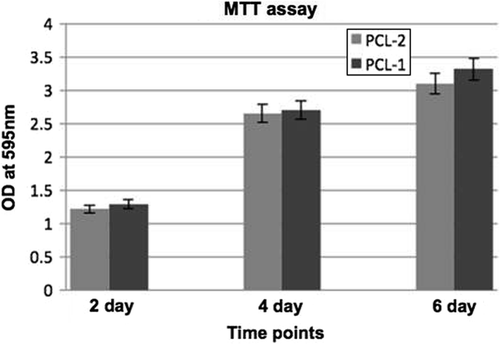
PCL scaffolds were cut into 1 cm squares, washed in three washes of sterile PBS, followed by sterilization with serial dilutions of ethanol. Scaffolds were washed well with sterile PBS and stored in PBS supplemented with antibiotics, at 4°C. Rat skeletal L6 myoblasts were cultured in DMEM high glucose medium supplemented with 10% fetal bovine serum and antibiotics. Bovine collagen (0.2%) was coated on PCL scaffolds prior to cell seeding for 1 h. L6 cells were seeded onto scaffolds in non-adherent culture dishes at a seeding density of 2.5 × 104 cells/scaffold and incubated at 37°C in an atmosphere containing 5% carbon dioxide. The culture medium was refreshed every 2 days. The MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide, a tetrazole) test assesses the cytotoxicity by measuring the cell viability and proliferation (CitationMi et al. 2000). After culturing for 2, 4 and 6 days, the scaffolds were transferred to fresh wells of a 12-well plate and incubated for 4 h with 1 ml of medium and 100 μl of MTT reagent at 5 mg/ml. After 4 h, the medium was discarded and 500 μL of DMSO was added, incubated for a further hour, and the resulting mixture was gently shaken for 10 min. Then, 200 μL of the solutions were removed and measured at 595 nm using a spectrophotometer.
Immunofluorescence staining with muscle-specific actin
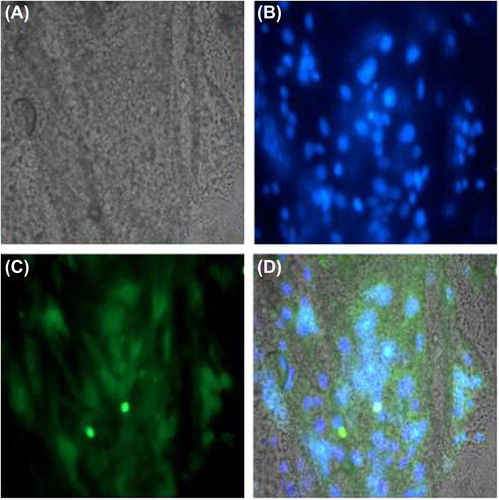
Seeded scaffolds were cryopreserved for a 6-day time point and cryosectioned. The sections were fixed with 3.7% formaldehyde for 10 min, rinsed twice with PBS, and permeabilized with 0.5% triton X 100 in PBS for 10 min. After blocking for 1 h, monoclonal anti muscle-specific actin antibody (Biogenex, USA) was added at (1:50) and incubated overnight. Sections were washed well and stained with Goat anti Mouse FITC (Merck), counter stained with DAPI to highlight the nuclei, and analyzed on a Zeiss LSM 510 Laser Scanning Confocal Microscope. The slides were rinsed in PBS and followed by nuclear staining with DAPI at 5 μg/μl added for 30 min. The slides were then observed on a Nikon TE Eclipse inverted fluorescence microscope, and images were analyzed using Image Pro Software.
