Abstract
This study was aimed at comparing the osseointegration of titanium (Ti)-based Küntscher nails (K-nails) and plates with modified nanostructured and hydroxyapatite-coated surfaces in a rat femur model. Material surfaces were first modified via a simple anodization protocol in which the materials were treated in hydrogen fluoride (1% w/w) at 20 V. This modification resulted in tubular titanium oxide nanostructures of 40–65 nm in diameter. Then, hydroxyapatite-deposited layers, formed of particles (1–5) μm, were produced via incubation in a simulated body fluid, followed by annealing at 500°C. Both surface modifications significantly improved cell proliferation and alkaline phosphatase (ALP) activity as compared to the control (non-modified Ti implants). The controls and modified nails and plates were implanted in the femur of 21 male Sprague-Dawley rats.
The implants, with surrounding tissues, were removed after 10 weeks, and then mechanical tests (torque and pull-out) were performed, which showed that the modified K-nails exhibited significantly better osseointegration than the controls. Histologic examinations of the explants containing plates showed similar results, and the modified plates exhibited significantly better osseointegration than the controls. Surface nanostructuring of commercially available titanium-based implants by a very simple method – anodization – seems to be a viable method for increasing osseointegration without the use of bioactive surface coatings such as hydroxyapatite.
Introduction
Titanium (Ti)-based materials are widely used by orthopedic surgeons for joint replacement and fixation; however, they can negatively affect surgical outcome when not properly osseointegrated, which causes the loosening of the implants and instability of the bone fragment. Osseointegration of Ti implants are essential, especially in young patients (20–30 years old), and can be achieved via surface modifications.
It is well known that initial cell attachment and proliferation, followed by transformation into osteoblasts, is very important for the success of the implant and osseointegration. These processes can be accelerated and designed to achieve better osseointegration, by optimization of the surface chemistry (e.g., an oxide layer, chemical composition of coatings, etc.) and morphology (a crystalline structure, roughness, etc.) (CitationAnselme et al. 2000a, CitationDiniz et al. 2002, CitationGiavaresi et al. 2003, CitationPark et al. 2007).
Most commercially available Ti implants have anatase and rutile phase surface properties, and mainly an amorphous oxide layer. Some studies have reported that surface Ti crystal structure affects bone response to implants, whereas others have reported that there are no significant differences (CitationFeng et al. 2003, CitationRipamonti et al. 2012, CitationWheeler 1996). Numerous in vitro and in vivo studies have reported that cell proliferation increases as the roughness of the Ti implant surface increases (CitationCastellani et al. 1999). It has been posited that not only surface roughness, but also the method used to increase surface roughness, affect cell attachment and proliferation (CitationDiniz et al. 2002). Although many studies report that cell proliferation increases as the roughness of the implant surface increases, others report that micro roughness does not increase cell proliferation (CitationAnselme et al. 2000b). The primary parameter indicative of the transformation of osteogenic cells into osteoblasts is alkaline phosphatase (ALP) activity. It has been shown that cells cultured on rough surfaces produce more (ALP) activity than cells cultured on smooth surfaces (CitationKokubo et al. 2006, CitationBäche and Kohal 2004, CitationCooper 2000, CitationRipamonti et al. 2012, CitationSpivak et al. 1990).
As hydroxyapatite (HA) has very similar properties to the apatite present in bone, it is widely used for biofunctionalization of inert metallic implants, to accelerate bone formation during the early osseointegration period. Nonetheless, HA-coating is associated with some disadvantages (Citationde Sena et al. 2002). A common problem with HA coatings is that the HA layer weakly adheres to metal surfaces. Although some studies indicate that there is a chemical bond formed between the HA–Ti interfaces (CitationFiliaggi et al. 1991, CitationJi et al. 1992, CitationYu-Liang et al. 1999), it has been shown that the bond is not sufficiently strong and can cause implant failure in the long term (CitationFiliaggi et al. 1991, CitationTsui et al. 1998). The sintering process is used to overcome this problem; however, sintering can cause HA decomposition. Another approach to increase HA– metal bonding is to increase the roughness of metal surfaces (CitationKokubo et al. 1990, Citationda Silva et al. 2001). It should be noted that loosening of HA-coated Ti implants is also a common problem that limits the use for dental applications (CitationGiavaresi et al. 2003, CitationYoon et al. 2010, CitationZhu et al. 2004).
