Abstract
Efavirenz is a non-nucleoside reverse transcriptase inhibitor, and is classified as BCS Class II API. Its erratic oral absorption and poor bioavailability make it a potential candidate for being formulated as a nanosuspension. The objective of this study was to formulate efavirenz nanosuspensions employing the antisolvent precipitation-ultrasonication method, and to enhance its solubility by reducing particle size to the nanometer range. The effects of different process parameters were studied and optimized with respect to particle size and poly dispersity index (PDI). The optimized formulation was also subjected to lyophilization, to further increase the solubility and stability, and the technology is potentially suited to a range of poorly water-soluble compounds.
Introduction
Poorly water-soluble drugs (BCS Class 2 & 4) often give rise to problems like low bioavailability and erratic oral absorption (CitationDahan and Miller 2012). Parameters like solubility, compatibility with excipients, vehicles, stability, and photostability are paramount, and play a crucial role in the formulation of a nanosuspension. Currently, a large share of active pharmaceutical ingredients have poor aqueous solubility, and formulating them as nanosuspensions can surely solve the solubility issues and enhance the bioavailability (CitationPatel and Agarwal 2011).
Formulation strategies include two approaches, namely the top-down approach (particle size reduction of larger crystals), and the bottom-up approach (building up crystals by precipitation of dissolved molecules). High pressure homogenization and media milling fall under the category of top-down techniques (CitationVan Eerdenbrugh et al. 2008), while ultrasonication, supercritical anti-solvent precipitation (SAS) (CitationReverchon 1999), or rapid expansion of a supercritical solution into a liquid solvent (RESOLV) (CitationPathak et al. 2006) are bottom-up techniques. Currently, five commercial products are available in the market based on the media milling technique, namely Rapamune®, Emend®, Tricor, Megace, and Invega®. Disadvantages associated with the media milling technique include wear or deformation of the wells, and incomplete recovery of the drug from the milling media due to stickiness to the surfaces (CitationVan Eerdenbrugh et al. 2009). For the preparation of lesser amounts of drug nanosuspensions, the bottom-up liquid precipitation approach is exploited, for which optimization of solvent/antisolvent is a critical prerequisite that is certainly a shortcoming in the context of a tight drug supply (CitationZhao et al. 2007). Moreover, the organic solvents must be eliminated from the formulation to avoid toxic effects (CitationGao et al. 2008). Another technique, microfluidization technology also produces stable and well dispersible nanosuspensions e.g., budesonide nanosuspensions (CitationZhang and Zhang 2014).
During the process, the drug that is dissolved in the solvent is poured into the antisolvent, leading to the precipitation of drug crystals to the desired size range by manipulating precipitation conditions (CitationKesisoglou et al. 2007). Antisolvents should contain some stabilizers, such as surfactants and polymers, to avoid stability issues. Polymers usually act by forming strong hydrogen bonds with active pharmaceutical ingredients and get adsorbed on to the particle surface, thus inhibiting crystal growth. The major utility of a stabilizer is in wetting the drug particles thoroughly, so as to prevent Ostwald ripening (a phenomenon in which small crystals dissolve and again deposit onto larger crystals) and agglomeration of the particles, in order to yield a physically stable formulation by providing a steric or ionic barrier (CitationPeltonen and Hirvonen 2010). The type and amount of stabilizer have a noticeable effect on the physical stability and in vivo behavior of the nanosuspension (CitationPrasanna and Giddam 2010).
Globally, around 34 million people are struggling with HIV. AIDS is a pandemic disease, spreading dynamically, covering a large proportion of population (CitationKallings et al. 2008). Efavirenz is a non-nucleoside reverse transcriptase inhibitor (NNRTI) and is used in the HAART regimen (Highly Active Antiretroviral Therapy) for the treatment of infection by the human immunodeficiency virus (HIV) type 1 (CitationShafer and Vuitton 1999). Efavirenz is classified as Class II API (poorly soluble and highly permeable) by BCS, with aqueous solubility less than 10 μg/ml (practically insoluble). Its erratic oral absorption and poor bioavailability of nearly 40% make it a potential candidate for being formulated as a nanosuspension.
