Abstract
In the present study, we report a green methodology for the synthesis of silver and gold nanoparticles, using the root extract of the herbal medicinal plant Korean red ginseng. The silver and gold nanoparticles were synthesized within 1 h and 10 min respectively. The nanoparticles generated were not aggregated, and remained stable for a long time, which suggests the nature of nanoparticles. The phytochemicals and ginsenosides present in the root extract assist in reducing and stabilizing the synthesized nanoparticles. The red ginseng root extract-generated silver nanoparticles exhibit antimicrobial activity against pathogenic microorganisms including Vibrio parahaemolyticus, Staphylococcus aureus, Bacillus cereus, and Candida albicans. In addition, the silver nanoparticles exhibit biofilm degrading activity against S. aureus and Pseudomonas aeruginosa. Thus, the present study opens up a new possibility of synthesizing silver and gold nanoparticles in a green and rapid manner using Korean red ginseng root extract, and explores their biomedical applications.
Introduction
Recently, nanoparticles have been the focus of intense research, owing to their fascinating electronic, optical, and chemical properties, and promising biomedical applications (CitationKholoud et al. 2010). An important and challenging task in nanoparticle synthesis is the development of a simple, rapid, and ecofriendly methodology. Conventionally, physiochemical techniques underlie the reduction of silver ions, followed by surface modification, addition of additional capping ligands for stability, and use of organic solvents which raise environmental concerns, because of the toxic compounds used and hazardous by-products (CitationKholoud et al. 2010, CitationMarimuthu et al. 2011). These limitations invite a new eco-friendly methodology for the synthesis of nanoparticles in an eco-friendly manner. Several biological entities like bacteria, fungi, and plants have been explored for the ability to synthesize nanoparticles in an eco-friendly way (CitationSriram et al. 2012, CitationChauhan et al. 2011, CitationElavazhagan et al. 2011). The naturally synthesized enzyme, protein, flavonoid, and antioxidant compounds from microorganisms and plant extracts help in reducing and stabilizing the synthesized nanoparticles (CitationSriram et al. 2012, CitationChauhan et al. 2011, CitationElavazhagan et al. 2011, CitationKrishnaraj et al. 2014).
Here, we report the use of natural and herbal red ginseng medicinal roots for the synthesis of silver and gold nanoparticles. The plant belongs to the Araliaceae family, and is well known for its pharmacologic importance in humans (CitationKiefer and Pantuso 2003). The use of the herbal medicinal plant for nanoparticle synthesis avoids the contamination of some toxic chemicals and solvents in the nanoparticles, and hazardous by-products, and thus is an additional advantage for the production, and for the pharmaceutical and biomedical applications, of nanoparticles (CitationKim et al. 2014). The main constituents of red ginseng are ginsenosides or polysaccharides, amino acids, flavones, peptide glycans, acetylenic compounds, and pyran derivatives (CitationChoi et al. 2008). The ginsenosides have the ability to improve immunity, insulin sensitivity, and impaired memory, and have anti-aging and anti-cancer components, along with anti-stress and antioxidant activity as well (CitationKim et al. 2014). Thus, due to the tremendous benefits, we explored the plant for application in the field of nanotechnology, for the synthesis of nanoparticles in a rapid and economical way.
The development of antibiotic resistance in microorganisms is of major concern (CitationPal et al. 2007, CitationHumberto et al. 2010). Silver nanoparticles are well-known for their antimicrobial potential against a wide range of microorganisms. In the present study, we applied the synthesized nanoparticles for antimicrobial and biofilm degrading activity against pathogenic microorganisms.
This green method involves the production of silver and gold nanoparticles, further characterization by ultraviolet-visible spectroscopy (UV-vis), field emission transmission electron microscopy (FE-TEM), energy dispersive X-ray analysis (EDX), elemental mapping and dynamic light scattering (DLS). Furthermore, the silver nanoparticles were applied for antimicrobial and antibiofilm activity against the pathogenic microorganisms Vibrio parahaemolyticus, Staphylococcus aureus, Bacillus cereus, Candida albicans, and Pseudomonas aeruginosa.
