Abstract
In this report, the oligosaccharides of hyaluronan (oHA)-histidine-menthone 1,2-glycerol ketal (MGK) (oHM) carried pH-sensitive MGK as hydrophobic moieties and oHA as the target of the CD44 receptor. The oHM could self-assemble, with a diameter of 65 nm. The cellular uptake, indicated by the fluorescent signals, was higher at 4 h. The ex vivo imaging indicated that micelles have a longer blood circulation, beyond 5 h. The fluorescence intensity of the micelles in the liver and spleen was much higher from 5 to 24 h. The CD44 receptor-targeting, indicated by the fluorescence signals of A549 and MDA-MB-231 group, were higher than those of the HepG2 and the control.
Introduction
Many studies on hyaluronan (HA) and its derivatives have reported that HA can have good biocompatibility and unique biological characteristics, suitable for use in tumor-targeting drug delivery systems, such as the HA receptor CD44-targeting (CitationMorra 2005, CitationStern et al. 2006, CitationGerecht et al. 2007, CitationToole 2004, CitationJaracz et al. 2005, CitationGötte and Yip 2006). Some research studies have reported that the native high molecular weight HA (nHA) and oligosaccharides of HA (oHA) can induce different biological effects upon binding to CD44 (CitationYang et al. 2012). For example, nHA binding to CD44 selectively induces CD44 clustering, while oHA can be inhibited (CitationYang et al. 2012). This is very important and interesting for the future use of oHA in drug delivery.
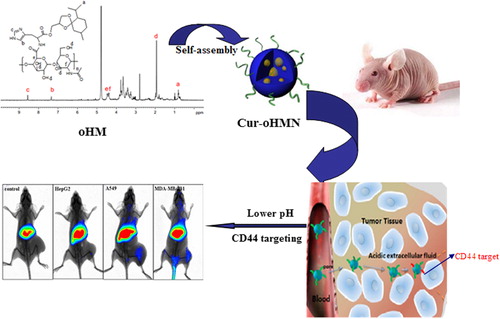
Because of the “Coating Saccharide” (CD44 clustering) on the tumor surface and the acidic condition of the tumor, it is difficult to target the chemotherapeutic drugs to the tumor site and achieve good results from the drug. To solve this problem, we prepared a multifunctional oHA-histidine-ketal menthone 1,2-glycerol ketal (oHM) nano drug delivery system, with the CD44 receptor as the target, based on our previous work on pH-sensitive nanocarriers (CitationYu et al. 2013, CitationChen et al. 2014, Citation2010, Citation2011, Citation2012a, Citation2012b, CitationChen and Wang 2014). In this study, as shown in , our aim was to develop the in vivo behavior of oHM nanoparticles (oHMN), such as in vitro stability, cellular uptake of oHMN (micelles), and ex vivo imaging of curcumin-loaded oHMN.
Experiments
As shown in , oHA was obtained by the chemical conjugation of hydrophobic MGK to the backbone of oHA, with histidine as the linker. The multifunctional Cur-oHMN micelles were prepared by the enhanced thin-film hydration method (CitationChen et al. 2014).
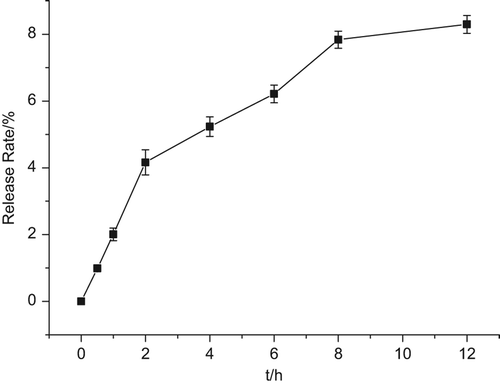
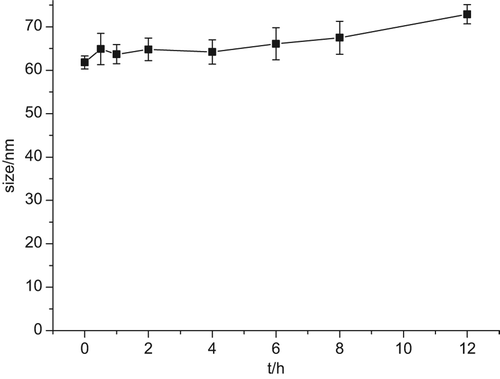
The particle size and leakage rate (LR) play an important role in the change of in vivo behavior and pharmacokinetics. However, it is also important to study the stability of Cur-oHMN before the research on cellular uptake. Nutrient solutions have many ingredients such as NaCl and protein, which can also affect the stability. The stability of Cur-oHMN after freeze-drying was observed by investigating the leakage rate, with the nutrient solution at 37°C. The change of the mean particle size and LR were measured after 0, 0.5, 1, 2, 3, 4, 6, 8, and 12 h of incubation. The particle sizes were measured with a particle sizer 3000HS (Malvern Instruments, UK) and analyzed by the Zetasizer 3000H (Malvern software). From and , we can see that the LR was about 8.5% at 12 h incubation. However, the particle sizes were not obviously changed (). Therefore, the cellular uptake test should be no more than 8 h.
