Abstract
Microemulsions have gained significant attention from formulation scientists since the time they have been discovered, because of their excellent properties related to their stability, solubility, simplicity, and formulation aspects. The application of microemulsions is not limited to drug delivery via the oral, topical or ocular routes, but may also be seen in cosmetics, immunology, sensor devices, coating, textiles, analytical chemistry, and spermicide. Finally, the objective of this review is to discuss briefly the applications of microemulsions in advanced drug delivery.
Introduction
Historically, microemulsions were first discovered by Rodewald, in 1928, in the form of liquid waxes. Hoar and Schulman introduced the word microemulsion, which they defined as a transparent solution obtained by titrating a normal coarse emulsion with medium chain alcohols. Microemulsions are simple carrier systems because of their thermodynamic stability and simple technology related to the ease of their preparation (CitationChaudhary et al. 2014). In today's world, we can discuss the definition given by Attwood, as “a microemulsion is a system of water, oil, and amphiphilic compounds (surfactant and cosurfactant), which is a transparent, single, optically isotropic, and thermodynamically stable liquid”. Microemulsions are isotropic and transparent systems, thermodynamically stable, and are comprised of oil, water, and a surfactant, mostly in association with a cosurfactant. The droplet size may vary from 10 to 200 nm (CitationGagandeep et al. 2014). Broadly, they are classified into 3 types: O/W microemulsion, W/O microemulsion, and a bicontinuous microemulsion with high solubilizing power. The mechanism of action of microemulsions on skin penetration is unique, since they tend to react with lipids on the skin, as a result of which the intercellular space is changed and hence the drug is transported. The main distinguishing feature between the emulsion and microemulsion is the droplet size of the dispersed phase (CitationShalviri et al. 2011). Microemulsions are promising tools as delivery systems, allowing both types of drug release, controlled as well as sustained, for various routes of administration. Microemulsions have various distinguishing features as a delivery system, with the main features of being less toxic, facilitating enhanced absorption of drugs, and regulating the drug release rates (CitationGarg 2014).
Advantages of microemulsions over other dosage forms
Microemulsions have a broad spectrum of applications in drug targeting and controlled drug release (CitationGarg and Goyal 2012).
They have unique distinguishing features like enhanced bioavailability, due to their ability to solubilize lipophilic drugs.
Microemulsions can carry water-soluble drugs into aqueous phase, and hence demonstrate the ability to carry both lipophilic as well as hydrophilic drugs.
Microemulsions have a wide range of applicability, as they can be delivered by all major routes of drug delivery (CitationGarg and Goyal 2014b).
Microemulsions demonstrate greater longevity as compared to other biphasic dosage forms.
Microemulsions are designed keeping in mind the utilization of their unique properties like minimum toxic side effects and reduction in the volume of carrying vehicle.
Ease of application makes them far better dosage forms than others.
They provide protection from hydrolysis and oxidation.
They facilitate increased patient compliance (CitationGarg and Goyal 2014c).
Theories of microemulsion formation
The interfacial tension between oil and water has to approach zero, in order to form a microemulsion. Microemulsions are termed as the biphasic liquid dosage forms, and therefore, are distinct from molecular solutions of hydrocarbons and water (CitationBurrows and Miguel 2001). The different theories and approaches behind the formation of microemulsions are listed in sequence below:
Thermodynamics of microemulsion formulation
The various thermodynamic properties of microemulsions, like free energy, surface tension, and interfacial area, are interdependent on each other by means of a simple algebraic equation, as given below. There is a well-defined relationship between the properties of free energy, surface tension, and interfacial area of microemulsions.
Where DG f is the free energy of formation, γ is the surface tension of the oil–water interface; DA is the change in interfacial area on microemulsification, DS is the change in entropy of the system, which is effectively the dispersion entropy, and T is the temperature. Negative values of free energy of formation are obtained where large reductions in surface tension are accompanied by positive entropy changes. Hence, microemulsification proves to be a spontaneous and thermodynamically stable process (CitationChandra et al. 2009).