These findings indicate that even though Ti-based implants with and without HA coating are among the most widely used materials for clinical bone repair, they have some important limitations, especially those related to osseointegration/loosening, that should be eliminated or reduced. As such, the present study aimed to determine whether creating nanostructures on the surface of Ti implants improves HA-Ti interfacial interaction forces, or whether the use of nanostructured surfaces only (without HA coating) promotes successful osseointegration.
We produced tubular nanostructures on the surface of Ti-based alloys, namely K-nails and plates, by anodization, and then deposited HA layers on top of these nanostructures, in order to investigate the effect of these surface modifications on osseointegration in a rat femur model.
Materials and methods
Materials preparation
We used highly purified Ti-based biomaterials (99.7%) for clinical bone repair (Trimed® Ankara, Turkey). K-nails of 20 mm height and 2 mm diameter, and Ti plates of 10 mm length, 3 mm width and 1 mm height, were prepared and donated by Trimed®. Non-modified K-nails and Ti plates (Group I - control), and modified K-Nails (Group 2) and modified plates (Group 3), were utilized. Modifications (nanostructuring) of these material surfaces was achieved by two methods, anodization, and HA deposition.
Before anodization, the materials were mechanically cleaned using silicon carbide (SiC) sand paper, and were polished with a diamond polishing material (Struers, Germany). They were then sonicated in a detergent solution for 5 min, followed by sonication in a 70% ethyl alcohol solution. In order to remove the oxide layer on the so-called pacified Ti surfaces, the materials were placed in an etching solution (1% HF; 1.5% HNO3) for 5 min, washed with deionized water, and then dried in an oven at 70°C. Lastly, the materials were subjected to an anodization procedure using a potentiostat (Micro Medical Electronics, Ankara, Turkey) in an HF solution (1% w/w) at 20 V and 5A for 20 min at room temperature. These anodized K-nails and Ti plates (NanoTi) constituted Group 2.
Following the anodization process, some of the materials were further modified by a deposit of HA on their surfaces. These materials were incubated for 3 h in a 10x simulated body fluid (SBF) solution containing 1000 mM NaCl, 5 mM KCl, 25 mM CaCl.2H2O, 5 mM MgCl2.6H2O, 3.62 mM NaH2PO4.H2O, and 10 mM NaHCO3, and were sintered at 500°C in ambient air for 1 h, as previously described [14, 15, 17, 20]. These HA-coated K-nails and Ti plates (NanoTiHA) constituted Group 3.
The morphology of the material's surface was investigated by scanning electron microscopy (SEM) (Quanta 200 FEG, FEI Instruments, Oregon, USA). Contact angles were measured using the sessile drop technique (Krüss, DSA 100, Hamburg, Germany). Deionized water droplets of about 3 μL volume were dropped on the surfaces, and measurements were recorded at 20°C. For each surface, at least 8 measurements at different points were obtained. The results are presented as mean values.
In vitro tests
The human osteosarcoma cell line (Saos-2/An1, SAP Institute, Ankara, Turkey) was used to investigate cell proliferation. The cells were cultured as a monolayer in Dulbecco's Modified Eagle's Medium (DMEM), (Sigma-Aldrich, USA) supplemented with 10% fetal bovine serum (FBS), (BIOCHROM, Germany), and 1% L-glutamine (Invitrogen, USA), together with 100 U mL− 1 of penicillin (Invitrogen, CA, USA) and 100 mg mL− 1 of streptomycin (Invitrogen, USA). The medium was replaced every 3 d, and the cultures were maintained in 5% CO2 at 37°C. When the cells reached 80% confluence, they were trypsinized with 0.25% trypsin containing 1 mM ethylene-diamine tetraacetic acid (EDTA) (Invitrogen, CA, USA), and were enumerated using a hemocytometer (Hausser Scientific, PA, USA) prior to use. These cells were then used in the following cell culture tests.
Cell culture tests were performed only with the Ti plates (both modified and non-modified). Samples (n = 6) were placed in 24-well plates, and 50 μL of cell suspension (2 × 103 cells) was transferred into each well. The well plates were incubated at room temperature for 2 h, and then 1 mL of culture medium was transferred to the wells; the culture medium was replaced with fresh medium every 3 d. The samples were cultured for 3, 5 and 7 d, and cell proliferation on the sample surfaces was measured using the MTT test. Briefly, the samples were washed with PBS buffer, followed by 4 h of incubation in 200 μL of culture medium and 30 μL of MTT solution. Then, the culture medium was removed and 200 μL of pure isopropanol containing 0.04 M HCl was transferred into each well. The wells were then incubated at room temperature for 15 min. This solution was then transferred to 96-well plates and formazan absorbance was measured at 570 nm, using a microplate reader (Biochrom, Asys Expert Plus Microplate Reader, Cambridge, England).