The aim of the present work was to overcome the solubility-related issues of drug by formulating it in the form of nanosuspensions. Nanosuspensions were developed using the bottom-up technique, employing the antisolvent precipitation-ultrasonication method, so as to enhance their solubility by reducing the particle size to nanometer range. The effect of process parameters like drug concentration, stabilizer concentration, time duration of ultrasonication, and the solvent/antisolvent ratio, were studied and optimized with respect to particle size and PDI. The formulation was lyophilized to increase its physical stability. The novelty of the present work lies in the fact that the size of formulations is controlled by ultrasonication in a specific range, by using a combination of stabilizers and surfactants in order to maximize stability. Thus, formulating a nanosuspension of a hydrophobic drug is definitely a potential approach to deal with drug delivery of poorly soluble drugs.
Methods
Materials
Efavirenz was provided as a kind gift from Emcure Pharmaceuticals Ltd. (India); Poloxamer 407 was purchased from Sigma-Aldrich (USA), while soya lecithin and mannitol were procured from Central Drug House, New Delhi (India). Analytical grade acetone and methanol were purchased from Rankem Laboratory Reagents, New Delhi (India). Purified water from Elix-3/Millipore was used. To stabilize the nanosuspensions formed, poloxamers and soya lecithin were used. Mannitol was used as a cryoprotectant in the freeze drying process.
Methods
Preparation of efavirenz nanosuspensions
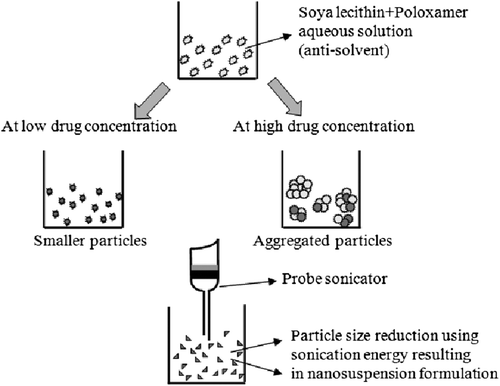
Efavirenz nanosuspensions were prepared by the bottom-up technique, employing the antisolvent precipitation- ultrasonication technique (CitationXia et al. 2010). Acetone was selected as the solvent, and an aqueous solution of poloxamer 407 and soya lecithin was used as the antisolvent. The drug was dissolved in organic phase in different concentrations ranging from 15 to 25 mg/ml. Poloxamer 407 and soya lecithin in different concentrations and ratios were dissolved in purified water, to obtain a series of antisolvents. The antisolvent was cooled to below 4°C in an ice-water bath. Then, 2 ml of organic solution was introduced drop-wise into 20 ml of the precooled antisolvent, under continuous probe sonication (DP 120, Dakshin, Mumbai). As the drug was completely insoluble in aqueous media, it immediately precipitated, leading to the production of submicron particles. A probe with a tip diameter of 8 mm was immersed 10 mm in the liquid, resulting in the wave traveling downwards and reflecting upwards. The period of ultrasound burst was set to 4 s (on-time), with a pause of 6 s (off-time) between two ultrasound bursts. Cool conditions were maintained during the process, by using an ice-water bath. The nanosuspensions obtained were subjected to centrifugation at 15,000 rpm for 40 mins, using a refrigerated centrifuge (Model: 3K-30, Sigma, USA). After the centrifugation, the supernatant was replaced with antisolvent solution. The nanoparticle pellet was redispersed using a vortex shaker, and the final drug content was adjusted to 15 mg/ml (drug weight/volume of nanosuspension), using an appropriate volume of stabilizer solution. In order to further increase solubility and stability, efavirenz nanosuspensions were rapidly frozen in liquid nitrogen and freeze-dried for 10 h. Mannitol as a cryoprotectant was added in the ratio of 1/1 to that of drug. The schematic representation of the mechanism behind particle size reduction is shown in .
