Abstract
The aim of this study was to prepare and characterize spray dried inhalable chitosan nanoparticles (CNPs) for sustained delivery of anti-tubercular drugs, isoniazid (INH) and rifampicin (RIF), to the lungs. CNPs were prepared by ionic gelation technique followed spray drying. Results showed that the CNPs obtained had a smooth spherical shape with an average size of 230 ± 4.5 nm, with a poly dispersity index of 0.180 ± 0.021. Both drugs, were detected in various organs (lungs, liver, spleen and kidney) until 24 h post nebulization. The chemotherapeutic efficacy of a single dose of drug-loaded CNPs suggested that they are more effective against the mycobacterium than free drugs.
Introduction
Tuberculosis (TB) is a contagious disease containing the tubercle bacilli, Mycobacterium tuberculosis (MTb) (CitationKaur et al. 2014a, CitationChaubey and Mishra 2014). It mainly spreads by inhalation of bacilli-containing airborne droplets (CitationKaur et al. 2014b, CitationPatel et al. 2011). The failure of antitubercular drug (ATD) therapy is mainly due to patient non-compliance (CitationKaur et al. 2014c, CitationPourshahab et al. 2011), higher cost (CitationChaudhary et al. 2014, CitationSaraogi et al. 2011), lengthy treatment duration (CitationGagandeep et al. 2014), and the side-effects caused (CitationGarg 2014). In recent years, developments of nanoparticle-based formulations are being widely considered for direct delivery of ATDs to the lungs via the pulmonary route (CitationGarg and Goyal 2014, CitationSaraogi et al. 2010). Numerous biocompatible and biodegradable natural origin polymers (starch, gelatin, cellulose) are used as drug carriers, but chitosan was considered for this study due to its biocompatible and biodegradable nature as a non-toxic natural cationic polymer which has good mucoadhesive and membrane permeability-enhancing properties. It controls the drug release rate, prolonging the duration of therapeutic effects and delivering the drug to the specific sites in body. Chitosan also shows anti-microbial activity, which has also been reported for several years (CitationGarg et al. 2012, CitationSemete et al. 2010). The pulmonary route avoids the GI tract problems such as poor solubility, thereby enabling a reduction in the dose as well as in dosing frequency, and an improvement in patient compliance. The present study was planned to develop chitosan nanoparticles (CNPs) containing isoniazid (INH) and rifampicin (RIF), and characterized on the basis of morphology, entrapment efficiency and in vitro drug release. Chemotherapeutic efficacy and toxicity were also investigated against experimental murine TB.
Materials and methods
Materials
INH and RIF were received from BV Patel Centre (Ahmedabad, India) as gift samples. Chitosan with low molecular weight, Guar gum and Tripolyphosphate (TPP) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Mannitol and leucine were purchased from HiMedia Pvt. Ltd., Mumbai, India. All other reagents were of analytical grade, and were obtained from standard companies.
Animals
Female BALB/c mice (6–8 of weeks age, 20–25 g) obtained from the ISF College of Pharmacy, Moga, Punjab, India, were used in the study. The project was approved by the Institute Animal Ethics Committee. All efforts were made to reduce animal suffering.
Methods
Development and characterization of drug-loaded CNPs
CNPs were prepared by the ionotropic gelation technique (CitationKaur et al. 2014e). Briefly, TPP solution was added to chitosan solutions (0.5% w/v) containing different amounts of INH and RIF, under magnetic stirring. To prepare inhalable nanoparticles, the drug-loaded CNPs were spray-dried with mannitol (1% w/v) and leucine (0.25% w/v), using a spray dryer (LU 222 advanced, Labultima, Mumbai, India). The powders were then removed from the collector vessel and characterized (CitationKaur et al. 2014f).