Mixed film theory of microemulsion formation
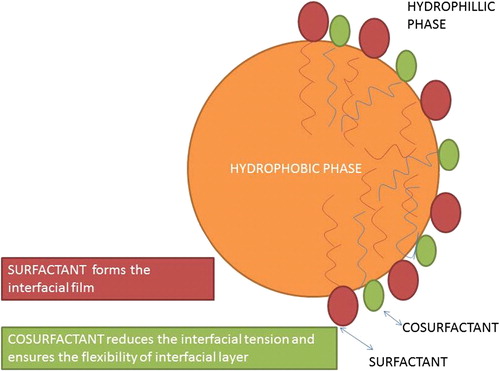
According to the interfacial mixed film theory, there is an assumption that the film at the interface is a dual film made up of surfactant and cosurfactant, and the type of microemulsion formed depends on bending of the curvature of the this film (CitationSchulman et al. 1959). The formation of the surfactant-cosurfactant dual film at the interface causes a reduction in oil–water tension to extremely low values. Spreading pressure of the dual film exceeds the value of the oil–water tension, and hence the negative interfacial tension causes a reduction in the size of droplets. The resulting net interfacial tension is a process that takes place at dynamic equilibrium and finally attains zero or a very small positive value ().
Solubilization theory of microemulsion formation
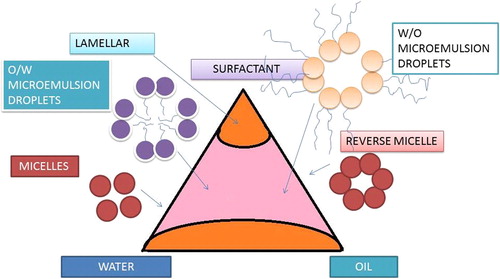
The solubilization theory has very clear-cut assumptions, according to which two types of micelles exist – normal and inverse, as illustrated in the picture above. Oil is solubilized by normal micelles, and water is solubilized by reverse micelles (CitationGillberg et al. 1970). Solubilization is very closely and directly related to micelle concentration, as CMC solubilization is directly proportional to micelle concentration. There are numerous factors affecting the solubilization process related to microemulsions (CitationSchott 1985). Temperature is one such important parameter (CitationShinoda and Kunieda 1971). Solubilization is closely related to micellization, and CMC solubilization increases in direct relation to the micelle concentration. Several important factors affect the solubilization, including temperature. Generally, solubilization increases with increased temperature. Incorporation of electrolytes as well as non-electrolytes like alcohols cause an increase in the micelle size, and hence, causes an increase in the solubilizing power (CitationSchulman et al. 1959). Generally, two approaches have been considered in the description of microemulsion systems, to have them considered as higher swollen micelles or to involve changes that occur at the O/W interface. Microemulsion droplets are formed in the following two ways: 1. The breakdown of larger droplets on lowering of the interfacial tension. 2. Swelling of the interior of the micelle by molecular diffusion of the interior phase throws a bulk of the medium (CitationGarg and Goyal 2014a).
Types of microemulsion systems
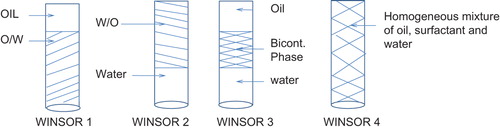
According to Winsor, there exist 4 types of microemulsion phases at equilibrium, and these phases are called Winsor phases ().
WINSOR 1: In this type of microemulsion system, the lower O/W microemulsion phase is in equilibrium with the upper excess oil.
WINSOR 2: It exists in the form of two phases, the upper microemulsion phase (W/O) in equilibrium with the lower excess water phase.
WINSOR 3: It contains 3 phases, the middle bicontinuous phase is in equilibrium with the upper excess oil and the lower excess water.
WINSOR 4: It consists of a single phase only, that is oil, water, and surfactants are homogeneously mixed.
Microemulsion preparation techniques
Microemulsions differ from the traditional emulsions in the fact that they are made by suitable quantities of ingredients at optimum temperature (CitationShalviri et al. 2011). The preparation of microemulsions is a straightforward process. The method of preparation of the O/W type of microemulsion starts with the W/O emulsion containing a lipophilic surfactant. During the process, a hydrophilic surfactant is added, with stirring, which initially forms a cubical structure, but on further addition of hydrophilic surfactant results in the formation of an O/W microemulsion. The exactly opposite procedure can be adopted for the preparation of the W/O type of microemulsion (CitationGarg et al. 2012a).