Characterization of efavirenz nanosuspensions
Size, size distribution, and zeta potential
The Beckman Coulter DelsaTM Nano C particle analyzer was used to measure size, size distribution (PDI), and zeta potential of the optimized formulation. It is an instrument that employs photon correlation spectroscopy (PCS), which determines particle size by measuring the rate of fluctuations in laser light intensity scattered by particles as they diffuse through a fluid. For the analysis, a cuvette was filled up to the appropriate amount with the formulation, and analyzed for the size and PDI (CitationPatel et al. 2013).
Morphology of nanosuspensions
The scanning electron microscope (SEM) was utilized for the visualization of shape and morphology of the prepared nanosuspensions. The sample was scanned with a focused beam of electrons to produce an image (CitationCosta et al. 2013). The electrons interacted with atoms in the sample, producing various signals that were detected, and gave information about the sample's surface topography.
X-ray powder diffraction characterization
To analyze the potential changes in the crystalline structure of API and the freeze dried nanosuspensions, X-ray powder diffraction (XRPD) was employed (CitationEerdenbrugh et al. 2009). Samples were analyzed by the X-ray diffractometer system (XPERT-PRO) at Punjab University, Chandigarh, with Cu Kα radiation at a wavelength of 1.542 Å, generated at 40 mA and 45 kV. The scanning speed was 10°/min. from 5° to 50° of 2θ, with a step size of 0.017°.
pH
The pH was determined by a MAX-ME 962-P pH meter. Initially, the pH meter was calibrated with buffers of pH 4, 7.4, and 9. Once calibrated, the electrode was washed with distilled water and dipped in the formulation. The reading was noted, and in this way, the pH of all the formulations were analyzed.
Determination of saturation solubility
The saturation solubility was determined by spiking an excess amount of drug and lyophilized nanosuspensions separately in purified water, to obtain a saturated solution. Both the test tubes were sealed and agitated on a vortex shaker for 24 h at 37°C. After centrifugation and filtration through a 0.1 μm membrane filter (Millipore Corporation), the filtrate was diluted and finally analyzed using a UV spectrophotometer at 247 nm (CitationBindu et al. 2011).
Drug content
The percent drug content was analyzed by UV spectroscopy at 247 nm. The nanosuspensions developed were subjected to centrifugation at 25,000 rpm for 20 mins. The supernatant thus obtained was assayed to determine the amount of drug, using a UV spectrophotometer at 247 nm. By subtracting the amount of drug present in supernatant from the total amount of drug, the amount of drug actually dispersed in nanosuspensions was calculated. The percentage drug content can be calculated by the formula mentioned underneath (CitationDeepali and Elvis. 2010).
In vitro drug release
The in vitro release profiles of the drug from the nanosuspensions was studied in PBS (pH 7.4), using a dialysis bag. The dialysis membranes were pre-treated, according to the manufacturer's recommendations.
Each dialysis bag was loaded with 5 ml of nanosuspension (equivalent to 5 mg of the drug). In vitro drug release studies were performed using a dialysis bag dipped in 200 ml of PBS (pH 7.4). The flask containing the dialysis bag and dissolution media was placed in a shaking incubator, at a rotation speed of 100 rpm and a temperature of 37.0 ± 0.2°C. Samples (2 ml) were withdrawn periodically and replaced with the same volume of fresh media and filtered, and were finally assayed for drug content at 247 nm against the blank, using the UV-visible spectrophotometer (CitationMuthu and Singh 2009).
Results
Effects of drug concentration on particle size
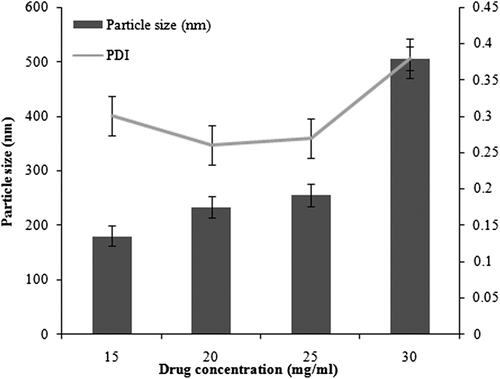
Efavirenz nanosuspensions were prepared with different drug concentrations in the organic solutions (15–25 mg/ml), and poured drop-wise into the aqueous phase containing stabilizers (mixture of soya lecithin and poloxamer 407). When the duration of ultrasonication was fixed at 8 min, the smallest particle size was obtained when the drug concentration was 15 mg/ml and the concentrations of both soya lecithin and poloxamer 407 were 0.3%. The theory of crystallization, or Ostwald ripening, explains the reason behind the smaller particle size at particular concentrations. Steps included in it are particle nucleation, molecular growth, and aggregation of crystals. If Ostwald ripening is controlled in nanosuspensions, avoiding steps of molecular growth and aggregation, then smaller particle size can be attained. At lower drug concentration, the particle size was observed to be 182.4 ± 18.7 nm (PDI = 0.249). However, as the drug concentration was increased, particles with a size of 1.5 μm (PDI = 0.542) were obtained. This was probably because of higher aggregation rate due to increased supersaturation. shows optimization of drug concentration with respect to particle size and PDI.