The samples were treated with lysis buffer containing 1% Triton X-100. The pellets and supernatants of the cell lysates were separated out via centrifugation at 12,000 rpm for 10 min, and then the supernatant was used to measure ALP activity. P-nitrophenylphosphate (pNNP) was used as the substrate for this purpose. ALP activity of the control samples (without supernatant) was measured at 405 nm by reading the reaction (Jasco V-530, Tokyo, Japan). The ALP activity was measured using a SensoLyte® pNPP Colorimetric Alkaline Phosphatase Assay Kit (Anaspec, CA, USA)
Animal model
The study protocol was conducted in accordance with the local laws on animal research and was approved by the Hacettepe University Animal Experimentation Ethics Committee (date: 14.05.2010, #:2010/30-2). Male Sprague-Dawley rats, (n = 21) aged 7 weeks and weighing 300–350 g, were kept in standard cages with a 12 h light/dark cycle. The room temperature and humidity were maintained at 20 ± 10°C and 40 ± 10%, respectively. Care was taken to avoid unnecessary stress and discomfort to the animals throughout the experimental period. The animals were divided into 3 groups, each with 7 rats: control – Group 1, NanoTi – Group 2, and NanoTiHA – Group 3.
Surgery was performed under aseptic conditions. Food was withheld from the animal for 8 h prior to surgery. The rats were anesthetized via intraperitoneal injection of Ketamine HCL at 10 mLkg− 1 (Parke Davis, 50 mg ml− 1, Taiwan) and Rompun at 1 mLkg− 1 (2%; Bayer, Germany). Anesthesia was performed by an independent animal handler in accordance with the standard protocol, under the supervision of a veterinarian. The rats’ legs were shaved and disinfected using a10% iodine solution (Batticon, Droksan, Turkey). Skin-subcutaneous tissues were cut from a 2 cm incision made on the lateral side of the right and left femurs so that it passed the intermuscular plane of the flexor and extensor muscles. The femur and the bone crest were exposed via subperiosteal dissection. The left femur was divided in two from 1/3 proximal under saline irrigation, using a 1 mm trephine bur inserted on the tip of a rotary cutting accessory. K-nails were implanted in the left femurs that were divided in two along the medulla (). The crest was pierced, and stainless steel nails (No. 4) were passed through the holes, so that rigid bone fixation was attained. With this method, axial and lateral movements of the femur were prevented. Using this animal model, the aim was to measure the effect of implant surface modification on implant integration with cancellous bone and the formation of new bone tissue, based on torque and pull-out tests.
Figure 1. Representative photographs showing implantation of Ti-based materials in rats. A. K-nails. B. Plates.
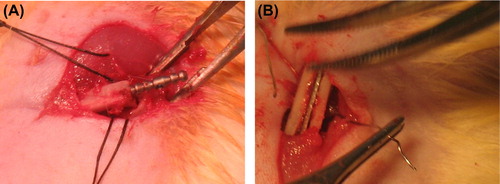
On the lateral side of the right femur, a longitudinal opening the same size as the plate implants was made at the lateral cortex using a 1 mm bur, and then the plates were inserted at the single-layer osteotomy line (). Using this animal model, the aim was to measure histologic changes and the rate of osseointegration of surface-modified implants, and to compare these findings with those in the control groups.
All incisions were closed using 5-0 silk sutures. The animals were sacrificed after 10 weeks, and the left femurs were removed with implants and stored in formaldehyde (4%) for histologic analysis, or in cold saline for mechanical testing the next day (pull-out and torque tests).
Mechanical tests
After removal of the stainless steel wire used for bone fixation, the femurs implanted with K-nails were mounted in a quick-setting epoxy resin (Buehler, IL, USA) to 8 mm barrels fitting the torque testing apparatus, so that a standard 1-cm length of bone centered on the implant site was exposed for testing. The test was performed on only 1 side of the explant. The torque failure force test was conducted using a CDI torque-meter (CDI Suretest, CDI Torque Products, CA, USA) with a 4 Nmm load cell. After torque testing, the femurs were carefully removed from the sample holder and then subjected to pull-out testing.
