Abstract
Selenium acts as an important element in the prevention and treatment of cardiovascular diseases but their health-related effects have not been fully explored. As a novel attempt, zebrafish embryos were treated separately with SeNPs (5–25 μg/ml) and sodium selenite (5–25 μg/ml) starting at early blastula stage. Abnormalities were also observed in the morphology of the zebrafish embryos. The SeNPs-treated embryos exhibited concentration-dependent increased in mortality, pericardial edema, and cardiac arrhythmia. In contrast, sodium selenite showed no significant malformation effect in developing zebrafish embryos. The results of the present study conclude that the SeNPs were more toxic than sodium selenite. The results also suggest that lower concentrations of SeNPs and sodium selenite can be used as possible therapeutic agents for cardiovascular-related problems.
Introduction
Epidemiologic studies indicate that low levels of selenium in serum are associated with increased risk of cardiovascular disease mortality (CitationRayman 2012, CitationMurphy et al. 2002). There is a correlation between the levels of serum selenium and the cardiomyopathy-related diseases (CitationLoscalzo 2014). The beneficial role of selenium in myocardial injury and cardiac remodeling was reported (CitationAlexaniana et al. 2014). The World Health Organization recommended daily intake of 53 and 60 μg of selenium for women and men per day, respectively (CitationWHO 1996). Selenium nanoparticles (SeNPs) have been identified in human cells as a metabolite of selenite and it is formed in nanosized (5–550 nm) or larger aggregates (CitationWeekley and Harris 2013). SeNPs can be synthesized biologically using bacteria like Klebsiella pneumoniae (CitationFesharaki et al. 2010), Pseudomonas alcaliphila (CitationZhang et al. 2011), Bacillus cereus (CitationDhanjal and Cameotra 2010), Shewanella sp. HN-41 (CitationLee et al. 2007), Bacillus megaterium (CitationMishra et al. 2011), and Dugenella sp. (CitationBajaj et al. 2012). However, unfortunately the bacteria-mediated synthesis of SeNPs also contains some drawbacks such as sonication and ultracentrifugation, and this is an expensive process (CitationLü and Cui 2010, CitationKitching et al. 2014). Here we report protein-mediated synthesis of SeNPs as they are highly stable; large number of particles is formed, the particles are uniformly distributed, and the process is easily reproducible. As an innovative attempt, keratin was used as a reducing agent for the synthesis of SeNPs as they have many advantages in controlling NPs size and capping agent (CitationLü and Cui 2010, CitationMartin et al. 2011). Using H9C2 Heart cell line, researchers have demonstrated the therapeutic potential of SeNPs (69–173 nm) especially for cardiac-related diseases (CitationSoumya et al. 2013, Citation2014). SeNPs were also shown to induce the differentiation of mesenchymal stem cells into osteoblast cells, which could pave way for potential new therapy for the restoration of damaged or diseased tissue (CitationZheng et al. 2014). Further, sodium selenite supplement restores the antioxidative capacity of in vitro bone marrow stromal cells and reduces the micronuclei formation (CitationEbert et al. 2006). These studies lead to the conclusion that sodium selenite can be used for tissue engineering and transplantation of stem cells to overcome cardiovascular diseases. In spite of increasing popularity of sodium selenite and SeNPs in biological applications, there is still lack of in vivo data for prediction of comparative toxicity of sodium selenite and SeNPs in cardiovascular toxicity. Therefore, the present study focuses in creating a better understanding of the toxicity of sodium selenite and SeNPs in both embryos and larvae of zebrafish. The zebrafish embryos are well established and widely used as in vivo model in developmental toxicity due to its small size, transparency, rapid embryogenesis and continuous reproduction (CitationGroh et al. 2014). Cardiac functions and cardiovascular toxicity in zebrafish embryos could be assessed visually without dissecting the animal. Zebrafish embryos were used to evaluate the developmental toxicity of SeNPs and sodium selenite. Selenium is a strong protective agent against cardiovascular diseases and overcomes the risk of cardiovascular death by 2–3 times when its concentration is below 45 μg/ml in serum (CitationHatfield et al. 2014, CitationStoppe et al. 2013). Hence the current work is planned to evaluate SeNPs as a possible therapeutic agent in cardiovascular-related diseases and to study their toxicity effect in zebrafish embryos.