Effect of stabilizer concentration on particle size
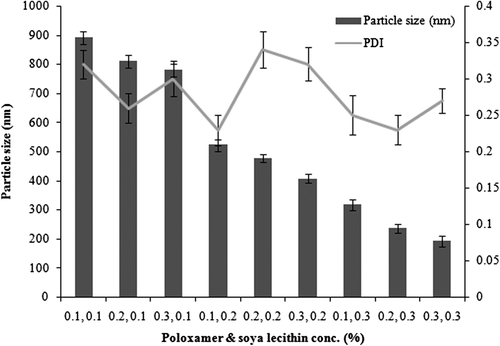
In the present study, the optimum concentrations of soya lecithin and poloxamer 407 were used to obtain smaller particle size. The main role of a stabilizer is particle wetting, so as to prevent Ostwald ripening and agglomeration of nanosuspensions, in order to get a physically stable formulation. The effect of the type and the amount of stabilizer on the physical stability is considerable. Agglomeration or aggregation is promoted with a smaller amount of stabilizer, while using it in higher quantities will result in Ostwald ripening. Particle size with soya lecithin and poloxamer 407 (0.1% for both) was approximately 892.6 ± 22.3 nm (PDI = 0.320), while with a higher concentration of 0.5%, it was 359.8 ± 14.8 nm (PDI = 0.17). The least particle size, of 182.4 ± 18.7 nm (PDI = 0.249), was observed at a concentration of 0.3% (for both the stabilizers). depicts the optimization of poloxamer and soya lecithin concentrations with respect to particle size and PDI.
Effect of duration of ultrasonication
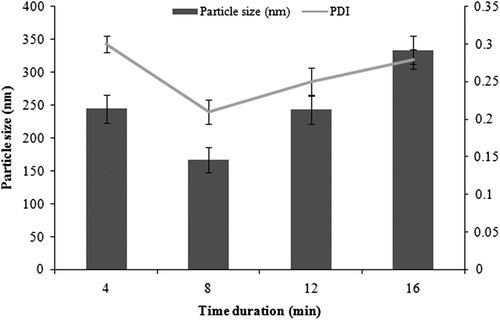
Particle size at varying time durations was observed at constant amount of drug, soya lecithin, and poloxamer, that is 15 mg/ml, 0.3%, and 0.3% respectively. Increased particle size was seen initially, but the size was reduced by increasing the time duration to 8 min. Interestingly, with further increase, no reduction in particle size was evident, as aggregates might be formed if the formulation was exposed to a longer ultrasonication duration. Such a phenomenon occurs because an accelerated aggregation reaction is caused by increased nucleation by ultrasonication. Finally, strong aggregates are formed at longer time durations (CitationYamaguchi et al. 2012). It is well demonstrated in that the optimal time duration was found to be 8 min.