Table I. Compatibility and stability of Cur-oHMN with the culture medium.
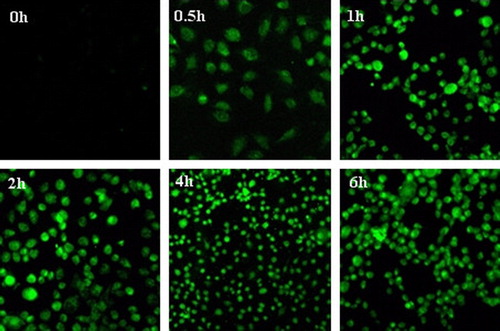
In vitro cellular uptake of Cur-oHMN was assayed by confocal laser scanning microscopy (CLSM) (CitationChoi et al. 2011, CitationCoradini et al. 2004, CitationLee et al. 2007, CitationPeer and Margalit 2004, CitationSleeman et al. 1996). As a novelty, curcumin (Cur), was incorporated into oHMN, both as a therapeutic drug and a fluorescence marker. The Cur was dissolved in ethanol as the control (5 mg/mL). A375 human melanoma cells were seeded at a density of 105 cells/well. After 24 h, the medium was augmented by micelles diluted in the medium (5 mg/mL). At the different time points (0.5, 1, 2, 4, and 6 h after incubation), the cells were washed three times with PBS and fixed with 4% paraformaldehyde for 15 min. Finally, the cells were observed with the Olympus FluoView FV 1000 (Ex 488 nm). As shown in , the fluorescence signals were higher at 4 h, increased by the cellular uptake. Then, the cells presented atrophy or apoptosis, decreasing at 6 h due to the therapeutic effect of the encapsulated Cur. This finding demonstrated that oHMN (micelles) could be good intracellular carriers for the anti-tumor drugs.
Figure 4. Fluorescent imaging of cellular uptake of Cur-HMN at different times after incubation with the A 785 cells. Available in colour online.
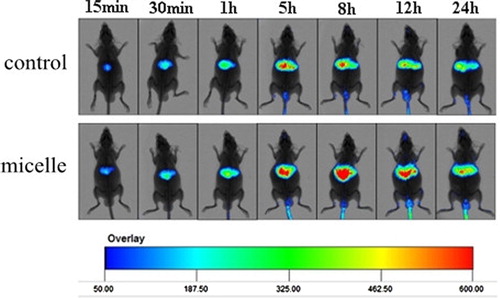
In studies of ex vivo imaging, Cur-oHMN, with DiR as the marker, was tested with a noninvasive optical imaging system (CitationChoi et al. 2011, CitationCoradini et al. 2004, CitationLee et al. 2007, CitationPeer and Margalit 2004, CitationSleeman et al. 1996). Kunming mice (n = 12) were injected with free DiR (50 ug/ml) as the control and DiR-labeled PCK oHMN micelles (50 μg/ml) through the tail vein. The imaging tests were carried out at 10 min, 30 min, 1, 5, 8, 12, and 24 h post-injection, using the Carestream Molecμlar Imaging FX PRO (Carestream Health, Inc., USA). Images were analyzed using the Molecular Imaging Software 5.X. shows the real-time images of the DiR micelles and the free DiR in the mice at 5 h. The abundant fluorescence signals of micelles were found in the liver at about 5 h. However, a few fluorescence signals of free DiR were found in the liver at about 5 h. This could indicate that the micelles have a longer blood circulation, after the 5-h detection. Then, the further studies at different times were carried out. shows that the fluorescence signals of free DiR reduced more quickly. The fluorescence intensity of the micelles in the liver and spleen was much higher from 5 to 24 h. These results indicate that oHM micelles had improved blood circulation time and caused the selective accumulation in the liver and spleen.