The pull-out test was performed on the samples that previously underwent torque testing, to determine the degree of integration of the implant with the medulla, using a Lloyd Universal Test LRX Internal Extensometer (Lloyd, UK) and a 500 N load cell, at a pull-out rate of 1 mm sec− 1.
Histology and histomorphometry
Histologic and histomorphometric analyses were only applied to the Ti plates implanted in the rat's right femur. The explants were cut with a 10 mm hand bur (Black and Decker, USA) and were fixed in a formalin solution in PBS buffer (pH 7.0). Samples were dehydrated sequentially in 30%, 60%, 80% and 99.8% ethanol solutions (obtained by dilution of 99.8% ethanol, Sigma-Aldrich, USA) and dried under pressurized air. The samples were then embedded into epoxy resin (Buehler, IL, USA) inside a 2 × 2 × 3 cm silicon mold, and were degassed under vacuum for 1 h. They were maintained at 40°C for 8 h, so that the resin could completely harden. Then, they were cut into sections 700 μm thick, using a diamond-tipped rotary-blade cutter (IsoMet 4000, Linear Precision Saw, Buehler, USA). For the histologic examination, 700 μm thick ground sections were further thinned to 50 μm using a polishing instrument (Phoenix Beta, Buehler, CA, USA).
The non-decalcified ground sections were stained with hematoxylin & eosin and Masson’ s trichrome, and were then evaluated as previously described (CitationRipamonti et al. 2012, CitationYoon et al. 2010). In brief, photomicrographs of each implant-containing region were generated using a light microscope (Leica, Germany) attached to a computerized digital camera (Model DFC 500, Leica, Germany). The entire implant-containing region was visible at 4x magnification. Bright-field images were captured and analyzed quantitatively using an image-processing program (LAS, Leica, Germany), in order to calculate the Bone to Implant Contact (BIC) percentage. The percentage of BIC was calculated in four consecutive sections from the bone cortex. Bone/implant BIC percentage ratios were considered as the mean for each sample.
Statistical analysis
The results of the torque and pull-out tests were compared by one-way ANOVA (95% significance level), and then the Tukey's test was used to identify statistically significant differences between the groups. A P value of < 0.05 was considered statistically significant, where the independent variables were the surface treatment methods and the dependent variables were torque failure and pull-out forces.
Histomorphometric data were analyzed using the nonparametric Kruskal-Wallis test for multiple comparisons, and the Mann Whitney U test with Bonferroni correction was used to identify significant differences between two groups. A P value of < 0.05 was considered statistically significant, with the independent variables as the surface treatment methods and the dependent variables as the BIC percentages.
Results
Surface characterization
Representative SEM images of the control, NanoTi, and NanoTiHA surfaces are shown in . As seen, anodization in HF (1% w/w) at 20 V resulted in tubular TiO2 structures of 40–65 nm in diameter. Note that the anodization process conditions were optimized in a preliminary study, to reach the present size range with homogeneous distribution.
Figure 2. Representative SEM micrographs of Ti surfaces before surface modification (control) (A), after anodization (NanoTi) (B), and after HA deposition (NanoTiHA) (C).
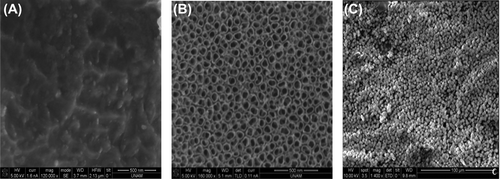
shows that the deposition of HA particles varying in size between sub-micron and 5 μm was achieved by treating anodized Ti materials with incubation in SBF solution, followed by annealing at 500°C.
Water contact angle measurements showed that the untreated Ti surfaces of the control had a water contact angle of 83.9°, versus 40.3° for the NanoTi surface (due to oxidation of the surfaces as a result of anodization), and 34.9° for the NanoTiHA surface (due to a more hydrophilic HA surface layer).
Cell proliferation
Cell culture studies were conducted to investigate the effects of Ti implant surface nanostructures with and without HA deposition, on cell proliferation. Cell proliferation findings are shown in . Cell proliferation steadily increased on all surfaces, and the differences between the surfaces increased with time. Cell proliferation was the highest on the surface with HA coating. The nanostructured surface (NanoTi) had significantly greater cell proliferation than the control on d-7.