Reverse transcription polymerase chain reaction (RT-PCR)
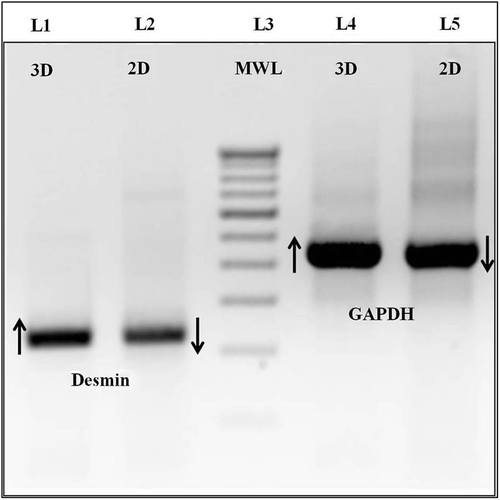
Using RNA Plus (MP Biomedicals), total RNA was isolated and converted to cDNA using the Verso cDNA synthesis kit (ABgene) according to manufacturer's protocol, from PCL matrices seeded with myoblasts for 6 days. Polymerase chain amplification (PCR) was performed as follows: 94°C for 30 s for denaturation, 60°C and 63°C for 30 s for annealing of GAPDH and desmin respectively, and 72°C for 30 s for extension. PCR was carried out with 35 cycles, which were analyzed using agarose gel electrophoresis. GAPDH primers 5’-ACC ACA GTC CAT GCC ATC-3’ (forward) and 5’-TCC ACC ACC CTG TTG CTG-3’ (reverse) with 556bp product size; desmin primers 5’-CAA CCT TCC GAT CCA GAC CT-3’ (forward) and 5’-GAG TGG AAA AGG CTG GCT TC-3’ (reverse) with 224 bp product size.
Statistical analysis
All numerical data were expressed as mean ± standard deviation (SD).
Results
Characterization of PCL scaffolds
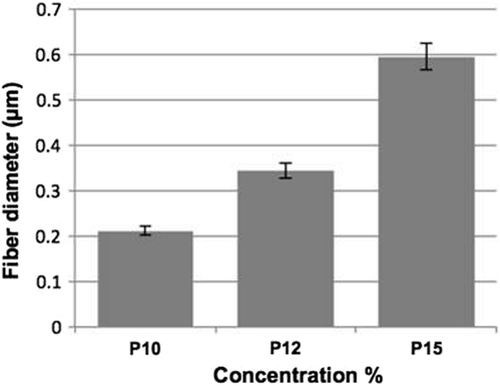
PCL fibers were generated in all three concentrations that were tested. However, the scaffolds varied in optimal characteristics. Beaded fibers were observed at the concentration of 10% and were not present at higher concentrations. The fiber diameters were increased at higher concentrations. 10% PCL matrices showed fiber diameter ranging from 150 to 210 nm, 12% PCL matrices showed fiber diameter ranging from 200 to 350 nm, and 15% PCL matrices showed fiber diameter ranging from 250 to 595 nm (). The average fiber diameter of the three different concentrations, plotted as a graph, showed the increase in fiber diameter (). Scaffolds generated with 12% polymer concentration were chosen for further tests. Porosity measurements carried out using the methods of Mobarakeh et al. revealed an average porosity percentage of 52.8 ± 2.36 (), which is optimal for tissue engineering applications. Tensile measurements of PCL scaffolds revealed a remarkably low modulus of elasticity with a mean value of 36.05 ± 13.38 kPa (). The mechanical properties of the PCL scaffolds indicate that they are ideal for soft tissue engineering applications.
Biocompatibility assays
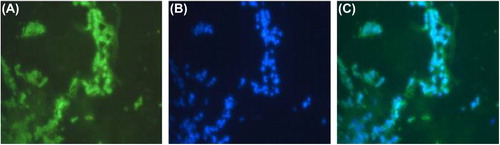
Rat skeletal myoblasts adhered onto PCL scaffolds and proliferated well, as examined by the MTT assay. The average was 3 samples per scaffold, at 2, 4, and 6-day time points (). The seeded scaffolds were compatible and the 3D environment was optimal for the proliferation of muscle cells, as analyzed over defined time points of 2, 4, and 6 days, with no reduction in colorimetric signal. Cryosections of the seeded scaffolds on day-6 revealed the presence of muscle-specific actin and desmin, indicating that the matrices were conducive for maintaining the native architecture of myoblasts ( and ). This was also confirmed by gene expression of desmin on 3D PCL matrices using Reverse Transcription Polymerase Chain Reaction (RT-PCR) (). Briefly, 250 ng of cDNA of day-6 of matrices with myoblasts were harvested and compared with 2D cultures of the same time point, for expression levels of both desmin and GAPDH, as described. We observed that there was moderate increase in expression levels of desmin in 3D PCL matrices when compared to the 2D culture, showing active expression of factors important for the function of muscle. Our study confirms that the electrospinning of polycaprolactone using acetic acid and DMSO can generate favorable nanofiber matrices with suitable modulus for application in muscle tissue engineering applications.