Materials and methods
SeNPs synthesis from keratin
A total of 0.1g of sodium selenite was dissolved in 10 mL of deionized water kept in a 50-mL beaker. A total of 0.1g of keratin was dissolved in 10 mL of Tris (pH: 11.0) buffer under magnetic stirring and sodium selenite was added at 65°C for 20 min.
Purification of SeNPs
The SeNPs carrying keratin suspension was centrifuged at 12,000 rpm for 10 min and then the supernatant was discarded. The keratin-containing SeNPs were washed and resuspended in deionized water and again centrifugation was done at 12,000 rpm for 10 min. The above step was repeated three times at room temperature (CitationKalishwaralal et al. 2014).
Characterization
The shape and size of SeNPs were measured using a scanning electron microscope (Philips JSM 6390 model, USA). One drop of the sample suspension was taken for analysis. The selected areas were undergone to elemental composition analysis using an energy-dispersive X-ray spectroscopy (EDX) microanalysis system.
Zebrafish husbandry and exposure of SeNPs
The fertilized eggs were collected and visualized under an optical microscope (Primo Star Carl Zeiss, Germany). Zebrafish embryos in the early blastula stage (24 h post fertilization [hpf]) were chosen and kept in a 50-mL beaker (10 embryos in 10 mL of deionized water) in triplicate. The developing embryos and larvae were maintained at 27 ± 1°C constantly. Then the embryos were treated with SeNPs (5–25 μg/mL) and sodium selenite (5–25 μg/mL) separately up to 24 hours.
Apoptosis assay for whole embryo
The embryos (96 hpf) were transferred to 30-mm cell culture Petri dish and stained with acridine orange (5 μg ml− 1) for 20 min at room temperature. The stained embryos were washed quickly in phosphate-buffered saline and observed under a fluorescence microscope (Carl Zeiss, Axio; Germany) (CitationAsharani et al. 2008).
Embryonic malformation, heart rate, and mortality
For toxicologic studies, the zebrafish embryos were exposed to SeNPs and sodium selenite for 24-h duration at a concentration range of 5–25 μg/mL. The embryonic mortality was recorded up to 96 hpf and percentage of malformation was calculated. For heart rate assay, 10 embryos at 96 hpf were taken for each treated group and, finally, they were compared with that of control.
Statistical analysis
Each experiment was performed with three replicates, and the values represent the means ± standard deviation of three experiments. Significant differences between the treatments were determined using one-way analysis of variance (P < 0.05). All statistical analyses and calculations were carried out using GraphPad Prism 5 (GraphPad Software, San Diego, USA). Differences were considered statistically significant with P value < 0.0001.