Figure 3. Changes in formazan activity in cell cultures containing Ti plates show SAOS proliferation (both non-modified [control] and modified).
![Figure 3. Changes in formazan activity in cell cultures containing Ti plates show SAOS proliferation (both non-modified [control] and modified).](/cms/asset/c75d0e45-d1bd-4287-b88d-ff05a9930b52/ianb_a_1008512_f0003_b.gif)
As mentioned earlier, one of the primary properties of implant surfaces that facilitate the transformation of osteogenic cells into osteoblasts is ALP activity; therefore ALP activity in cell cultures was also studied. shows the changes in ALP activity over time in the cell culture experiments, which was quite similar to that observed via the MTT test. ALP activity was highest on the surfaces with HA coating (NanoTiHA); interestingly the nanotubular structures (NanoTi) also had much higher ALP activity that increased with time, compared to the controls.
Mechanical tests
Increasing osseointegration of Ti implants via surface modifications, namely nanostructuring and HA coating, was the primary target of this study, which was examined via mechanical testing (torque and pull-out).
Femurs implanted with K-nails were removed from the rats and first underwent torque tests, the results of which are given in the . The highest torque to failure value was obtained for the Ti nails with HA coating (NanoTiHA), which was significantly higher than that for the control. Nanostructuring alone (NanoTi) also improved the integration of the nails to the surrounding bone. Note that the mean torque to failure for intact bone of the rats was 417 ± 36.9 Nmm. The relative torque value in the control group was 45.17% of the intact femur, whereas the value was significantly higher for NanoTi (55.96%) and for NanoTiHA (60.86%) (P < 0.05).
Table I. Mechanical test.
Pull-out tests
Following torque testing, the pull-out failure forces were measured and statistically evaluated as described earlier (). The mean ± SD pull out-forces for the 3 implant materials tested was as follows; control: 2.4 ± 0.5 N, NanoTi: 4.4 ± 0.8, and NanoTiHA: 3.3 ± 0.5 N. Interestingly, the force needed for separation (pull-out) of the implants was the highest in the case of the Ti nails with a nanostructured surface (NanoTi group), which was 87.2% higher than that in the control group, whereas the pull-out force for the NanoTiHA implants was only 41.1% higher than that in the control group.
Histologic and histomorphometric analyses
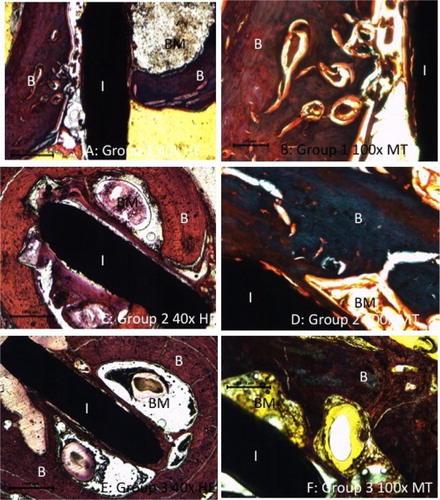
The effects of nanostructuring and HA deposition on osseointegration were histologically and histomorphometrically analyzed. Histologic analysis was only performed for the explants containing Ti plates (modified and non-modified). shows some representative images, t. The Ti implant is seen filling the marrow cavity and the entire layer of the compact bone in all groups. Bone marrow depletion and replacement by fibrous and/or bony tissue adjacent to the implant was observed in all groups. The implants were well integrated, with their surfaces having established intimate contact with mature cortical bone, indicating osseointegration. Nonetheless, in addition to integrated surfaces, compact bone containing some empty lacunae-lacking osteocytes was observed near the implant in several specimens of each group.
Figure 5. Micrographs of the non-decalcified sections show bone marrow depletion and replacement by fibrous and/or bone tissue adjacent to the implant. The implant exhibits osseointegration at several contact points. I: implant; B: bone; BM: bone marrow; H&E: hematoxylin-eosin; MT: Masson's trichrome.