Optimization of solvent/antisolvent ratio
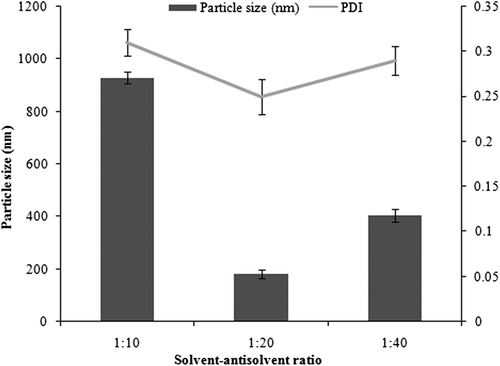
Optimization of the solvent/antisolvent ratio was done at a fixed drug concentration of 15 mg. The concentrations of both soya lecithin & poloxamer were fixed at 0.3%. Ultrasonication was carried out for 8 min. The ratio of 1/20 was observed to be the optimal value of solvent/antisolvent ratio. It was found that larger particles were obtained on decreasing the antisolvent concentration (1/10), as well as on increasing antisolvent concentration (1/40). This could be explained on the basis of the fact that stabilizers cover the particle surface to prevent their aggregation to each other to form bigger particles. A lower solvent/antisolvent ratio led to insufficient surface coverage by the stabilizers, which further led to production of aggregated particles with a size of 930 nm. However, a higher solvent/antisolvent ratio hindered the transmission of ultrasonic waves, leading to reduced ultrasonication efficiency, and ultimately larger particle size. shows the results of solvent/ antisolvent optimization.
Particle size, polydispersity, and zeta potential
The average size, polydispersity, and zeta potential of developed nanosuspensions were found to be 182.4 ± 18.7 nm, 0.249, and − 12.28 mV, respectively.
Morphology
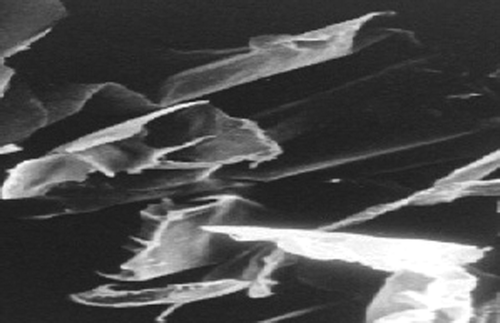
SEM was used for visualizing surface topography of the optimized formulation. Nanosuspensions were found to be flaky in nature. SEM images of lyophilized nanosuspensions are shown in .
XRPD
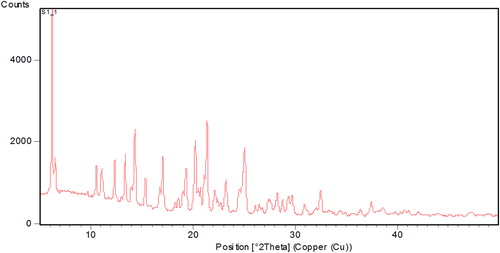
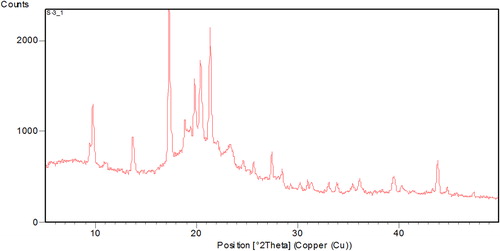
XRPD patterns of efavirenz presented sharp multiple peaks at 2θ-scattered angles, indicating the crystalline nature of the drug, as shown in . The diffraction patterns of physical mixtures revealed the presence of free crystalline drug (data not shown). Most of the crystalline peaks of efavirenz were absent in the lyophilized nanosuspensions (), indicating that the drug was not merely in crystalline form. The intensity of peaks was also decreased in the freeze dried nanosuspension formulations, indicating the decreased crystallinity or drug amorphization. In general, crystalline substances are physically more stable compared to amorphous forms, and are expected to be stable during the storage time (CitationDetroja et al. 2011). The reason behind the improved solubility is not simply the change in the crystalline state, it is also due to the reduction in size and the presence of a surfactant.
pH
The pH of the nanoformulation was found to be 7.54 ± 0.21, which shows that the formulation is in a neutral range and inert by nature.
Percent drug content
The nanosuspensions were assayed by UV spectroscopy for calculating the percent drug content, and the value was found to be 98.52 ± 0.21%. The yield values for nanosuspensions were between 89% and 94%.