Phase titration method
The phase titration method is one of the methods to prepare microemulsions, and it can be explained with the help of a triangle-shaped phase diagram. These triangular phase diagrams are called as ternary diagrams. They are easy to interpret, being less time consuming, and have three corners, each corner representing 100% of the pure component. Ternary diagrams of water, oil and Smix are made at fixed weight ratios of Smix. The ingredients are weighed into clean and dry vials, titrated with water, and stirred. Visual inspection is used to see and confirm the formation of the monophasic or biphasic system. Samples are observed, and assumed to be biphasic in case turbidity is followed by phase separation. In case of monophasic systems, we manage to obtain clear and transparent systems after stirring. These are considered as the points in the phase diagrams. The area occupied by these points is considered the microemulsification zone. The phase diagrams are handy tools to describe the microemulsion system (CitationGarg et al. 2014a). A simple ternary diagram shows the blending of oil, water, and surfactant. Each corner of the triangle represents a pure compound. Each corner point of the triangle represents the composition of the blend of two components, and the point inside the diagram represents the blend of 3 components. When the water content of the microemulsion is less, near the oil corner of the phase diagram, the local structure consists of swollen inverse micelles. The oil phase is continuous, and water micellar droplets are the dispersed phase. Surfactant and cosurfactant are deposited on the interface between the water and the oil phase. As we increase the water content, the shape and volume of the hydrophilic core of the hydrophilic micelles expands. At the water corner, the microemulsion looks like a direct micellar solution, because water is the continuous phase (CitationGarg et al. 2014d). In the intermediate phase at which the water and oil phase exist in almost the same quality, the system exists as a lamellar phase ().
Phase inversion method
It is a method of converting the dispersed phase into a continuous phase, and vice versa. Phase inversion is a phenomenon by which the dispersed phase becomes the continuous phase, and vice versa. Surfactant arrangements at the O/W interface undergo a spontaneous change, accompanying the phenomenon of phase inversion (CitationChakraborty 2007). Phase inversion can be brought about by means of the 4 methods, as below:
1.Change in the temperature of the system
2.Addition of salts
3.Change in the volume fractions
4.Introduction of particular flows (CitationAkay et al. 2005).
Change in the experimental conditions induces a sudden and dramatic change in the morphology of the emulsion system. Catastrophic phase inversion is a term that was introduced by Galindo Alvarez and coworkers. They described the influence of oil viscosity and process conditions of the phase inversion phenomenon (CitationSadtler et al. 2012). Another scientist, Jhanzad, showed that the formation of a microemulsion follows a catastrophic event (CitationJahanzad et al. 2007). Sajjadi pointed out the catastrophic phase inversion of abnormal emulsions in the vicinity of the phase inversion point, and nanoemulsion formation by catastrophic phase inversion (CitationJahanzad et al. 2007).
Composition of microemulsions
Microemulsions are known to increase the absorption of the applied drug at the site of application. The reason for this is the penetration enhancement effect of the carrier, mostly composed of saturated as well as unsaturated fatty acids as the oil phase.
Surfactants
Surfactants used to stabilize microemulsions may be (i) nonionic, (ii) zwitterionic, (iii) cationic, or (iv) anionic surfactants. Ionic surfactants are not used generally due to toxicologic concerns, and nonionic surfactants are considered suitable for oral ingestion (CitationGarg et al. 2014b). Nonionic surfactants are available commercially in solubilized oral formulations, including Cremophor EL, Cremophor RH 40, Tween 20, Tween 80, TPGS, Span 80, Labrafil M-1944CS, Labrasol, Gelucire 44/14, etc. (CitationNarang et al. 2007). Surfactants having low hydrophilic-lipophilic balance (HLB) values ranging from 3–6 are preferred for the formulation of W/O microemulsions; surfactants with high HLB values ranging from 8–18 are preferred for the formation of O/W microemulsions. Surfactants whose HLB values are greater than 20 often require the presence of the cosurfactant to reduce the HLB value to within the desired range (CitationGarg et al. 2014c). Surfactants can be modified with the intermediate polarity groups such as polypropylene oxide and polyethylene oxide inserted between the hydrophilic head and the hydrophobic tail. With this modified structure, they are capable enough to provide enhanced interaction with both oil and water phases. This results in the formation of microemulsions with very low interfacial tension and high solubilization properties (CitationGarg et al. 2011a).