Results and discussion
SeNPs synthesis from keratin
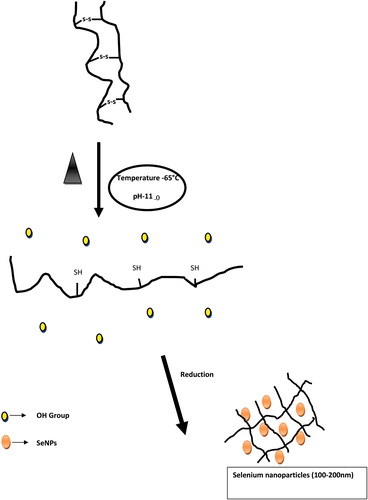
The synthesis of SeNPs was carried out using keratin as a reducing agent. When an aqueous sodium selenite solution was added to keratin at 65°C with pH: 11.0 and incubated for 20 min it turns light brown to orange (CitationJia et al. 2015). This result indicated the formation of SeNPs. Most of the keratin molecules were denatured at a temperature of 65°C and pH: 11.0, which results in the eradication of disulfide group in cysteine (). The eradication of the disulfide bond can be a driving power in achieving protein solubility via alkaline hydrolysis. Furthermore, the thiol (–SH)-containing peptide sites are strong reducing and stabilizing agents for smaller-size SeNPs (CitationVallee et al. 2010). In silk fibroin, the cysteine-containing peptide sequences are responsible for the reduction process of high-valent Au, Ag, Pd and Pt resulting in NPs formation. During this process, cysteine (–SH group) present in silk fibroin could play an essential role in NPs synthesis at high temperature and pH of 10 (CitationDas and Dhar 2014). Previous studies have shown that keratin in alkaline condition (pH: 10) dynamically change the size of the AgNPs (CitationLü and Cui 2010, CitationMartin et al. 2011). In the present study, when hydroxide ions were added very good changes occur in the size of SeNPs, and 65°C with pH: 11.0 for 20 min was found to be the suitable conditions for the synthesis of SeNPs.
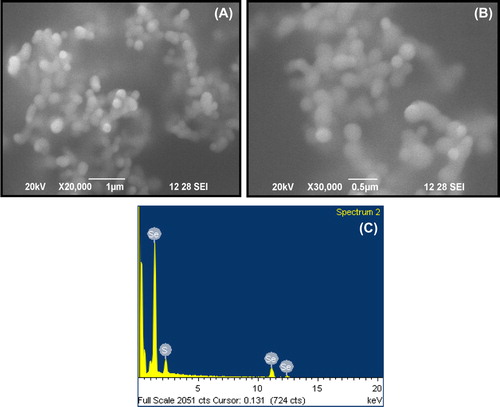
The SeNPs were characterized using scanning electron microscopy ( and ). At higher magnification, SeNPs were seen as small spherical particles with the dimensions of 100–200 nm (). The EDX measurements of SeNPs indicated the presence of Se as 75.14% (wt) and S as 24.86% (wt) with the atomic ratio of S/Se increasing with decreasing the size of the particles (). This correlates with the increase in surface-to-volume ratio when particles become smaller, which correspond to a larger amount of stabilizing thiol molecules (S) on the particle surface relative to the number of (Se) atoms in the particle core (CitationVallee et al. 2010, CitationDuan et al. 2013). This corroborates with the observation of CitationDas and Dhar (2014), who demonstrated that the thiol group absorbed on the surface of the metal NPs like Ag, Au, Pt, and Pd NPs improved the stabilizing power against NPs aggregation.
Figure 2. Scanning electron microscope (SEM) analysis of the SeNPs. (A) SEM image of the SeNPs produced by keratin (20,000 X magnification) (B) Particles size analysis at 30,000 X magnification (C) EDX analysis of ratio between thiol and SeNPs.
In contrast to the previous reports, selenite-resistant bacteria synthesize Se nanospheres (approximately 300 nm); this was observed with both Escherichia coli cells expressing recombinant Se factor A and purified recombinant protein in controlling SeNPs (CitationDebieux et al. 2011). However, recombinant biological methods have some disadvantages such as they are expensive and use complicated techniques and apparatus (CitationDurán et al. 2011).