Materials and methods
Materials
Silver nitrate (AgNO3) and gold (III) chloride trihydrate (HAuCl4·3H2O) were purchased from Sigma-Aldrich Chemicals, USA. All the media were purchased from Difco, MB Cell, Seoul, Republic of Korea. The pathogenic bacterial strains Vibrio parahaemolyticus [ATCC 33844], Staphylococcus aureus [ATCC 6538], Bacillus cereus [ATCC 14579], Candida albicans [KACC 30062], and Pseudomonas aeruginosa [ATCC 27853] were used. The bacterial strains were cultured on nutrient agar media at 37°C and preserved at − 70°C in glycerol stock vials. Candida albicans was cultured on Sabouraud dextrose agar at 28°C and preserved at − 70°C in glucose yeast peptone broth (GYP) glycerol stock vials for further study.
Preparation of red ginseng root extract
Red ginseng root powder was obtained after grinding the red ginseng root. First, 10 g of root powder was mixed with 100 ml of sterile water and boiled for 30 min, so that components leached out into the water. After 30 min of boiling, the collected liquid was filtered using a Whatman filter paper and was centrifuged at 10,000 rpm for 10 min, to remove any suspended material. The total volume of filtrate was maintained at 100 ml, with sterile water at the end of the process, and stored at 4°C for further use. This 100 ml of stock filtrate was used further for the synthesis of silver and gold nanoparticles.
Synthesis of silver and gold nanoparticles
For the synthesis of silver and gold nanoparticles, 5 ml from the 100 ml of red ginseng stock filtrate was mixed with 25 ml of sterile water. The silver nitrate and gold (III) chloride trihydrate was added with a final concentration of 1 mM in the corresponding reaction mixtures, separately. The reaction mixture was then kept at 80°C for reduction of Ag+ ions to Ag atoms and Au3+ to Au°. The change in color of the reaction mixture was observed continuously, which indicated the formation of silver and gold nanoparticles in the relevant reaction mixtures. For the collection and purification of nanoparticles, the reaction mixture was first centrifuged at 2000 rpm for 10 min, to remove any other unwanted components. Then, the nanoparticles were collected by centrifugation at 16,000 rpm for 15 min, in the form of a pellet, which was washed several times with sterile water to remove the other material and unwanted components. Then, the washed particles were finally kept overnight, air dried, and obtained in powder form. This respective product was used for further characterization and confirmation of nanoparticles.
Characterization of silver and gold nanoparticles
To verify the reduction of silver and gold ions, each solution was scanned in the range of 300–800 nm in a UV-vis spectrophotometer (Ultrospec 2100 Pro, Amersham Biosciences). The size, shape, morphology and distribution of the nanoparticles were analyzed using FE-TEM, EDX, and elemental mapping with a JEM-2100F (JEOL) instrument operated at 200 kV. The sample was prepared by placing a drop of the collected nanoparticles on a carbon-coated copper grid and subsequently drying in an oven at 60°C, before transferring it to the microscope. The size distribution profile of the nanoparticles was studied using DLS, by the particle size analyzer (Photal, Otsuka Electronics, Japan). The hydrodynamic diameters and polydispersity index (PDI) were analyzed at 25°C. As a reference, a dispersive medium of pure water with a refractive index of 1.3328, viscosity of 0.8878, and dielectric constant of 78.3 was used. The stability of the nanoparticles was observed before and after the addition of sodium hydroxide (base) to the nanoparticles. The effect of the change in pH on the stability of the nanoparticles was studied, over the pH range of 3–12.
Antimicrobial activity
The antimicrobial activity of the silver nanoparticles against pathogenic microorganisms such as V. parahaemolyticus, S. aureus, B. cereus, and C. albicans was measured on Muller-Hinton agar (MHA) plates using the well-diffusion method. In this assay, the MHA medium plates were spread evenly with 100 μL of an overnight log culture of test organisms. Wells were made on the MHA plates by using a gel puncture. Next, 50 μL of the biosynthesized silver nanoparticle reaction mixture was added into each of the four wells, and the plates were incubated at 37°C for 24 h. After incubation, the susceptibility pattern of the test organisms was determined by measuring the diameter of the zone of inhibition of each wells.