Discussion
The physical and mechanical properties of the scaffolds are dependent on the choice of the solvent. We have shown in our work that it is possible to generate nanofibers of PCL using an economical and benign solvent combination, which also results in matrices with a reduced modulus of elasticity, thus making it favorable to use the FDA-approved biomaterial PCL in soft tissue engineering applications. CitationKanani and Bahrami 2011, studied the different solvents and their combinations, such as glacial acetic acid, 90% acetic acid, methylene chloride/dimethyl formamide, and formic acid/acetone, and compared them with the morphology of the PCL nanofibrous matrices; they concluded that the usage of glacial acetic acid creates a non uniform fiber diameter (CitationKanani and Bahrami 2011). CitationSchueren et al. (2011), also evaluated the morphology with solvents such as chloroform, methanol, ethanol, and formic acid/acetic acid, and concluded that the optimal solvent combination is a mixture of formic acid and acetic acid. A review of the literature shows that ideal solvents for electrospinning PCL are dichloromethane, tetrahydrofuran, trifluoroethanol and hexafluoro isopropanol, which are generally considered as toxic and not environmentally friendly solvents (CitationSchueren et al. 2011). CitationJuliana Dias et al. (2013), conducted a study with acetic acid and triethylamine, and also emphasized the accuracy of the polymer concentration. The benign binary solvent combination, acetic acid and dimethyl sulfoxide, used to electrospin PCL, resulted in beaded fibers at lower concentrations and smooth nanofibers at higher concentrations of the polymer. The matrices prepared from a 12%wt concentration had smooth fibers without beads between 200 and 350 nm, with a modulus 50 times lower when compared with those generated by other groups with commonly used solvents, as reported in the literature (). The novelty in the study is the preparation of PCL substrates of lower modulus, without blending with natural polymers, which can be beneficial in soft tissue engineering.
Desmin is an intermediate protein of the contractile filament which is expressed in all stages of the development of muscle, seen in both proliferating as well as in differentiated muscle. It connects different components in cytoplasm, to maintain the cell architecture and structure with the contractile apparatus. In the current study, myoblast-seeded electrospun PCL nanofibrous matrices showed an appreciable increase in proliferation upto 6 days in growth medium, in comparison with the 2D culture. Boontheekul et al., showed C2C12 cell adherence, spread, and proliferation on hydrogel matrices, with a stiffness between 1 and 45 kPa, in contrast to the primary myoblast which showed adherence and proliferation only on matrix modulus between 12 and 45 kPa and not in the 1–10 kPa range (CitationBoontheekul et al. 2007). The current study establishes electrospun PCL matrices by using acetic acid and dimethyl sulfoxide with a modulus of elasticity of 36.05 ± 13.38 kPa, which is compliant for myoblast cultures, and also in the range for primary myoblast culture. Generally, such a low modulus is achieved with hydrogels, but we were able to establish such a low modulus in nanofibrous scaffolds, which can be effectively used as a tissue patch. Seeded matrices actively express muscle actin and desmin after a week in culture, and RT-PCR results revealed a healthy expression of desmin on day-6. This correlates well with the study conducted by Boontheekul et al., and the elasticity of the matrices was found suitable for 3D muscle culture. Shin et al. have shown contracting cardiomyocytes on PCL nanofibrous membranes, with the thickness of 10 μm, and concluded that the stacking of cell sheets would potentially lead to a cardiac graft (CitationShin et al. 2004a, CitationIshii et al. 2005). Our PCL substrates generated with the benign solvent combination thereby open the possibility for their use in soft tissue and organ-specific tissue engineering applications.