CNPs were characterized for their size and polydispersity index (PDI) on a particle size analyzer (Beckman Coulter Pvt. Ltd., Delsa Nano C) (CitationKaur et al. 2014d). Scanning electron microscopy (SEM) was employed for visualization of the optimized nanoparticle shape. The amount of entrapped anti-tubercular drugs in the CNPs was calculated by estimating the amount of unentrapped drug recovered in the supernatant after centrifugation (CitationSingh et al. 2014a, Citation2014b, CitationSharma et al. 2014c). Drug release from CNPs was determined using the dialysis tube diffusion method at 37 ± 1°C. In this technique, nanoparticulate dispersions (10 mL) were separately placed into the dialysis membrane (MWCO 12000–14000 Da, HiMedia, India), tied at both the ends, and suspended in a beaker containing 50 mL of PBS (pH 7.4). The formulations were stirred at 50 rpm using a shaking incubator (LSB-1005RE, Daihan Labtech. Co. Ltd., Korea). At predetermined time intervals, samples were withdrawn and analyzed spectrophotometrically (UV-1601 Shimadzu, Japan) for INH and RIF content, at 262 nm and 473 nm respectively. The studies were performed in triplicate (CitationSharma et al. 2014a, Citation2014b, CitationRohilla et al. 2014).
In vivo drug disposition studies
For in vivo studies, female Balb/c mice (20–30 gm) were used for organ distribution studies of optimized formulations. A compressor nebulizer system (Pan Century, USA) was used in this study. For single dose drug disposition studies, female mice were grouped as follows, with six animals in each group: Group 1, free drug, aerosol; and Group 2, nanoparticle-loaded ATDs, aerosol. Animals were sacrificed at different time points, and drug levels were determined in lungs, liver, kidney and spleen tissue homogenates (CitationPabreja et al. 2014, CitationMorie et al. 2014, CitationModgill et al. 2014).
Experimental infection and chemotherapy
Experimental infection and chemotherapy studies were done in the National Jalma Institute for Leprosy and other Mycobacterial Diseases (JALMA), Agra, UP, India. The animals were infected with 4.2 × 107 bacilli/ml of M. tuberculosis H37Rv in 0.1 ml of 0.9% NaCl, through the aerosol route (Glas-Col inhalation exposure system, USA). The animals were then divided into the following groups: Group 1, control group; Group 2, naïve group; Group 3, free drug, aerosol; and Group 4, nanoparticle-loaded ATDs, aerosol. The animals were sacrificed after 2 and 4 weeks of chemotherapy; lungs and spleen were removed, homogenized in sterile normal saline solution and plated on Middlebrook 7H10 Agar plates for colony-forming unit (CFU) estimation on the 14th and 28th days post inoculation (CitationMarwah et al. 2014, CitationMalik et al. 2014, CitationKaur et al. 2014h).
Statistical analysis
Statistical tests were performed with GraphPad Instat Software. A difference with a value of p ≤ 0.05 was considered statistically significant.
Results and discussions
Physicochemical characterization of chitosan nanoparticles
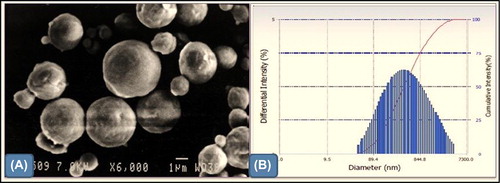
CNPs had a smooth spherical shape with an average size of 230 ± 4.5 nm, with a PDI of 0.180 ± 0.021 (). Smooth-surface nanoparticles are easily captured by the alveolar macrophage of lungs, and the low PDI of nanoparticles suggested a homogenous dispersion (CitationKaur et al. 2014g, CitationKataria et al. 2014).