Effect of sodium selenite and SeNPs on zebrafish embryos
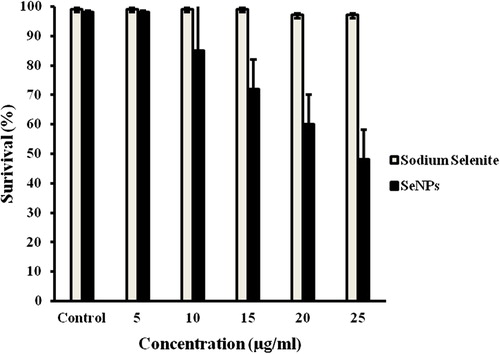
Zebrafish is one of the recent animal models and is being increasingly used in testing the toxicity of NPs (Duan et al. 2011). As chorion pore canals of zebrafish embryos are approximately 500–700 nm in diameter (CitationLee et al. 2007), agglomerates of SeNPs of about 100–200 nm can easily be incorporated. Zebrafish embryos were exposed to different concentrations of either sodium selenite (5–25 μg/mL) or SeNPs (5–25 μg/mL) and their effects were compared. When sodium selenite and SeNPs were treated at a concentration of 25 μg/ml, only 50% of the zebrafish embryos were viable in the case of SeNPs-treated embryos but in the case of sodium-selenite-treated embryos the viability was 90% at the same concentration (). More specifically, SeNPs appeared to be more toxic than sodium selenite in fish exposed before 48 hpf. Others have shown that, under comparable conditions, AgNPs were more toxic than Ag bulk for almost all the concentrations tested (CitationGroh et al. 2014).
SeNPs induce malformation in zebrafish embryos
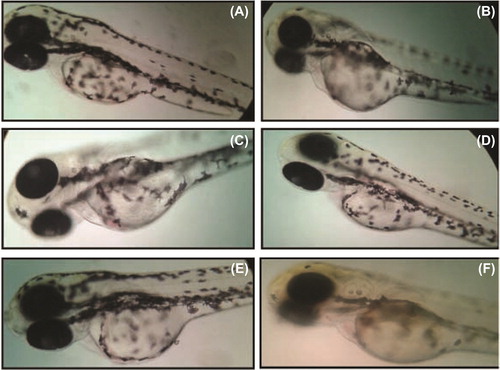
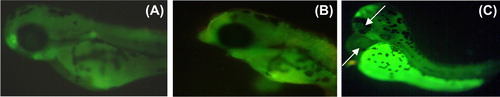
After 24-h SeNPs treatment, zebrafish per group were randomly selected and observed for cardiovascular-toxicity-associated morphology including pericardial edema. Malformations such as pericardial edema and tail malformation were observed in embryos incubated with SeNPs at 5–25 μg/mL, whereas no significant malformation was observed at lower concentrations ( and ). In contrast, ventricles of the SeNPs-treated zebrafish embryos were located anterior to the atrium, so the chambers could be easily illustrious with little overlap (CitationDuan et al. 2013). Cardiac abnormalities including string-like morphology, elongated ventricle, and edema increased in a dose-dependent manner (15–25 μg/mL) and the malformations were significantly increased at higher concentrations compared with those of the control group (). Interestingly, sodium selenite (5–25 μg/mL)-treated zebrafish embryo did not exhibit any morphological abnormality ( and ). Acridine orange (AO) staining to study apoptosis showed no significant staining in control embryos and 25 μg/ml of sodium selenite (), whereas SeNPs (25 μg/ml) ()-treated embryos showed green fluorescent spots on the heart and eye region of the body. This observation corroborates with that of other authors who also observed that different nanomaterials led to different types of malformations; AO staining revealed the induction of apoptosis around the heart region (CitationAsharani et al. 2008, CitationZhu et al. 2014). In a previous study, we also observed that 500-nm SeNPs led to formation of tail malformation and pericardial edema (CitationKalishwaralal et al. 2014).
Figure 4. Zebrafish embryos exposed to different concentrations of 100–200-nm SeNPs. (A- control; B-5 μg/ml; C- 10 μg/ml; D-15 μg/ml; E- 20 μg/ml; F- 25 μg/ml).
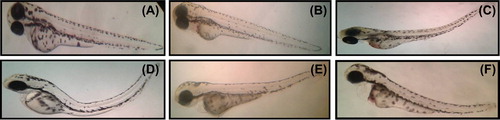
Figure 5. Malformations (e.g., pericardial edema) induced by SeNPs (A) Control; (B) 5 μg/ml; (C) 10 μg/ml; (D) 15 μg/ml; (E) 20 μg/ml; and (F) 25 μg/ml.