Cosurfactant
Microemulsions often contain a cosurfactant, which is an amphiphilic molecule. It accumulates at the interfacial region, along with the main surfactant. The combination of a low HLB surfactant with a high HLB surfactant is tried, in case the HLB of the system is to be modified. A cosurfactant is used with a purpose of modifying the HLB of the system. The amount of cosurfactant used in the formulation is generally less than that of the surfactant. Alcohols can function as the cosurfactant. Similarly, nonionic surfactants can function as the cosurfactant (CitationNarang et al. 2007). Mostly, single chain surfactants are themselves unable to reduce the O/W interfacial tension to a sufficient level, and hence, a cosurfactant is used. Substances used as natural cosurfactants are phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol, and their derivatives, synthetic ethanol, isopropanol, propanol (short chain alcohols), transcutol, polyethylene glycol, propylene glycol, etc. (CitationGarg et al. 2011b).
Oil phase
One of the most challenging tasks while designing the microemulsion formulation is careful selection of the oil phase. It has various advantages, as it not only solubilizes the lipophilic drug, but also enhances the absorption of the lipophilic drug from the GIT. Lipophilic drugs are preferably solubilized in O/W microemulsions. The main criterion for selection of the oil phase are that the drug should be highly soluble in it. Examples of oils are olive oil, castor oil, corn oil, coconut oil, etc. (CitationGarg et al. 2012b).
Aqueous phase
Water used in the formulation of microemulsions is highly processed. It is deionized, double-distilled, and further purified by passing through the ion exchanger. In the aqueous phase, it may accommodate a hydrophilic active drug and some preservatives. Few formulation scientists make use of the buffer solution developed by some researchers. The droplet structure of the O/W microemulsion is retained on dilution by the biological aqueous phase, while exactly the opposite happens in case of the W/O microemulsions. As far as the W/O microemulsions are concerned, an increase in the volume of the aqueous phase leads to a decrease in the surfactant/water ratio, and hence, droplet growth. In case further dilution continues, it might lead to phase inversion or separation, or a return of the original microemulsion properties, and the integrity might be lost (CitationGarg et al. 2013).
Buffer
As far as the use of buffers in microemulsions is concerned, phosphate and borate buffers and their mixtures are used at low concentrations. Zwitterionic microemulsions are made to be put into use to allow higher voltages for faster separation, as they decrease the intensity of current produced during the migration. Examples of zwitterionic buffers are ACES, CAPS, and CAPSO. The pH of the buffer media has a pronounced effect on the separation selectivity. Buffers have been and are being used mostly in the pH range of 7 to 9. Low pH values below 3 are applied to cancel the negative voltages that are applied through the capillaries, as a result of which hydrophobic compounds are easily detected first. Conditions of pH above 12 have two advantages: in case of basic compounds, they are used to avoid chances of ionization, and in case of compounds like estrogens, they induce their ionization and improve their detection (CitationGoyal et al. 2013a).
Applications in advanced drug delivery
Applications in oral drug delivery
Microemulsion formulations are normally beneficial over the conventional oral formulations for oral administration. They offer increased absorption, enhanced clinical potency, and less drug toxicity. Hence, microemulsions have been reported to be ideal delivery carriers of drugs such as hormones, steroids, antibiotics, and diuretics (CitationCharman 2000). The oral route is the major route for drug delivery in many diseases. The major problem that we face in the delivery of oil-soluble drugs in the oral route is the poor aqueous solubility. One way to sort out this problem is to deliver the drug in the microemulsion form. Designing an effective oral delivery system has always been problematic for researchers, because of poor solubility and instability of the drugs in the GI fluid. Microemulsions have the unique ability to circumvent these problems (CitationGoyal et al. 2013b). Microemulsions encapsulate the drugs with varying solubility because of the presence of polar, nonpolar, and interfacial domains in them. Microemulsions protect the incorporated drugs from oxidative and enzymatic degradation. Commercially available microemulsion formulations are of cyclosporin A, saquinavir, and ritonavir, available in the market (CitationFricker et al. 2010). A new microemulsion-based system of myricetin has been developed for oral delivery. The myricetin microemulsion improves the oral bioavailability of the drug myricetin, which was a poorly water-soluble drug. In vivo absorption studies of myricetin showed that microemulsification effectively promotes the absorption of the drug. Microemulsified forms reduce the dose of the drug and improve the bioavailability (CitationGoyal et al. 2014a). Self-microemulsifying drug delivery systems in soft capsule form have been prepared for oral delivery for improved intestinal absorption of drugs. Forty percent of the newly discovered drugs possess little or low water-solubility and hence low bioavailability. These drugs are good candidates to be formulated in the form of SMEDDS (self-microemulsifying drug delivery system) (CitationGibaud and Attivi 2012). Lovastatin lowers the cholesterol level by inhibition of the enzyme involved in the synthesis of cholesterol. The oral bioavailability of cholesterol is less than 5%, because of rapid metabolism in the gut and liver. In vivo studies have revealed that there is increased bioavailability after oral administration of lovastatin in the microemulsion form (4.7 times), as compared to the commercially available lovastatin. The absorption efficiency of levofloxacin hemihydrate, a synthetic quinolone antibiotic, was found to be enhanced by the use of a water-in-oil microemulsion system (CitationGoyal et al. 2014b).