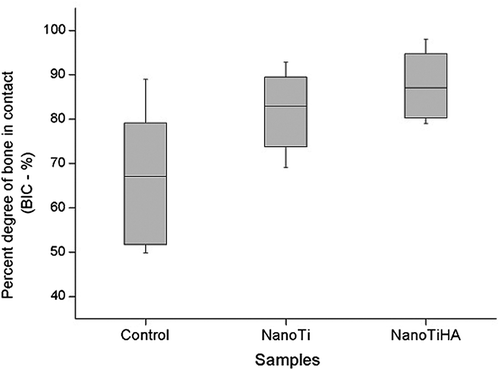
shows the BIC percentage. Specimens in the NanoTiHA group had a significantly higher BIC percentage than those in the control group (P = 0.007). Histomorphometric analysis showed that there wasn't a significant difference in the BIC percentage between the control and NanoTi groups or between the NanoTi and NanoTiHA groups.
Discussion
The primary aim of this study was to determine the effect of Ti-based implants treated with two surface modifications – at the nano and micro levels – with different surface topographies and chemistries, on osseointegration. The first Ti surface modification was the formation of TiO2 nanotubes 40–65 nm in diameter, via a simple anodization protocol in HF (CitationHuang and Yao 2005, CitationMacák et al. 2005). The diameter of the nanotubes was arranged/optimized based on preliminary research, in consideration of earlier studies which reported that nanotubes < 30 nm in diameter do not increase osteoblast functions, but induce cellular apoptosis (CitationGutwein and Webster 2004, CitationPark et al. 2007).
As mentioned in the Introduction, both the structure and composition of the oxide layer on Ti affect cell-surface interactions including osseointegration (CitationZhu et al. 2004) Published findings are inconsistent; however, there is general consensus that TiO2 surface layers facilitate better cell proliferation, which was considered during the planning of the present study (CitationFeng et al. 2003, CitationSul et al. 2001). In addition to oxidized surfaces, we aimed to create nanotubular structures by etching the surface layers, which resulted in an oxide layer.
In vitro cell culture experiments in the present study showed that cells proliferated significantly better on the modified Ti surfaces than on the non-modified (control) Ti surfaces, which could be considered an indication of improved osseointegration. Cells not only attached and proliferated better on modified Ti implant surfaces, but also exhibited higher ALP activity on the Ti implants further modified with HA coating (NanoTiHA), which was expected and is in agreement with the literature (CitationMoroni et al. 1999 CitationSato et al. 2008, CitationMavis et al. 2009). Interestingly, the TiO2 nanotubular structures that were prepared via a simple anodization process resulted in much higher cell proliferation and ALP activities than in the control samples.
Mechanical testing of the K-nails in the present study clearly showed that the TiO2 nanostructures positively affected osseointegration. According to the pull-out test results, the highest resistance to separation was observed for NanoTi, which was significantly better than that for both the control and NanoTiHA samples, which might have been because the HA layer deposited on top of the nanostructures reduced the binding forces between the material and bone surface; however, NanoTiHA samples showed the best mechanical resistance to torque, which is inconsistent with the pull-out test results, and difficult to explain. K-nails with different properties have been tested in numerous studies using similar animal models (primarily Sprague-Dawley rats); the torque values reported were in the range of 290–680 Nmm and were dependent on age, gender, fixation method, and fracture model (CitationRipamonti et al. 2012, CitationSul et al. 2001).
As it is similar to its biological counterpart, synthetic HA is one of the most widely used agents for surface modification of Ti-based materials. HA particles varying in size from nano to micron (varying in surface roughness) have been deposited (coated) on the Ti surface, with varying measures of thickness and via multiple techniques (CitationKokubo et al. 1996, Citationda Silva 2001). As reported earlier, an HA layer on implant surfaces accelerates bone formation during the early osseointegration period; however, depending on the size of the particles, surface layer thickness, and method of application to increase adherence of HA on Ti surfaces (such as sintering), several problems, including the loosening of the implant, have been observed in preclinical studies and in clinical uses. The method used in the present study is to obtain similar adherence and osseointegration, via nanostructuring alone or deposition of HA particles on nanotubes.
As shown in , micron-sized HA particles were deposited using a very simple incubation technique in SBF. In addition, sintering at 500°C, as suggested in the literature, was used to increase the binding strength of the HA-deposited layer and its interaction with the Ti sub-layer (CitationKusakabe et al. 2004, CitationMoroni et al. 1994, CitationMoroni et al. 1999, CitationSchliephake et al. 2003, CitationSøballe et al. 1990, CitationTsui et al. 1998). The present study's mechanical test and histologic findings showed that HA coating improved osseointegration and the binding forces between the implant and surrounding bone tissue. Binding was strongest with the HA-coated implants (NanoTiHA); however, nanostructuring alone produced a very similar effect on integration of the Ti implants to bone, which is the primary conclusion of this study.