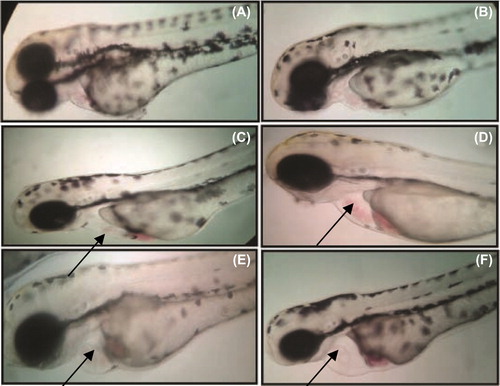
Figure 6. Percentage of malformation (tail, pericardial edema) induced by different concentrations of SeNPs (100–200nm) and sodium selenite.
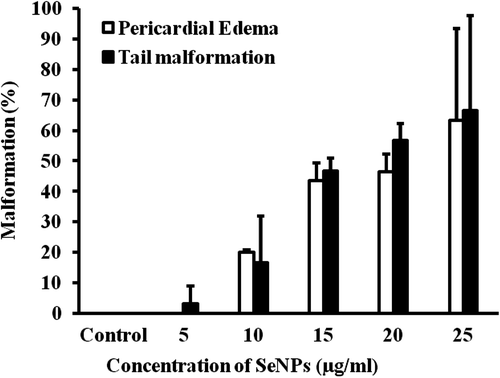
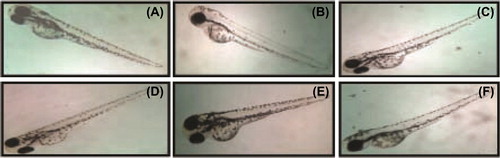
Figure 7. Zebrafish embryos exposed to different concentrations of sodium selenite. (A- control; B-5 μg/ml; C- 10 μg/ml; D-15 μg/ml; E- 20 μg/ml; F- 25 μg/ml).
Cardiac effects of SeNPs and sodium selenite
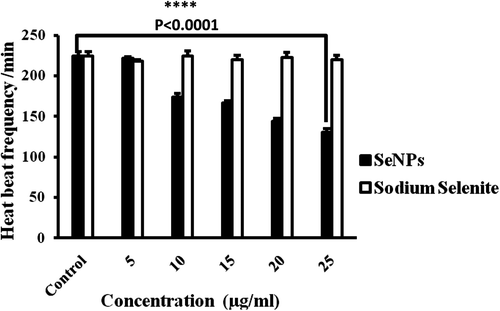
We compared the sodium selenite- and SeNPs-induced cardiovascular toxicity results in zebrafish with those derived from humans. We examined the heart rate of embryos exposed to SeNPs (5–25 μg/mL) at 96 hpf time point. As shown in , the heart rate decreased in a dose-dependent manner. At the highest concentration (25 μg/mL), the heart rate of embryos was 130 beats/min compared with 220 beats/min of control at 96 hpf (Supplementary Movie 1 & 2 to be found at online http://informahealthcare.com/doi/abs/10.3109/21691401.2015.1008507). In contrast, at the same concentration (25 μg/mL) of sodium selenite, the heart rates of the treated zebrafish embryos were comparable with those of the control group (220 beats/min); this indicates that there is no significant difference in the heart rate of embryos (Supplementary Movie 1 & 3 to be found at online http://informahealthcare.com/doi/abs/10.3109/21691401.2015.1008507). In the present study, exposure to high doses of SeNPs causes some cardiac defects, such as distorted looping and string-like chambers characterized by the ventricle located particularly anterior to the atrium, in zebrafish embryos and this may lead to cardiac failure, which are similar to those described for silica NPs and pyrene compounds (CitationDuan et al. 2013, CitationZhu et al. 2014). In contrast, sodium selenite exposure could still lead to no heart failure. Based on the present findings using in vivo zebrafish model, it can be concluded that low doses of SeNPs and sodium selenite treatment may contribute as therapeutic agents for cardiovascular tissue engineering and transplantation.