Applications in topical delivery
Microemulsions have an excellent ability to deliver large amounts of water and topical agents into the skin, greater than the ability of water alone or other traditional vehicles such as creams and lotions, because they act as an excellent reservoir for poorly soluble drugs by their capacity for enhanced solubilization. Microemulsions of poorly soluble anti-fungal agents have been successfully developed, like miconazole nitrate (CitationHussain et al. 2014). Microemulsion-based gels for vaginal delivery of clotrimazole and fluconazole were developed and compared with marketed clotrimazole gel by in vitro methods. Microemulsions have proved to increase the cutaneous absorption of both hydrophilic and lipophilic medicaments as compared to the conventional vehicles like pure oils, emulsions, and aqueous solutions (CitationJohal et al. 2014). This type of behavior of microemulsions is generally attributed to the high solubility of medicament in the microemulsions, with an enhanced concentration gradient towards the skin. Microemulsions have received great importance in terms of applications for transdermal drug delivery. The use of curcumin for treating various diseases like skin cancer, psoriasis, and scleroderma has been extensively reported (CitationJoshi et al. 2014a). Microemulsions can be considered a suitable carrier system for the application of ascorbic acid as whitening agent. In addition, a good passage of the drug-in-drug could be interesting for the relative oxygen matrix damage. A microemulsion containing diethyl glycol monoethyl ether was found to be the best formulation to deliver the whole dose and also enhanced the skin penetration (CitationJoshi et al. 2014b). This microemulsion showed best results in the antifungal activity test against Candida albicans. Hence, these types of microemulsions prove to be very promising for clinical evaluation. A microemulsion gel containing betamethasone dipropionate and salicylic acid provides good antiinflammatory activity for the treatment of psoriasis (CitationKalia et al. 2014).
Applications in ocular drug delivery
Drugs are delivered topically to treat eye diseases. O/W microemulsions have been under the radar for ocular administration to dissolve poorly soluble drugs, to increase absorption, and also to prolong the release profile (CitationKataria et al. 2014). Pilocarpine microemulsions were formulated using lecithin, propylene glycol, and PEG 200 as cosurfactants, and isopropylmyristate as the oil phase. Microemulsions have several excellent advantages as ocular delivery carriers, as they offer very low surface tension, thermodynamic stability, phase transition to liquid crystal state, and small droplet size, which may lead to improved ocular retention, high absorption, long duration of action, and enhanced permeation of loaded drugs (CitationKaur et al. 2014a). Eye drops are the most used form of dosage by the ocular route. The ocular route has two disadvantages – one is low bioavailability, and the other is the pulsed release. Microemulsions have advantageous properties like ease of sterilization, specific structures, stability, and capacity to dissolve the drugs. The results of tests on healthy volunteers in vivo have shown enhanced bioavailability (CitationKaur et al. 2014b). Eye drops account for 90% of the available ophthalmic formulations due to their convenience, simplicity, and suitability. Rapid precorneal loss caused by drainage and high tear fluid turnover is one of the major concerns associated with topical delivery of ophthalmic drugs. Only 5% of the drug drops penetrate the cornea and reach the intraocular tissue (CitationKaur et al. 2014c). This results in the production of undesirable side effects. Microemulsions are a promising tool, with improved ocular retention, reduced side effects, and increased corneal drug absorption, maintaining the simplicity and convenience of eye drops as dosage forms. The formulations in microemulsion form are low viscosity fluids with a refractive index lending them to ophthalmic applications. The tested microemulsions have proved to be nonirritant in hen egg membrane and rabbit eye. Long-term pharmacologic effects were observed, when the drug was compared with aqueous solution in vivo (CitationKaur et al. 2014d).