Conclusion
There are many titanium implants in the market, for use in orthopedic clinics. Several surface modifications are implemented on these implants. Studies carried out on the effects of these surfaces are present in latest studies. It has been observed that tissue compatibility increases when implant surface characteristics change. In order to increase the long-term success and decrease the recovery time following surgery, several solutions are being applied.
In this study, anodic oxidation has been chosen from these methods of surface modification, and K-nails, used in orthopedic clinics, are used as the model. Initially, material cell compatibility has been studied through in vitro tests, and whether these surfaces are optimum for clinical use or not has been investigated through in vivo studies. According to both in vitro and in vivo test results, cell and tissue interaction has increased via anodic oxidation applied to surfaces. This reaction shows that osseointegration has increased. This surface modification, frequently used in dental implants, is also suggested to orthopedic clinics. This low-cost and reliable method can be used against early period losses and to increase implant-bone tissue compatibility. Osseointegration will be an essential parameter for even more successful implants in the future. This study shows that osseointegration increases through surface modification.
Acknowledgement
Prof. Erhan Pişkin has been supported by Turkish Academy of Sciences as a full member.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ágata de Sena L, Calixto de Andrade M, Malta Rossi A, de Almeida Soares G. 2002. Hydroxyapatite deposition by electrophoresis on titanium sheets with different surface finishing. J Biomed Mater Res. 60:1–7.
- Anselme K, Bigerelle M, Noel B, Dufresne E, Judas D, Iost A, Hardouin P. 2000a. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J Biomed Mater Res. 49:155–166.
- Anselme K, Linez P, Bigerelle M, Le Maguer D, Le Maguer A, Hardouin P, et al. 2000b. The relative influence of the topography and chemistry of TiAl6V4 surfaces on osteoblastic cell behaviour. Biomaterials. 21:1567–1577.
- Bächle M, Kohal R. 2004. A systematic review of the influence of different titanium surfaces on proliferation, differentiation and protein synthesis of osteoblast like MG63 cells. Clin Oral Implants Res. 15:683–692.
- Castellani R, De Ruijter A, Renggli H, Jansen J. 1999. Response of rat bone marrow cells to differently roughened titanium discs. Clin Oral Implants Res. 10:369–378.
- Cooper L. 2000. A role for surface topography in creating and maintaining bone at titanium endosseous implants 1. J Prosthet Dent. 84:522–534.
- Diniz M, Soares G, Coelho M, Fernandes M. 2002. Surface topography modulates the osteogenesis in human bone marrow cell cultures grown on titanium samples prepared by a combination of mechanical and acid treatments. J Mater Sci Mater Med. 13:421–432.
- Feng B, Weng J, Yang B, Qu S, Zhang X. 2003. Characterization of surface oxide films on titanium and adhesion of osteoblast. Biomaterials. 24:4663–4670.
- Filiaggi M, Coombs NA, Pilliar R. 1991. Characterization of the interface in the plasma-sprayed HA coating/Ti-6Al-4V implant system. J Biomed Mater Res. 25:1211–1229.
- Giavaresi G, Fini M, Cigada A, Chiesa R, Rondelli G, Rimondini L, et al. 2003. Mechanical and histomorphometric evaluations of titanium implants with different surface treatments inserted in sheep cortical bone. Biomaterials. 24:1583–1594.
- Gutwein L, Webster T. 2004. Increased viable osteoblast density in the presence of nanophase compared to conventional alumina and titania particles. Biomaterials. 25:4175–4183.
- Huang H, Yao X. 2005. Preparation and characterization of rutile TiO2 thin films by mist plasma evaporation: Surface and Coatings. Technology. 191:54–58.
- Ji H, Ponton C, Marquis P. 1992. Microstructural characterization of hydroxyapatite coating on titanium. J Mater Sci Mater Med. 3:283–287.
- Kokubo T. 1991. Bioactive glass ceramics: properties and applications. Biomaterials. 12:155–163.
- Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T. 1990. Solutions able to reproduce in vivo surface structure changes in bioactive glass ceramic A-W3. J Biomed Mater Res. 24:721–734.
- Kokubo T, Miyaji F, Kim HM, Nakamura T. 1996. Spontaneous formation of bonelike apatite layer on chemically treated titanium metals. J Am Ceram Soc. 79:1127–1129.