Determination of biofilm degrading activity
The biofilm degrading activity of silver nanoparticles was determined by the colorimetric method against S. aureus and P. aeruginosa (CitationGurunathan et al. 2014). Briefly, the wells of 96-well micro-titer plates were filled with 100 μL of overnight-grown log phase of S. aureus and P. aeruginosa. After culturing for 24 h, different concentrations of silver nanoparticles ranging from (1–4 μg) were added. The cell culture plates were then incubated for 4 h at 37°C. After incubation, the media were removed and the wells were washed three times with 200 μL of sterile water. Then, the microtiter plate was kept for air drying, for 45 min. Then, 200 μL of a 0.1% (v/v) crystal violet solution in water was added in each well and kept for 45 min. The wells were then washed three times with 300 μL of sterile water to remove excess stain. The dye incorporated by the adherent cells was solubilized with 200 μL of 95% (v/v) ethanol. The absorbance of each well was measured at 595 nm, using a microtiter ELISA reader. The absorbance was measured and the percentage inhibition of biofilm activity was calculated using the following equation: [1−(A595 of test/A595 of control)] × 100 (CitationWei et al. 2006). The experiments were performed in duplicate and data were interpreted in terms of mean ± SD.
Results and discussion
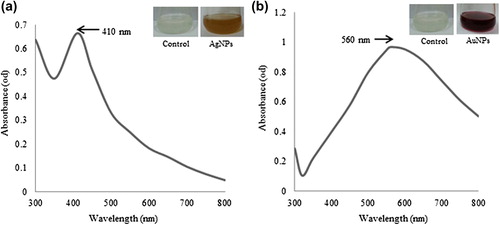
Various plants have been successfully used for the synthesis of silver and gold nanoparticles. The nontoxic phytochemicals, enzymes, proteins, flavonoids, and other biological molecules released by the living moieties assist in the synthesis of nanoparticles (CitationSriram et al. 2012, CitationChauhan et al. 2011, CitationElavazhagan et al. 2011, CitationKrishnaraj et al. 2014). In the present study, we have synthesized silver and gold nanoparticles by using the red ginseng root extract, without any capping and stabilizing agent. The reduction of silver nitrate and auric chloride trihydrate was visually evident from the color change of the reaction mixture after the incubation period at 80°C. For silver and gold nanoparticles, the reaction mixture completely turns to brown and ruby red within 1 h and 10 min, respectively. The characteristic surface plasmon resonance band of the nanoparticles was observed in the UV-vis spectrum, which showed maximum absorption peaks at 410 and 560 nm for the silver and gold nanoparticles, correspondingly (, ) (CitationElavazhagan et al. 2011).
Figure 1. UV-vis spectra of the contents of the reaction mixture for silver nanoparticles (a) and gold nanoparticles (b), respectively.
Results of the TEM analysis showed that the particles synthesized were spherical in shape, with sizes ranging from 10–30 nm (for both silver and gold nanoparticles) (, ). Moreover, the particles were evenly distributed and quite monodisperse in nature, which evades the complications associated with polydispersity of nanoparticles; this is a supplementary advantage of this methodology. The spherical-shaped nanoparticles have been synthesized using green methodology (CitationAtta et al. 2014, CitationMostafa et al. 2014) previously, and our results were in the same line.
Figure 2. TEM image of spherically shaped silver nanoparticles (a) and gold nanoparticles (b). EDX spectra of silver nanoparticles (c) and gold nanoparticles (d), respectively.
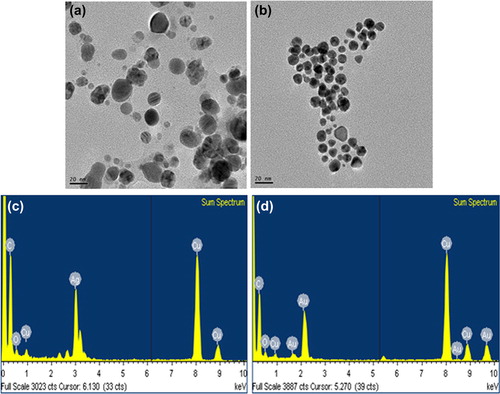
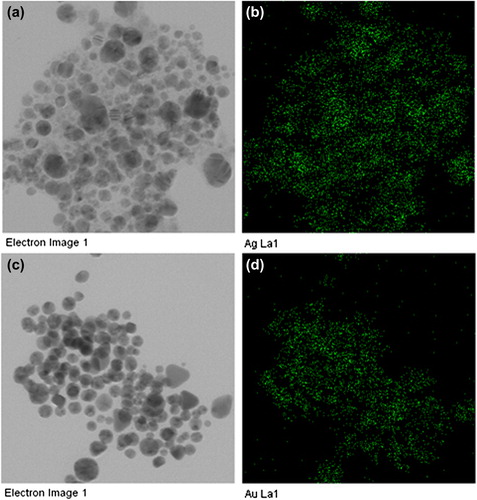
The purity of the biosynthesized silver and gold nanoparticles was examined by EDX (, ). The EDX spectrum displayed optical absorption band peaks at 3 keV and 2.3 keV, which are the characteristic peaks of nanosized metallic silver and gold, corresponding to surface plasmon resonance (CitationElavazhagan et al. 2011). In addition, the elemental mapping results reflect the distribution profile of the nanoparticles in their respective electron micrograph images, and showed that the silver and gold were the predominant elements in the corresponding products, which further confirm the purity of the nanoparticles () (CitationShankar et al. 2012).