Immunological applications
Tacrolimus is a potent immunosuppressive agent. It has limited corneal penetration. Microemulsions increase the drug's solubility and enhance drug absorption in the eye. Betamethasone-dipropionate has immunomodulatory, antiproliferative and antiinflammatory activity (CitationKaur et al. 2014f). The addition of corticosteroids such as BD, and keratolytic agents such as salicylic acid in microemulsion formulations, results in an enhanced and sustained corticosteroid delivery rate, leading to better antipsoriatic activity. Hydrogels as immuno therapeutics have great potential for improving the efficacy of vaccines and immuno therapeutics for diseases such as cancer (CitationKaur et al. 2014e).
Phytochemical applications
The delivery of plant protection agents to the leaves, and the process of their application, are still to be optimized (CitationKaur et al. 2014g). Till now, plant protection agents contain harmful emulsion or suspension concentrates that often contain environmentally harmful organic solvents and adjuvants (CitationKaur et al. 2014h). Emulsified microemulsions prepared from environmentally friendly components can be loaded with the plant protection agent fenpropiomorph up to 48% without organic solvent (CitationEdris and Malone 2012). The solubilization behavior of a number of essential oils containing volatile phenolic constituents was investigated in 5 different micellar solutions (CitationKaur et al. 2014i). These oils include Eugenia caryophyllata, Thymus serpyllum, and Thymus capitates. The results of these studies showed different applications in personal hygiene, fragrance, pharmaceutical products, and cosmetics. In a new study, a microemulsion system with cassia oil as oil, ethanol as cosurfactant, Tween 20 as surfactant, and water, was made and its in vivo and in vitro activity against Geotrichum citri-aurantii was assessed (CitationKaur et al. 2014j). The results indicated that the microemulsion was effective in preventing postharvest diseases of citrus fruits caused by G. citri-aurantii (CitationXu et al. 2012).
Biotechnological applications
The microemulsion-based organogels have numerous potential biotechnological applications. A highlighting example is the use of various lipase microemulsion systems for synthetic and hydrolytic reactions (CitationKaur et al. 2014k). These microemulsion-based gels are media for bioorganic reactions using lipases as catalyst, formulated and optimized microemulsion-based organogels containing propanol hydrochloride, using experimental design methods (CitationMalik et al. 2014). The results of the study concluded that the choice of lecithin/iso propyl myristate weight ratio, and the amount of drug incorporated, may be crucial in determining the performance of the organogel (CitationMarwah et al. 2014). Enzyme catalysis in microemulsions is used for many reactions, and similarly, for the synthesis of sugar acetal, peptides, esters, transesterification, steroid transformation, and a variety of hydrolytic reactions (CitationModgill et al. 2014a). The most widely used class of enzymes in microemulsions consists of enzymes used for lipase catalysis. Microemulsions are used for the extraction of the proteins from fermenter liquids (CitationModgill et al. 2014b). These proteins and enzymes maintain and retain their normal activity. Solvent concentration, salt type, temperature, pH, and ionic strength affect the process of partition of proteins. One of the stereochemical advantages of the microemulsions is that with the help of biotechnology, we can produce the chiral epoxides using mycobacterium in W/O emulsions (CitationMorie et al. 2014). Many biotransformation processes are difficult to carry out because of poor water solubility of substrate or product. Candida rugosa lipase has been immobilized. It has been done on gelatin-containing AOT microemulsion-based organogels, and sometimes on silica gel as well. The activity of tyrosinase was studied both in aqueous buffer solution as well as in AOT/water/isooctane W/O microemulsion, for oxidation of 4-butyl catechol to 4-butylyquinone (CitationPabreja et al. 2014). In case of aqueous solutions, AOT is capable of activating the enzyme at low concentrations, but inhibits the activity at high concentration. The same is true for the W/O microemulsions. It has been reported that the enzyme organophosphorus hydrolase degrades organophosphorus pesticides in water/Tween 85 + isopropanol hexane microemulsion systems. Kinetics and stability studies were conducted, which showed the partitioning of enzyme between the micelle surfactant layer and aqueous core (CitationRohilla et al. 2014a).