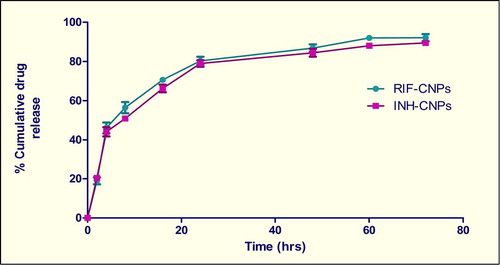
Drug encapsulation efficiency was observed to be 70.8 ± 6.62 for RIF and 68.8 ± 7.02 for INH. The higher drug encapsulation observed for CNPs in this study can be attributed to the higher drug/polymer ratio, as well as an excellent ionic gelation between TPP and chitosan (CitationJoshi et al. 2014, CitationJohal et al. 2014, CitationHussain et al. 2014). The release behavior of RIF and INH from the nanoparticulate formulation followed a biphasic pattern, that is, an initial burst (40–50% within 4 h) followed by a slower sustained release (90–95% for up to 72 h) (). Kinetic analysis of drug release from optimized formulations indicates that the drug is released from the nanoparticles by a diffusion mechanism. An initial burst release of RIF and INH from nanoparticles could be due to rapid dissolution of drug crystals located on the surface or present beneath the nanoparticle surface (CitationGoyal et al. 2013b, Citation2014a, Citation2014b).
In vivo drug disposition studies
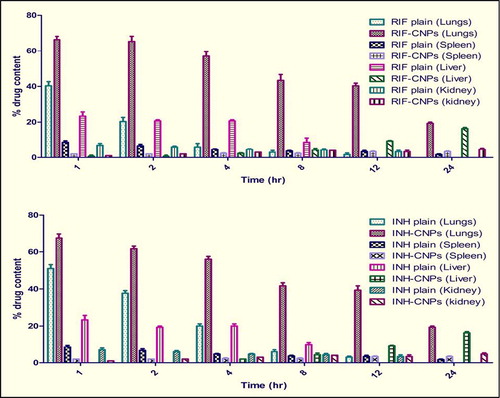
When the CNPs were administered through nebulization, the superiority of the ATD-loaded CNPs was seen in comparison to the plain drug in increasing the accumulation of ATDs within the organs rich in macrophages, that is, lungs. Both drugs were detected in tissues, that is, lungs, spleen, liver, and kidney, for up to 24 h (), thus prompting us to design the nebulized drug-loaded CNPs for chemotherapy. The higher lung uptake of CNPs may be attributed to preferential phagocytosis by macrophages and localization in alveolar macrophages (CitationGoyal et al. 2013a, CitationGarg et al. 2013, Citation2014b).
Chemotherapeutic efficacy
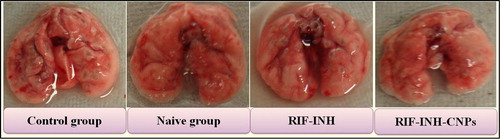
Administration of nebulized CNPs for 28 days to MTb- infected mice resulted in undetectable mycobacterial CFUs in lung and spleen homogenates (). These experimental observations suggested that CNPs are more effective against the mycobacterium than free drugs (). The efficacy of chitosan nanoparticle formulations shows an effective targeting to macrophage-rich organs in treating the disease (CitationGarg et al. 2014a, CitationGarg and Goyal 2014).
Table I. Chemotherapeutic efficacy of various formulations (mean ± S.D., n = 6).
Conclusions
In this study, drug-loaded CNPs were successfully synthesized and characterized as an effective delivery carrier for alveolar macrophage targeting. The optimized formulations showed lower cytotoxicity and significant reduction in the number of bacilli in the lungs, as compared to free drug. Finally, it may be concluded that CNPs may be exploited as a prospective tool for drug delivery to direct drugs to the lung tissues for the treatment of TB.
Acknowledgements
The author Mr. Tarun Garg is thankful to the Punjab Technical University (PTU), Kapurthala, Punjab, for providing an opportunity to carry out the research work. The author Dr. Amit K. Goyal (under IYBA scheme; BT/01/IYBA/2009 dated 24/05/2010) is thankful to the Department of Biotechnology (DBT), New Delhi, India, for providing financial assistance to carry out research work.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Chaubey P, Mishra B. 2014. Mannose-conjugated chitosan nanoparticles loaded with rifampicin for the treatment of visceral leishmaniasis. Carbohydr Polym. 101:1101–1108.
- Chaudhary S, Garg T, Murthy RS, Rath G, Goyal AK. 2014. Recent approaches of lipid-based delivery system for lymphatic targeting via oral route. J Drug Target. 22:871–882.