Conclusion
Green synthesis of 100–200-nm size of SeNPs from assorted compounds such as protein is a nontoxic eco-friendly effort with affordable cost under specific parameters of temperature and pH. Thus, the protein-mediated synthesis of SeNPs method acts as a favorable approach when compared with that of bacteria-mediated synthesis of SeNPs. Thus, this work paves way for selecting the therapeutic dose of SeNPs and sodium selenite.
Supplementary material available online
ianb_a_1008507_sm1126.zip
Download Zip (16.5 MB)Acknowledgments
The authors greatly thank ful to Kalasalingam University for the facilities provided. Mr. A. Raja of Karunya University, Coimbatore is acknowledged for his help with SEM and EDX analysis.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Rayman MP. 2012. Selenium and human health. Lancet. 379: 1256–1268.
- Murphy J, Hannon EM, Kiely M, Flynn A, Cashman KD. 2002. Selenium intakes in 18–64-y-old Irish adults. Eur J Clin Nutr. 56:402–408.
- Loscalzo J. 2014. Keshan disease, selenium deficiency, and the selenoproteome. N Engl J Med. 370:1756–1760.
- Alexaniana I, Parissisa J, Farmakisa D, Pantzioub C, Ikonomidisa I, Paraskevaidisa I, et al. 2014. Selenium contributes to myocardial injury and cardiac remodeling in heart failure. Int J Cardiol. 176:272–273.
- WHO/FAO/IAEA. 1996. Trace Elements in Human Nutrition and Health, World Health Organization, Geneva.
- Weekley CM, Harris HH. 2013. Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem Soc Rev. 42:8870–8894.
- Fesharaki PJ, Nazari P, Shakibaie M, Rezaie S, Banoee M, Abdollahi M, Shahverdi AR. 2010. Biosynthesis of selenium nanoparticles using Klebsiella pneumoniae and their recovery by a simple sterilization process. Braz J Microbiol. 41:461–466.
- Zhang W, Chen Z, Liu H, Zhang L, Gao P, Li D. 2011. Biosynthesis and structural characteristics of selenium nanoparticles by Pseudomonas alcaliphila. Colloids Surf B Biointerfaces. 88:196–201.
- Dhanjal S, Cameotra SS. 2010. Aerobic biogenesis of selenium nanospheres by Bacillus cereus isolated from coalmine soil. Microb Cell Fact. 9:52.
- Lee JH, Han J, Choi H, Hur HG. 2007. Effects of temperature and dissolved oxygen on Se (IV) removal and Se (0) precipitation by Shewanella sp. HN-41. Chemosphere. 68:1898–1905.
- Mishra RR, Prajapati S, Das J, Dangar TK, Das N, Thatoi H. 2011. Reduction of selenite to red elemental selenium by moderately halotolerant Bacillus megaterium strains isolated from Bhitarkanika mangrove soil and characterization of reduced product. Chemosphere. 84:1231–1237.
- Bajaj M, Schmidt S, Winter J. 2012. Formation of Se (0) Nanoparticles by Duganella sp. and Agrobacterium sp. isolated from Se-laden soil of North-East Punjab, India Microb Cell Fact. 11:64.
- Lü X, Cui S. 2010. Wool keratin-stabilized silver nanoparticles. Bioresour Technol. 101:4703–4707.
- Martin JJ, Cardamone JM, Irwin PL, Brown EM. 2011. Keratin capped silver nanoparticles– synthesis and characterization of a nanomaterial with desirable handling properties. Colloids Surf B Biointerfaces. 88:354–361.
- Groh KJ, Dalkvist T, Piccapietra F, Behra R, Suter MJ, Schirmer K. 2014. Critical influence of chloride ions on silver ion-mediated acute toxicity of silver nanoparticles to zebrafish embryos. Nanotoxicology. doi:10.3109/17435390.2014.893379.
- Hatfield DL, Tsuji PA, Carlson BA, Gladyshev VN. 2014. Selenium and selenocysteine: roles in cancer, health, and development. Trends Biochem Sci. 39:112–120.