- Kokubo T, Takadama H. 2006. How useful is sbf in predicting in vivo bone bioactivity? Biomaterials. 27:2907–2915.
- Kusakabe H, Sakamaki T, Nihei K, Oyama Y, Yanagimoto S, Ichimiya M, et al. 2004. Osseointegration of a hydroxyapatite-coated multilayered mesh stem. Biomaterials. 25:2957–2969.
- Macák J, Tsuchiya H, Schmuki P. 2005. High-aspect-ratio TiO2 nanotubes by anodization of titanium. Angew Chem Int Ed. 44: 2100–2102.
- Mavis B, Demirtas TT, Gümüsderelioglu M, Gündüz G, Çolak Ü. 2009. Synthesis, characterization and osteoblastic activity of polycaprolactone nanofibers coated with biomimetic calcium phosphate. Acta Biomater. 5:3098–3111.
- Moroni A, Caja V, Egger E, Trinchese L, Chao EY. 1994. Histomorphometry of hydroxyapatite coated and uncoated porous titanium bone implants. Biomaterials. 15:926–930.
- Moroni A, Rocca M, Faldini C, Stea S, Giardino R, Giannini S. 1999. Hydroxyapatite fully coated conic hip prosthetic stem: A long term animal study. Ann Chir Gynaecol. 88:198–204.
- Park J, Bauer S, von der Mark K, Schmuki P. 2007. Nanosize and vitality: Tio2 nanotube diameter directs cell fate. Nano Lett. 7:1686–1691.
- Prado da Silva M, Lima J, Soares G, Elias C, De Andrade M, Best S, Gibson I. 2001. Transformation of monetite to hydroxyapatite in bioactive coatings on titanium. Surf Coat Technol. 137:270–276.
- Ripamonti U, Roden LC, Renton LF. 2012. Osteoinductive hydroxyapatite-coated titanium implants. Biomaterials. 33:3813–3823.
- Sato M, Aslani A, Sambito MA, Kalkhoran NM, Slamovich EB, Webster TJ. 2008. Nanocrystalline hydroxyapatite/titania coatings on titanium improves osteoblast adhesion. J Biomed Mater Res A. 84:265–272.
- Schliephake H, Scharnweber D, Dard M, Röler S, Sewing A, Hüttmann C. 2003. Biological performance of biomimetic calcium phosphate coating of titanium implants in the dog mandible. J Biomed Mater Res A. 64:225–234.
- Søballe K, Hansen ES, Brockstedt-Rasmussen H, Pedersen CM, Bünger C. 1990. Hydroxyapatite coating enhances fixation of porous coated implants: a comparison in dogs between press fit and noninterference fit. Acta Orthopaedica. 61:299–306.
- Spivak JM, Ricci JL, Blumenthal NC, Alexander H. 1990. A new canine model to evaluate the biological response of intramedullary bone to implant materials and surfaces. J Biomed Mater Res. 24:1121–1149.
- Sul YT, Johansson CB, Jeong Y, Röser K, Wennerberg A, Albrektsson T. 2001. Oxidized implants and their influence on the bone response. Mater Sci Mater Med. 12:1025–1031.
- Tsui Y, Doyle C, Clyne T. 1998. Plasma sprayed hydroxyapatite coatings on titanium substrates. Part 1:Mechanical properties and residual stress levels. Biomaterials. 19: 2015–2030.
- Wheeler SL. 1996. Eight-year clinical retrospective study of titanium plasma-sprayed and hydroxyapatite-coated cylinder implant. Int J Oral Maxillofac Implants. 11:340–350.
- Yoon HI, Yeo IS, Yang JH. 2010. Effect of a macroscopic groove on bone response and implant stability. Clin Oral Implants Res. 21:1379–1385.
- Yu-Liang C, Lew D, Park JB, Keller JC. 1999. Biomechanical and morphometric analysis of hydroxyapatite-coated implants with varying crystallinit. J Oral Maxillofac Surg. 57:1096–1108.
- Zhu X, Chen J, Scheideler L, Reichl R, Geis-Gerstorfer J. 2004. Effects of topography and composition of titanium surface oxides on osteoblast responses. Biomaterials. 25:4087–4103.


![Figure 4. Changes in ALP activity in cell cultures containing Ti plates (both non-modified [control] and modified).](/cms/asset/b430544f-467f-4fd0-8f37-75b0637e8195/ianb_a_1008512_f0004_b.gif)