- Gagandeep, Garg T, Malik B, Rath G, Goyal AK. 2014. Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur J Pharm Sci. 53:10–16.
- Garg T. 2014. Current nanotechnological approaches for an effective delivery of bio-active drug molecules in the treatment of acne. Artif Cells Nanomed Biotechnol. 20:1–8.
- Garg T, Goyal AK. 2014. Biomaterial-based scaffolds—current status and future directions. Expert Opin Drug Deliv. 11:767–789.
- Garg T, Kumar A, Rath G, Goyal AK. 2014a. Gastroretentive drug delivery systems for therapeutic management of peptic ulcer. Crit Rev Ther Drug Carrier Syst. 31:531–557.
- Garg T, Rath G, Goyal AK. 2014b. Ancient and advanced approaches for the treatment of an inflammatory autoimmune disease-psoriasis. Crit Rev Ther Drug Carrier Syst. 31:331–364.
- Garg T, Singh O, Arora S, Murthy R. 2012. Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst. 29:1–63.
- Garg T, Singh S, Goyal AK. 2013. Stimuli-sensitive hydrogels: an excellent carrier for drug and cell delivery. Crit Rev Ther Drug Carrier Syst. 30:369–409.
- Goyal AK, Rath G, Garg T. 2013a. Nanotechnological approaches for genetic immunization. DNA and RNA Nanobiotechnologies in Medicine: Diagnosis and Treatment of Diseases.p. 67–120.
- Goyal G, Garg T, Malik B, Chauhan G, Rath G, Goyal AK. 2013b. Development and characterization of niosomal gel for topical delivery of benzoyl peroxide. Drug Deliv.
- Goyal G, Garg T, Rath G, Goyal AK. 2014a. Current nanotechnological strategies for an effective delivery of drugs in treatment of periodontal disease. Crit Rev Ther Drug Carrier Syst. 31:89–119.
- Goyal G, Garg T, Rath G, Goyal AK. 2014b. Current nanotechnological strategies for treating glaucoma. Crit Rev Ther Drug Carrier Syst. 31:365–405.
- Hussain T, Garg T, Goyal AK, Rath G. 2014. Biomedical Applications of Nanofiber Scaffolds in Tissue Engineering. J Biomater Tiss Eng. 4:600–623.
- Johal HS, Garg T, Rath G, Goyal AK. 2014. Advanced topical drug delivery system for the management of vaginal candidiasis. Drug Deliv.1–14.
- Joshi D, Garg T, Goyal AK, Rath G. 2014. Advanced drug delivery approaches against periodontitis. Drug Deliv.1–15.
- Kataria K, Sharma A, Garg T, Goyal AK, Rath G. 2014. Novel technology to improve drug loading in polymeric nanofibers. Drug Deliv Lett. 4:79–86.
- Kaur M, Garg T, Narang RK. 2014a. A review of emerging trends in the treatment of tuberculosis. Artif Cells Nanomed Biotechnol.1–7.
- Kaur M, Garg T, Rath G, Goyal AK. 2014b. Current nanotechnological strategies for effective delivery of bioactive drug molecules in the treatment of tuberculosis. Crit Rev Ther Drug Carrier Syst. 31:49–88.
- Kaur M, Malik B, Garg T, Rath G, Goyal AK. 2014c. Development and characterization of guar gum nanoparticles for oral immunization against tuberculosis. Drug Deliv.
- Kaur N, Garg T, Goyal AK, Rath G. 2014d. Formulation, optimization and evaluation of curcumin-beta-cyclodextrin-loaded sponge for effective drug delivery in thermal burns chemotherapy. Drug Deliv.1–10.
- Kaur R, Garg T, Das Gupta U, Gupta P, Rath G, Goyal AK. 2014e. Preparation and characterization of spray-dried inhalable powders containing nanoaggregates for pulmonary delivery of anti-tubercular drugs. Artif Cells Nanomed Biotechnol.1–6.