- Stoppe C, Spillner J, Rossaint R, Coburn M, Schälte G, Wildenhues A, Marx G, Rex S. 2013. Selenium blood concentrations in patients undergoing elective cardiac surgery and receiving perioperative sodium selenite. Nutrition. 29:158–165.
- Asharani PV, Wu YL, Gong Z, Valiyaveettil S. 2008. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology. 19:255102.
- Jia X, Liu Q, Zou S, Xu X, Zhang L. 2015. Contruction of selenium nanoparticles/β-glucan composites for enhancement of the antitumor activity. Carbohydr Polym. 117:434–442.
- Vallee A, Humblot V, Pradier CM. 2010. Peptide interactions with metal and oxide surfaces. Acc Chem Res. 43: 1297–1306.
- Duan J, Yu Y, Li Y, Yu Y, Sun Z. 2013. Cardiovascular toxicity evaluation of silica nanoparticles in endothelial cells and zebrafish model. Biomaterials. 34:5853–5862.
- Debieux CM, Dridge EJ, Mueller CM, Splatt P, Paszkiewicz K, Knight I, et al. 2011. A bacterial process for selenium nanosphere assembly. Proc Natl Acad Sci U S A. 108:13480–13485.
- Durán N, Marcato PD, Durán M, Yadav A, Gade A, Rai M. 2011. Mechanistic aspects in the biogenic synthesis of extracellular metal nanoparticles by peptides, bacteria, fungi, and plants. Appl Microbiol Biotechnol. 90:1609–1624.
- Lee KJ, Nallathamby PD, Browning LM, Osgood CJ, Xu XH. 2007. In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. ACS Nano. 1:133–143.
- Zhu JJ, Xu YQ, He JH, Yu HP, Huang CJ, Gao JM, et al. 2014. Human cardiotoxic drugs delivered by soaking and microinjection induce cardiovascular toxicity in zebrafish. J Appl Toxicol. 34: 139–148.
- Kalishwaralal K, Jeyabharathy S, Sundar K, Muthukumaran A. 2014. A novel one pot green synthesis of selenium nanoparticles and evaluation of its toxicity in zebrafish embryos Artif Cells Nanomed Biotechnol. doi: 10.3109/21691401.2014.962744.
- Kitching M, Ramani M, Marsili E. 2014. Fungal biosynthesis of gold nanoparticles: mechanism and scale up. Microb Biotechnol. doi: 10.1111/1751–7915.12151.
- Lü X, Cui S. 2010. Wool keratin-stabilized silver nanoparticles. Bioresour Technol. 101:4703–4707.
- Ebert R, Ulmer M, Zeck S, Meissner-Weigl J, Schneider D, Stopper H, et al. 2006. Selenium supplementation restores the antioxidative capacity and prevents cell damage in bone marrow stromal cells in vitro. Stem Cells. 24:1226–1235.
- Soumya RS, Vineetha VP, Salin Raj P, Raghu KG. 2014. Beneficial properties of selenium incorporated guar gum nanoparticles against ischemia/reperfusion in cardiomyoblasts (H9c2). Metallomics. 6:2134–2147.
- Soumya RS, Vineetha VP, Reshma PL, Raghu KG. 2013. Preparation and characterization of selenium incorporated guar gum nanoparticle and its interaction with H9c2 cells. PLoS One. 8:e74411.
- Zheng C, Wang J, Liu Y, Yu Q, Liu Y, Deng N, Liu J. 2014. Functional Selenium Nanoparticles Enhanced Stem Cell Osteoblastic Differentiation through BMP Signaling Pathways. Adv Funct Mater. 24:6872–6883. doi: 10.1002/adfm.201401263.
- Das S, Dhar BB. 2014. Green synthesis of noble metal nanoparticles using cysteine-modified silk fibroin: catalysis and antibacterial activity. RSC Adv. 4:46285–46292.