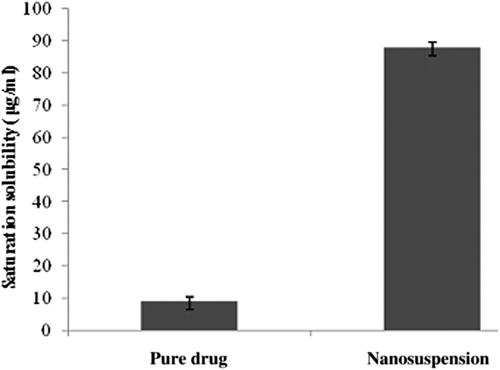
Saturation solubility studies
Though saturation solubility is a physicochemical constant, it can be increased if the particle size is reduced to the nanometer range. The saturation solubility of efavirenz pure drug was found to be just 9.21 ± 0.36 μg/ml, while the saturation solubility of lyophilized nanosuspensions increased to 88.10 ± 0.24 μg/ml. This indicates a remarkable increase in solubility by almost 10-fold. represents the increased saturation solubility of freeze-dried nanosuspensions, as compared to pure drug.
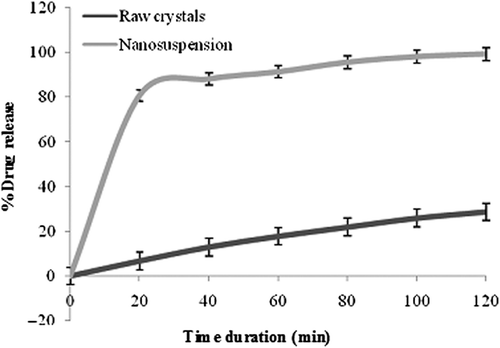
In vitro release studies
In vitro release studies were conducted for 2 h, in which only 28.6% of the drug was detected in PBS at a pH of 7.4, and effective dissolution was not attained, while more than 90% of nanosuspensions were dissolved at this time point. The reason behind this might be the large crystal size of pure drug, while nanosuspensions have a reduced size and thus a larger surface area. This significantly enhanced the dissolution rate, and decreased diffusion layer thickness was observed. A blend of all the stated factors suggested an enhanced dissolution rate that facilitated greater drug release by the nanosuspensions formulation (CitationXia et al. 2010). The dissolution profile of pure drug powder and lyophilized nanosuspensions is depicted in .
Discussion
Although many research studies have come up for developing efavirenz nanosuspensions using sonication energy, our group has developed nanosuspensions using a combination of soya lecithin and poloxamer 407, to control the particle size within a specific range. Thus, a precipitation–ultrasonication method was successfully employed to formulate efavirenz nanosuspensions. As the particle size of nanosuspensions was found to be dependent on process parameters, optimization of the parameters was a necessity. With the optimized process parameters, nanosuspensions with diameter of about 182.4 nm (± 18.7 nm) could be obtained. Marked enhancement in dissolution rate and saturation solubility was achieved by nanosuspensions as compared to plain drug suspension. The present study will be considered for further research in this area, as the precipitation-ultrasonication method (bottom-up approach) is easy to apply and needs only simple equipment to formulate nanosuspensions attaining controlled particle size and polydispersity, and has emerged as a promising technique for solubility enhancement.
Acknowledgements
The authors are thankful to M/s Emcure Pharmaceuticals Ltd. (India), for providing the gift sample of efavirenz. Author Sakshi Taneja acknowledges the All India Council for Technical Education, New Delhi for providing a JRF.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bindu M, Kusum B, Chatanya K, Naga M, Harsha VS, Banji D. 2011. Dissolution enhancement of efavirenz by solid dispersion and PEGylation techniques. Int J Pharm Investig. 1:29–34.
- Costa MA, Seiceira RC, Rodrigues CR, Hoffmeister CR, Cabral LM, Rocha HV. 2013. Efavirenz Dissolution Enhancement I: Co-Micronization. Pharmaceutics. 5:1–22.
- Dahan A, Miller JM. 2012. The Solubility–Permeability Interplay and Its Implications in Formulation Design and Development for Poorly Soluble Drugs. AAPS J. 14:244–51.