Figure 3. The results of elemental mapping indicate the distribution of elements. TEM micrograph of silver nanoparticle pellet solution (a), and silver element (b), respectively. TEM micrographs of gold nanoparticle pellet solution (c), and gold element (d), respectively.
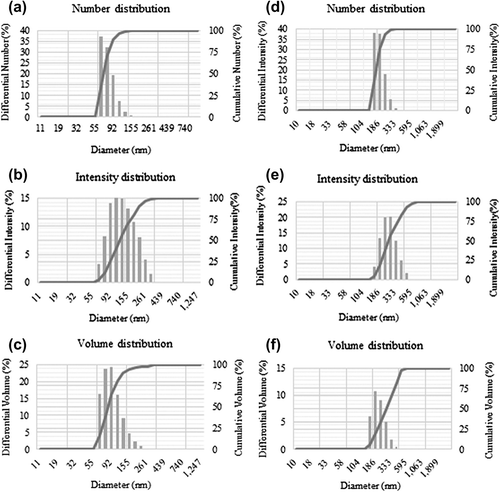
The DLS analysis of the nanoparticles showed the distribution of nanoparticles from three different aspects, which include size, number, and intensity. The results showed that the average particle size of silver nanoparticles was 83 nm, with a PDI value of 0.190 () and for gold nanoparticles 183 nm, with a PDI value of 0.159 ().
Figure 4. Particle size distribution of silver nanoparticles according to number (a), intensity (b), and volume (c), respectively. Particle size distribution of gold nanoparticles according to number (d), intensity (e), and volume (f), respectively.
The stability of the nanoparticles was examined by analyzing their UV-vis absorbance spectrum, after keeping them for several periods at room temperature. There was no apparent difference observed in the results, in the absorbance spectrum even after one month, which resembles the stability of nanoparticles in water. In addition, after adding the sodium hydroxide solution, no measurable difference was observed in the absorbance, which further confirms the stability.
The silver nanoparticles synthesized with red ginseng root extract displayed antimicrobial activity against pathogenic microorganisms such as V. parahaemolyticus, S. aureus, B. cereus, and C. albicans (). The mean diameter of the zone of inhibition of all the four wells was determined for each microorganism, showing that the silver nanoparticles had the greatest antimicrobial activity against C. albicans, followed by V. parahaemolyticus, S. aureus, and then B. cereus. The study has been done in four wells, and the average results were interpreted in . The results clearly indicate that the synthesized silver nanoparticles exert antimicrobial activity against tested microorganisms. For biofilm degrading activity, the results are interpreted in (), showing that the maximum biofilm degradation was observed for S. aureus and P. aeruginosa, at a 4 μg/ml concentration of silver nanoparticles (CitationGurunathan et al. 2014).
Figure 5. Antimicrobial activity of silver nanoparticles against Vibrio parahaemolyticus [ATCC 33844] (a), Staphylococcus aureus [ATCC 6538] (b), Bacillus cereus [ATCC 14579] (c), and Candida albicans [KACC 30062] (d), respectively.
![Figure 5. Antimicrobial activity of silver nanoparticles against Vibrio parahaemolyticus [ATCC 33844] (a), Staphylococcus aureus [ATCC 6538] (b), Bacillus cereus [ATCC 14579] (c), and Candida albicans [KACC 30062] (d), respectively.](/cms/asset/8c3d2cdb-a72d-4956-b3c5-26d252a792e5/ianb_a_1008514_f0005_oc.jpg)
Figure 6. Biofilm degrading activity of silver nanoparticles against Staphylococcus aureus [ATCC 6538] and Pseudomonas aeruginosa [ATCC 27853].