Conclusion
Our study concludes that the synthetic polymer PCL can be fabricated into scaffolds with tensile properties suitable for muscle and soft tissue engineering applications without blending with natural polymers. The benign solvent combination is non toxic and economical, and can electrospin matrices of low modulus of elasticity, similar to hydrogels, and can be potentially used as an ideal matrix for stem cell differentiation for organ-specific tissue engineering. These synthetic matrices have the potential to be further improved as functionally bioactive scaffolds with the inclusion of cytokines, cell adhesive peptides, and growth factors, to deliver therapeutic drugs and enhance their integration with native tissue in regenerative therapies using stem cell technology.
Acknowledgements
We are grateful to the Department of Biotechnology, Government of India, for funding this work (BT/PR11228/BRB/10/677/2008). We thank the members of the Biotechnology wing of Sree Chitra Thirunal Institute of Medical Science and Technology for their help with tensile testing.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Asran A, Salama M, Popescu C, Michler GH. 2010. Solvent influences the morphology and mechanical properties of electrospun poly(L-lactic acid) scaffold for Tissue Engineering Applications. Macromol Symp. Special issue: Layered Nanostructure-Polymer with improved properties. 294:153–161.
- Boontheekul T, Hill EE, Kong HJ, Mooney DJ. 2007. Regulating Myoblast Phenotype Through Controlled Gel Stiffness and Degradation. Tissue Eng. 13:1431–1442.
- Ciardelli G, Chiono V, Vozzi G, Pracella M, Ahluwalia A, Barbani N, et al. 2005. Blends of poly(ε-caprolactone) and polysaccharides in tissue engineering applications. Biomacromlecules. 6:1961–1976.
- Engler AJ, Sen S, Sweeney HL, Discher DE. 2006. Matrix elasticity directs stem cell lineage specification. Cell. 126:677–689
- Gaudio DC, Bianco A, Grigioni M. 2008. Electrospun bioresorbable trileaflet heart valve prosthesis for tissue engineering: in vitro functional assessment of a pulmonary cardiac valve design. Ann Ist Super Sanita. 44:178–186.
- Gunatillake PA, Adhikari R. 2003. Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater. 5:1–16.
- Hackett JM, Dang TT, Eve C, Tsai EC and Cao X. 2010. Electrospun Biocomposite Polycaprolactone/Collagen Tubes as Scaffolds for Neural Stem Cell Differentiation. Materials. 3:3714–3728.
- Ishii O, Shin M, Sueda T 2005. In vitro tissue engineering of a cardiac graft using a degradable scaffold with an extracellular matrix–like topography. J Thorac Cardiovasc Surg. 130:1358–1363.
- Jacot JG, McCulloch AD, Omens JH. 2008. Substrate Stiffness Affects the Functional Maturation of Neonatal Rat Ventricular Myocytes. Biophys J. 95:3479–3487.
- Johnson J, Niehaus A, Nichols S, Lee D, Koepsel J, Anderson D, Lannutti J. 2009. Electrospun PCL in vitro: a microstructural basis for mechanical property changes. J Biomater Sci Polym Ed. 20:467–481.
- Juliana RD, Filipe EA, Paulo JB. 2013. Influence of the rheological behaviour in electrospun PCL nanofi bres production for tissue engineering applications. In: Chemical Engineering Transactions. AIDIC 32:1015–1020.
- Kanani AG, Bahrami SH. 2011. Effect of changing solvents on Poly (e-caprolactone) nanofibrous webs morphology. J Nanomater. 2011:1–10.
- Kim MS, Park SJ, Gu BK, Kim CH. 2012a. Polycaprolactone-chitin nanofibrous mats as potential scaffolds for tissue engineering. J Nanomater. Article ID. 635212:doi:10.1155/2012/635212
- Kim HN, Kang DH, Kim MS, Jiao A, Kim DH, Suh KY. 2012. Patterning methods for polymers in cell and tissue engineering. Ann Biomed Eng. 40:1339–1355.
- Li WJ, Danielson KG, Alexander PG, Tuan RS. 2003. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolac- tone) scaffolds. J. Biomed Mater Res A. 67:1105.
- Li WJ, Tuli R, Okafor C, Derfoul A, Danielson, KG Hall, DJ, Tuan RS. 2005. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 26:599.