- Detroja C, Chavhan S, Sawant K. 2011. Enhanced Antihypertensive Activity of Candesartan Cilexetil Nanosuspension: Formulation, Characterization and Pharmacodynamic Study. Sci Pharm. 79: 635–651.
- Eerdenbrugh BV, Stuyven B, Froyen L, Humbeeck JV, Martens JA, Augustijns P,, Mooter GV. 2009. Downscaling Drug Nanosuspension Production: Processing Aspects and Physicochemical Characterization. AAPS Pharm Sci Tech. 10: 44–53.
- Gao L, Zhang D, Chen M. 2008. Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. J Nanopart Res. 10:845–862.
- Deepali G, Elvis M. 2010. UV Spectrophotometric Method for Assay of the Anti-Retroviral Agent Lamivudine in Active Pharmaceutical Ingredient and in its Tablet Formulation. J Young Pharm. 2:417–419.
- Kallings LO. 2008. The first postmodern pandemic: 25 years of HIV/AIDS. J Intern Med. 263:218–243.
- Kesisoglou F, Panmai S, Wu Y. 2007. Nanosizing Oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 59:631–644.
- Muthu MS, Singh S. 2009. Poly (D, L-Lactide) Nanosuspensions of Risperidone for Parenteral Delivery: Formulation and In-Vitro Evaluation. Curr Drug Deliv. 6:62–68.
- Patel GV, Patel VB, Pathak A, Rajput SJ. 2013. Nanosuspension of efavirenz for improved oral bioavailability: formulation optimization, in vitro, in situ and in vivo evaluation. Drug Deliv Ind Pharm. 40:80–91.
- Patel VR, Agarwal YK. 2011. Nanosuspension: An approach to enhance solubility of drugs. J Adv Pharm Technol Res. 2:81–87.
- Pathak P, Meziani MJ, Desai T, Sun YP. 2006. Formation and stabilization of ibuprofen nanoparticles in supercritical fluid processing. J Supercrit Fluids. 37:279–286.
- Peltonen L, Hirvonen J. 2010. Pharmaceutical nanocrystals by nanomilling: critical process parameters, particle fracturing and stabilization methods. J Pharm Pharmacol. 62:1569–1579.
- Prasanna L, Giddam AK. 2010. Nano-Suspension technology: a review. Int J Pharm Pharm Sci. 2:35–40.
- Reverchon E. 1999. Supercritical antisolvent precipitation of micro- and nano-particles. J Supercrit Fluids. 15:1–21.
- Shafer RW, Vuitton DA. 1999. Highly active antiretroviral therapy (HAART) for the treatment of infection with human immunodeficiency virus type 1. Biomed Pharmacother. 532: 73–86.
- Van Eerdenbrugh B, Stuyven B, Froyen L, Van Humbeeck J, Martens JA, Augustijns P, Van den Mooter G. 2009. Downscaling drug nanosuspension production: processing aspects and physicochemical characterization. AAPS Pharm Sci Tech. 10: 44–53.
- Van Eerdenbrugh B, Van den Mooter G, Augustijns P. 2008. Top down production of drug nanocrystals: nanosuspension stabilization, miniaturization and transformation into solid products. Int J Pharm. 364:64–75.
- Xia D, Quaa P, Piao H, Suna S, Yinc Y, Cui F. 2010. Preparation of stable nitrendipine nanosuspension using the precipitation– ultrasonication method for enhancement of dissolution and oral bioavailability. Eur J Pharm Sci. 40:325–334.
- Yamaguchi K, Matsumoto T, Kuwata K. 2012. Proper calibration of ultrasonic power enabled the quantitative analysis of the ultrasonication-induced formation process. Protein Sci. 21: 38–49.
- Zhang Y and Zhang J. 2014. Preparation of budesonide nanosuspensions for pulmonary delivery: Characterization, in vitro release and in vivo lung distribution studies. Artif Cells Nanomed Biotechnol. 6:1–5.
- Zhao H, Wang JX, Wang QA, Chen JF, Yun J. 2007. Controlled liquid antisolvent precipitation of hydrophobic pharmaceutical nanoparticles in a microchannel reactor. Ind Eng Chem Res. 46:8229–8235.