![Figure 6. Biofilm degrading activity of silver nanoparticles against Staphylococcus aureus [ATCC 6538] and Pseudomonas aeruginosa [ATCC 27853].](/cms/asset/af257d57-a7d8-4ff9-a775-bd84e6f3483e/ianb_a_1008514_f0006_b.gif)
Table I. Antimicrobial activity of silver nanoparticles against Vibrio parahaemolyticus [ATCC 33844] (a), Staphylococcus aureus [ATCC 6538] (b), Bacillus cereus [ATCC 14579] (c), and Candida albicans [KACC 30062] (d), respectively.
Conclusions
We report the green synthesis of silver and gold nanoparticles, by red ginseng root extract. The particles were synthesized in a rapid, economical and ecofriendly manner. Moreover, the particles were spherical and monodisperse in nature. Furthermore, the particles remain stable for over one month, without the addition of a stabilizing or capping agent. The high ginesenoside and phytochemical content of red ginseng played a role in the reduction and stabilization of nanoparticles. The synthesized silver nanoparticles showed antimicrobial activity against pathogenic microorganisms. The study also discloses the potential of silver nanoparticles in effective inhibition of biofilm formation against S. aureus and P. aeruginosa at 4 μg.
Acknowledgments
This work was supported by a post-doctoral fellowship grant from the Kyung Hee University in 20110213.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Atta AM, Al-Lohedan HA, Ezzat AO. 2014. Synthesis of silver nanoparticles by Green method stabilized to synthetic human stomach fluid. Molecules. 19:6737–6753.
- Chauhan A, Zubair S, Tufail S, Sherwani A, Sajid M, Raman SC, et al. 2011. Fungus-mediated biological synthesis of gold nanoparticles: potential in detection of liver cancer. Int J Nanomedicine. 6:2305–2319.
- Choi KT. 2008. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 29:1109–1118.
- Elavazhagan T, Arunachalam KD. 2011. Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int J Nanomedicine. 6:1265–1278.
- Gurunathan S, Han JW, Kwon DN, Kim JH. 2014. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res Lett. 31:373.
- Humberto HL, Nilda VAN, Liliana del CIT, Cristina RP. 2010. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J Microbiol Biot. 26:615–621.
- Kholoud MM, Abou El-Nour, Ala'a Eftaiha, Abdulrhman Al-Warthan & Reda AA Ammar. 2010. Synthesis and applications of silver nanoparticles. Arab J Chem. 3:135–140.
- Kiefer D, Pantuso T. 2003. Panax Ginseng. Am Fam Physician. 68: 1539–1542.
- Kim YJ, Jeon JN, Jang MG, Oh JY, Kwon WS, Jung SK, Yang DC. 2014. Ginsenoside profiles and related gene expression during foliation in Panax ginseng Meyer. J Ginseng Res. 38:66–72.
- Kim K. 2015. Effect of ginseng and ginsenosides on melanogenesis and their mechanism of action. J Ginseng Res. 39:1–6.
- Krishnaraj C, Muthukumaran P, Ramachandran R, Balakumaran MD, Kalaichelvan PT. 2014. Acalypha indica Linn: Biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDA-MB-231, human breast cancer cells. Biotechnol Rep. 4:42–49.
- Marimuthu V, Palanisamy SK, Sesurajan S, Sellappa S. 2011. Biogenic silver nanoparticles by Gelidiella acerosa extract and their antifungal effects. Avicenna J Med Biotechnol. 3:143–148.
- Mostafa MHK, Eman HI, Khaled ZEB, Doaa M. 2014. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arabian J Chem. 7:61131–61139.
- Pal S, Tak YK, Song JM. 2007. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A study of the gram-negative bacterium escherichia coli. Appl Environ Microbiol.73:1712–1720.
- Shankar C, Dao AT, Singh P, Higashimine K, Mott DM, Maenosono S. 2012. Chemical stabilization of gold coated by silver core–shell nanoparticles via electron transfer. Nanotechnology. 23:245704.
- Sriram MI, Kalishwaralal K, Gurunathan S. 2012. Biosynthesis of silver and gold nanoparticles using Bacillus licheniformis. Methods Mol Biol. 906:33–43.
- Wei GX, Campagna AN, Bobek LA. 2006. Effect of MUC7 peptides on the growth of bacteria and on Streptococcus mutans biofilm. J Antimicrob Chemother. 57:1100–1109.