- Kaur R, Garg T, Malik B, Gupta UD, Gupta P, Rath G, Goyal AK. 2014f. Development and characterization of spray-dried porous nanoaggregates for pulmonary delivery of anti-tubercular drugs. Drug Deliv.1–6.
- Kaur R, Garg T, Rath G, Goyal AK. 2014g. Advanced aerosol delivery devices for potential cure of acute and chronic diseases. Crit Rev Ther Drug Carrier Syst. 31:495–530.
- Kaur V, Garg T, Rath G, Goyal AK. 2014h. Therapeutic potential of nanocarrier for overcoming to P-glycoprotein. J Drug Target.1–12.
- Malik R, Garg T, Goyal AK, Rath G. 2014. Polymeric nanofibers: targeted gastro-retentive drug delivery systems. J Drug Target.1–16.
- Marwah H, Garg T, Goyal AK, Rath G. 2014. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv.1–15.
- Modgill V, Garg T, Goyal AK, Rath G. 2014. Permeability study of ciprofloxacin from ultra-thin nanofibrous film through various mucosal membranes. Artif Cells Nanomed Biotechnol.1–6.
- Morie A, Garg T, Goyal AK, Rath G. 2014. Nanofibers as novel drug carrier - An overview. Artif Cells Nanomed Biotechnol.1–9.
- Pabreja S, Garg T, Rath G, Goyal AK. 2014. Mucosal vaccination against tuberculosis using Ag85A-loaded immunostimulating complexes. Artif Cells Nanomed Biotechnol.1–8.
- Patel S, Chavhan S, Soni H, Babbar AK, Mathur R, Mishra AK, Sawant K. 2011. Brain targeting of risperidone-loaded solid lipid nanoparticles by intranasal route. J Drug Target. 19:468–474.
- Pourshahab PS, Gilani K, Moazeni E, Eslahi H, Fazeli MR, Jamalifar H. 2011. Preparation and characterization of spray dried inhalable powders containing chitosan nanoparticles for pulmonary delivery of isoniazid. J Microencapsul. 28:605–613.
- Rohilla R, Garg T, Goyal AK, Rath G. 2014. Herbal and polymeric approaches for liver-targeting drug delivery: novel strategies and their significance. Drug Deliv.1–17.
- Saraogi GK, Gupta P, Gupta UD, Jain NK, Agrawal GP. 2010. Gelatin nanocarriers as potential vectors for effective management of tuberculosis. Int J Pharm. 385:143–149.
- Saraogi GK, Sharma B, Joshi B, Gupta P, Gupta UD, Jain NK, Agrawal GP. 2011. Mannosylated gelatin nanoparticles bearing isoniazid for effective management of tuberculosis. J Drug Target. 19:219–227.
- Semete B, Booysen L, Lemmer Y, Kalombo L, Katata L, Verschoor J, Swai HS. 2010. In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomedicine. 6:662–671.
- Sharma A, Garg T, Aman A, Panchal K, Sharma R, Kumar S, Markandeywar T. 2014a. Nanogel-an advanced drug delivery tool: Current and future. Artif Cells Nanomed Biotechnol.1–13.
- Sharma R, Garg T, Goyal AK, Rath G. 2014b. Development, optimization and evaluation of polymeric electrospun nanofiber: A tool for local delivery of fluconazole for management of vaginal candidiasis. Artif Cells Nanomed Biotechnol.1–8.
- Sharma R, Singh H, Joshi M, Sharma A, Garg T, Goyal AK, Rath G. 2014c. Recent advances in polymeric electrospun nanofibers for drug delivery. Crit Rev Ther Drug Carrier Syst. 31:187–217.
- Singh B, Garg T, Goyal AK, Rath G. 2014a. Recent advancements in the cardiovascular drug carriers. Artif Cells Nanomed Biotechnol.1–10.
- Singh H, Sharma R, Joshi M, Garg T, Goyal AK, Rath G. 2014b. Transmucosal delivery of Docetaxel by mucoadhesive polymeric nanofibers. Artif Cells Nanomed Biotechnol.