Cosmetic applications
Cosmetics are another important field where microemulsions are being used. Microemulsions have great applications in the field of cosmetics. The microemulsions have advantages like high solubilization power, thermodynamic stability, and ease of preparation. They can enhance skin permeation of loaded substances (CitationRohilla et al. 2014b). Microemulsion formulations reduce the toxicity and increase the product efficiency. Microemulsions have potential use as cosmetic agents and pharmaceutical drug delivery systems. Because of their high oil content, they improve the bioavailability of hydrophobic drugs. There is evidence to show that microemulsions can be used to regulate and control the release pattern of drugs within a therapeutic situation. Microemulsions are also used in the development of novel cosmetics, and are used in the process of product preservation (CitationFriberg et al. 1994). It is believed that the skin adsorbs the cosmetics that are based on microemulsions. Cost and safety are the major criteria as far as microemulsion formulations in cosmetics are concerned, because some of the amphiphiles are not suitable for the personal healthcare products. Oligoglucoside, propanol, hexadecane, isopropyl myristate, lecithin, sodium alkyl sulfate, and alkyldimethylaminesulfate are used in microemulsion formulations for skin care (CitationShinoda et al. 1984). Fragrance and flavored oils are also added in microemulsion formulations. For the cosmetic use of microemulsions, vapor pressure over time and temperature is studied as a vital characteristic (CitationSharma et al. 2014a).
Microreactors and blood substitutes
The use of microemulsions as drug delivery vehicles has been an exciting and attractive area of research because of their many potential and extraordinary benefits. Microemulsions have found application as oral solid dosage forms as microreactors and blood substitutes (CitationSharma et al. 2014b).
Spermicides
Gel microemulsions are novel and non-toxic intravaginal spermicides that act as vehicles for lipophilic drug substances targeting sexually-transmitted pathogens. Spermicidal gel microemulsions have unique potential as dual-function microbicidal contraceptives to improve vaginal bioavailability of poorly soluble antimicrobial agents, without causing significant vaginal damage, as was demonstrated in an experiment conducted on contraceptive efficacy and safety studies of novel microemulsion-based lipophilic vaginal spermicides. The contraceptive activity of GM-4 with N-9 was compared, and it was concluded that the novel spermicide GM 4 formulation is quite safe and more significant than N 9 in terms of preventing contraception (CitationShi et al. 2007).
Analytical chemistry
The use of microemulsions for soil treatment has been reported. Rapeseed methyl-containing microemulsions have been consistently used in the extraction of the various compounds from the soil, like polychlorinated biphenyls and polycyclic aromatic hydrocarbons. Both O/W as well as bicontinuous microemulsion have been found equally reasonable and efficient in the extraction of these compounds from the soil. By making use of the chlorinated hydrocarbons and hydrophilic surfactants, these contaminants are removed with the help of microemulsions. Pesticides in O/W and W/O microemulsion forms have been used for the removal of impurities and also for detoxification of mustard compounds. A microemulsion medium at room temperature is used to measure the presence of polycyclic aromatic hydrocarbon by phosphorescence (CitationSharma et al. 2014c).
Microemulsions are used for two distinct purposes in the industry, i.e. coating, as well as textile finishing. They have the ability to produce monodispersion by polymerization of the W/O system, by utilizing the microemulsified monomers. They turn water-insoluble polymers into water-soluble ones. The dyeing of nylon 6.6 with azo dye has been reported, which is a novel method of dyeing and better than other traditional methods (CitationSingh et al. 2014a). Microemulsions containing siloxanes produce a good final finish, with dispersed microemulsions. Microemulsions are good for coating and textile functions for the following reasons: increased product abrasion, high stability, good product distribution, and high internal softness, etc. (CitationSingh et al. 2014b).