Figure 5. In vivo non-invasive NIRF images of free DiR (control) and DiR-labeled Cur-HMN (micelles) in mice, at 5 h. Available in colour online.
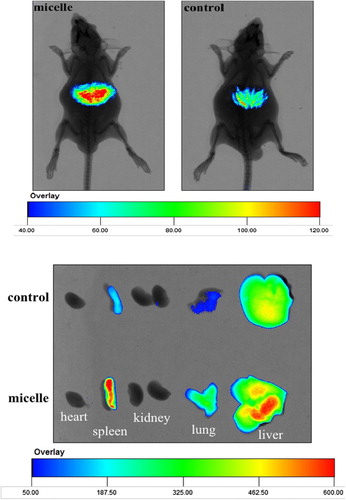
Figure 6. Time-dependent whole body images after intravenous injection in mice. Available in colour online.
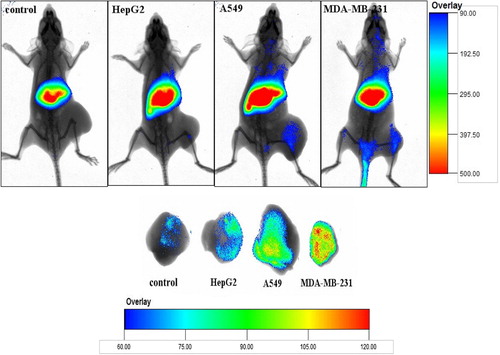
In order to study the CD44 receptor-targeting, the studies of ex vivo imaging of Cur-oHMN, with DiR as the marker, were tested with a noninvasive optical imaging system (CitationChoi et al. 2011, CitationCoradini et al. 2004, CitationLee et al. 2007, CitationPeer and Margalit 2004, CitationSleeman et al. 1996). Nude mice, with different tumor models of HepG2 (under-expression), A549 (over-expression), and MDA-MB-231(over-expression), were injected with free DiR (50 ug/ml) as the control and DiR-labeled PCK oHMN micelles (50 μg/ml), through the tail vein. shows that the fluorescence signals of the A549 (over-expression) group and the MDA-MB-231(over-expression) group were higher than those of the HepG2 (under-expression) group and the control group. These results indicate that the CD44 receptors with over-expression play an important role in the CD44-targeting drug delivery system.
Discussion
In this work, we have obtained a multifunctional targeting nanocarrier drug delivery system for curcumin. The oHM conjugates were synthesized by chemical conjugation of pH-sensitive MGK to the backbone of oHA, with histidine as the linker. The mechanism employed was that once oHM nanoparticles meet the CD44 receptor, the coating of CD44 clustering will not appear. The protonation of the histidine imidazole group is useful for the cellular uptake. After endocytosis, oHM nanoparticles will rapidly release the encapsulated drugs under the slightly acidic condition of the tumor, with the degradation of the micellar hydrophobic ketal group. Then, the lysosomal membrane will be destroyed via the proton sponge effect of the histidine imidazole group. The neutral ketones, alcohol degradation products of oHM, can also help the escape of drug from the lysosomes. The encapsulated drugs will escape from the lysosome to the cytoplasm with good stability, produce efficient results, and reduce side effects.
Conclusion
The oHM could self-assemble to nano-sized spherical shaped particles with the diameters of 65 nm, under the condition of a pH of 7.4, in PBS. The stability test indicated that the LR was about 8.5% at 12-h incubation, and that the particle sizes were not obviously changed (). Therefore, the cellular uptake test should last no more than 8 h. The cellular uptake test indicated that the fluorescence signals were higher at 4 h, increased by the cellular uptake. Then, the cells presented atrophy or apoptosis, decreasing at 6 h, due to the therapeutic effect of the encapsulated Cur. This finding demonstrated that oHMN (micelles) could be good intracellular carriers for the anti-tumor drugs. The results of the ex vivo imaging test indicated that micelles have a longer blood circulation, continuing after the detection at 5 h. The fluorescence intensity of the micelles in the liver and spleen was much higher from 5 to 24 h. These results indicate that oHM micelles had improved blood circulation time and caused the selective accumulation in the liver and spleen. The CD44 receptor-targeting test indicated that the fluorescence signals of the A549 (over-expression) group and the MDA-MB-231(over-expression) group were higher than those of the HepG2 (under-expression) group and the control group, which indicated that the CD44 receptors with over-expression play an important role in the CD44-targeting drug delivery system. These results present the promising potential of oHM as a multifunctional targeting nanocarriers in a drug delivery system for cancer treatment.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
This study is financially supported by the National Natural Science Foundation of China (no.81302718), Taishan Scholar Project (To Dr. Zimei Wu), Young Core Instructor Visitor Foundation of Shandong Province, China (2014), Young Core Instructor Training Program of Yantai University, China (2014).