- Li WJ, Mauck RL, Cooper JA, Yaun X, Tuan RS. 2007. Engineering Controllable Anisotropy in Electrospun Biodegradable Nanofibrous Scaffolds for Musculoskeletal Tissue Engineering. J Biomech. 40(8):1686–1693.
- Middleton, JC, Tipton AJ. 2000. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 21:2335–2346.
- Mi FL, Sung H, Shyu S. 2000. Synthesis and characterization of a novel chitosan-based network prepared using naturally occurring crosslinker. J Polym Sci Part A Polym Chem. 38:2804–2814.
- Mobarakeh LG, Semnani D, Morshed MA. 2007. A Novel method for porosity measurement of various surface layers of nanofibers mat using image analysis for tissue engineering applications. J Appl Polym Sci. 106:2536–2542.
- Mondal S. 2014. Influence of solvents properties on morphology of electrospun polyurethane nanofiber mats. Polymer Adv Tech. 25:179–183.
- Parekh SH, Chatterjee K, Lin-Gibson S, Moore NM, Cicerone MT, Young MF, Simon CG Jr. 2011. Modulus-driven differentiation of marrow stromal cells in 3D scaffolds that is independent of myosin-based cytoskeletal tension. Biomaterials. 32:2256–2264.
- Pham QP, Sharma U, Mikos AG. 2006. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 12:1197–1211.
- Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. 2008. Substrate modulus directs neural stem cell behavior. Biophys J. 95:4426–4438.
- Schueren LV, Schoenmaker B, Kalaoglu OI, Clerck K 2011. An alternative solvent system for the steady state electrospinning of polycaprolactone. Eur Polym J. 4:1256–1263.
- Sharma S, Mohanty S, Gupta D, Jassal M, Agrawal AK, Tandon R. 2011. Cellular response of limbal epithelial cells on electrospun poly-ε-caprolactone nanofibrous scaffolds for ocular surface bioengineering: a preliminary in vitro study. Molecular Vision. 17:2898–2910.
- Shin M, Ishii O, Sueda T, Vacanti JP. 2004. Contractile cardiac grafts using a novel nanofibrous mesh. Biomaterials. 25:3717–3723.
- Shin M, Yoshimoto H, Vacanti JP. 2004. In vivo bone tissue engineering using mesenchymal stem cells on a novel electrospun nanofibrous scaffold. Tissue Eng. 10:33.
- Sun H, Mei L, Song C, Cui X, Wang P. 2006. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials. 27:1735–1740.
- Thakkar S, Ghebes CA, Ahmed M, Kelder C, van Blitterswijk CA, Saris D, et al. 2013. Mesenchymal stromal cell-derived extracellular matrix influences gene expression of chondrocytes. Biofabrication. 5:doi:10.1088/1758-5082/5/2/025003.
- Yogeshwar CV, Gnanamani A, Giridev VR, Madhusoothanan M, Sekaran G. 2012. Electrospinning of Type I Collagen and PCL Nanofibers Using Acetic Acid. Journal of Applied Polymer Science 125:3221–3227.
- Yoshimoto H, Shin, YM, Terai H, Vacanti JP. 2003. A biodegradable nanofibers scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 24:2077.
- Zaman MH, Trapani LM, Sieminski AL, Mackellar D, Gong H, Kamm RD, et al. 2006. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell–matrix adhesion and proteolysis. Proc Natl Acad SciUSA. 103:10889–10894.
- Zeugolis DI, Khew ST, Yew ES, Ekaputra AK, Tong YW, Yung LY, et al. 2008. Electro-spinning of pure collagen nano-fibres Just an expensive way to make gelatin? Biomaterials. 29:2293–2305.
- Zhang Y, Ouyang H, Lim CT, Ramakrishna S, Huang ZM. 2005. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J Biomed Mater Res B Appl Biomater. 72:156–165.
- Zorlutuna P, Elsheikh A, Hasirci V.. 2009. Nanopatterning of collagen scaffolds improve the mechanical properties of tissue engineered vascular grafts. Biomacromolecules. 10:814–821.