References
- Chen D, Jiang X, Liu J, Jin X, Zhang C, Ping Q. 2010. In vivo evaluation of novel pH-sensitive mPEG-Hz-Chol conjugate in liposomes: pharmacokinetics, tissue distribution, efficacy assessment. Artif Cells Blood Substit Immobil Biotechnol. 38: 136–142.
- Chen D, Lian S, Sun J, Liu Z, Zhao F, Jiang Y, et al. 2014. Design of novel multifunctional targeting nano-carrier drug delivery system based on CD44 receptor and tumor microenvironment pH condition. Drug Deliv. 3:1–6.
- Chen D, Liu W, Shen Y, Mu H, Zhang Y, Ping Q. 2011. Effects of a novel pH-sensitive liposome with cleavable esterase-catalyzed and pH-responsive double smart mPEG lipid derivative on ABC phenomenon. Int J Nanomedicine. 6:2053–2061.
- Chen D, Mu H, Sun K, Liu W. 2012a. Synthesis, anticoagulant activity and potential drug carrier of biomimetic N-Cholesteryl hemisuccinate-O-sulfate chitosan polymer of blood cell membrane. J Control Release.172:e25.
- Chen D, Sun K, Mu H, Tang M, Liang R, Wang A, et al. 2012b. pH and temperature dual-sensitive liposome gel based on novel cleavable mPEG-Hz-CHEMS polymeric vaginal delivery system. Int J Nanomedicine. 7:2621–2630.
- Chen D, Wang H. 2014. Novel pH-sensitive biodegradable polymeric drug delivery systems based on ketal polymers. J Nanosci Nanotechnol. 14:983–989.
- Choi KY, Min KH, Yoon HY, Kim K, Park JH, Kwon IC, et al. 2011. PEGylation of hyaluronic acid nanoparticles improves tumor targetability in vivo. Biomaterials. 32:1880–1889.
- Coradini D, Zorzet S, Rossin R, Scarlata I, Pellizzaro C, Turrin C, et al. 2004. Inhibition of hepatocellular carcinomas in vitro and hepatic metastases in vivo in mice by the histone deacetylase inhibitor HA-But. Clin Cancer Res. 10:4822–4830.
- Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. 2007. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci USA. 104:11298–11303.
- Götte M, Yip GW. 2006. Heparanase, hyaluronan, and CD44 in cancers: a breast carcinoma perspective. Cancer Res. 66:10233–10237.
- Jaracz S, Chen J, Kuznetsova LV, Ojima I. 2005. Recent advances in tumor-targeting anticancer drug conjugates. Bioorg Med Chem. 13:5043–5054.
- Lee H, Mok H, Lee S, Oh YK, Park TG. 2007. Target-specific intracellular delivery of siRNA using degradable hyaluronic acid nanogels. J Control Release. 119:245–252.
- Morra M. 2005. Engineering of biomaterials surfaces by hyaluronan. Biomacromolecules. 6:1205–1223.
- Peer D, Margalit R. 2004. Loading mitomycin C inside long circulating hyaluronan targeted nano-liposomes increases its antitumor activity in three mice tumor models. Int J Cancer. 108:780–789.
- Sleeman J, Rudy W, Hofmann M, Moll J, Herrlich P, Ponta H. 1996. Regulated clustering of variant CD44 proteins increases their hyaluronate binding capacity. J Cell Biol. 135:1139–1150.
- Stern R, Asari AA, Sugahara KN. 2006. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 85:699–715
- Toole BP. 2004. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 4:528–539.
- Yang C, Cao M, Liu H, He Y, Xu J, Du Y, et al. 2012. The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J Biol Chem. 287:43094–430107.
- Yu H, Mu H, Xiu L, Sun K, Liu W, Chen D. 2013. A Novel Ketal-Based Chitosan as Nano-Vehicles for Potential pH-Sensitive Nanomedicine Delivery. Nanosci Nanotechnol Lett. 5:1007–1011.