Conclusions and future prospects
There is a strong need for edible delivery systems to protect, encapsulate and release bioactive lipids within medical, pharmaceutical and food industries. Emulsion technology is the best for fabrication and design of delivery systems for encapsulating bioactives. Microemulsions find applications in sustained drug delivery, targeted delivery, controlled delivery, enzyme immobilization, enhancing bioavailability, masking taste, etc. Since orally delivered hydrophilic drugs are unstable in the GIT, like peptides and proteins (CitationSingh et al. 2012a), new approaches consist of the use of biorecognizable moieties for active targeting in clinical trials. The advanced mechanism of action includes altering the mechanism of action of drugs with biomaterials. The cell signaling pathway, gene expression profile, and drug resistance can be altered by coupling bioactive agents with biomaterials (CitationSingh et al. 2012b). There are different objectives behind the selection of W/O microemulsions, since they protect water-soluble drug molecules from being metabolized. There are drug molecules that are heat-sensitive, and microemulsions do not demand high temperature conditions to form. W/O microemulsions, on addition of aqueous fluids, are converted to O/W micro emulsions, and hence, release the API. This allows W/O microemulsions to be designed to selectively release API at different regions of the GIT. Microemulsions have commercially important uses. The fluid used in some dry cleaning processes is a W/O microemulsion. Some floor polishes and cleaners, personal care products, pesticide formulations, and cutting oils are actually microemulsions. Much of the work done on these systems has been used to mobilize petroleum trapped in porous sandstone, for enhanced oil recovery. A fundamental reason for the use of these systems is that a micro emulsion phase sometimes has an ultralow interfacial tension between a separate oil and aqueous phase, which may release or mobilize them from solid phases even in conditions of slow flow and low pressure gradients. Supercritical CO2 is a handy replacement to organic solvents. Recently, efforts have been made towards making water and CO2 microemulsions. They have tremendous applications in cosmetics, catalysts, electronics, miniaturization and ceramics. Microemulsions are used in enhanced oil recovery from the sea by means of a method called chemical flooding (CitationSingh et al. 2014c).
Acknowledgements
The author Dr. Amit K Goyal is thankful to the Department of Biotechnology (DBT), New Delhi (under IYBA scheme; BT/01/IYBA/2009 dated May 24, 2010).
Declaration of interest
The authors confirm that the content of this article has no conflicts of interest.
References
- Akay S, Karasu Z, Akyildiz M, Tokat Y, Goker E. 2005. Successful treatment of liver transplant-associated Kaposi's sarcoma with long-term vincristine. Transplant Proc. 37:2188–2189.
- Burrows HD, Miguel MDG. 2001. Applications and limitations of uranyl ion as a photophysical probe. Adv Colloid Interface Scie. 89:485–496.
- Chakraborty S. 2007. Electroosmotically driven capillary transport of typical non-Newtonian biofluids in rectangular microchannels. Anal Chim Acta. 605:175–184.
- Chandra A, Sharma PK, Irchhiaya R. 2009. Microemulsion-based hydrogel formulation for transdermal delivery of dexamethasone. Asian J Pharm. 3:30–36.
- Charman WN. 2000. Lipids, lipophilic drugs, and oral drug delivery—some emerging concepts. J Pharm Sci. 89:967–978.
- Chaudhary S, Garg T, Murthy RS, Rath G, Goyal AK. 2014. Recent approaches of lipid-based delivery system for lymphatic targeting via oral route. J Drug Target. 22:871–872.
- Edris AE, Malone CF. 2012. Preferential solubilization behaviours and stability of some phenolic-bearing essential oils formulated in different microemulsion systems. Int J Cosmet Sci. 34:441–450.
- Friberg SE, Brancewicz C, Morrison DS. 1994. O/W microemulsions and hydrotropes: the coupling action of a hydrotrope. Langmuir. 10:2945–2949.
- Fricker G, Kromp T, Wendel A, Blume A, Zirkel J, Rebmann H, et al. 2010. Phospholipids and lipid-based formulations in oral drug delivery. Pharm Res. 27:1469–1486.
- Gagandeep GARG, T., Malik B, Rath G, Goyal AK. 2014. Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur J Pharm Sci. 53:10–16.
- Garg T. 2014. Current nanotechnological approaches for an effective delivery of bio-active drug molecules in the treatment of acne. Artif Cells Nanomed Biotechnol. 1–8.
- Garg T, Goyal AK. 2012. Iontophoresis: drug delivery system by applying an electrical potential across the skin. Drug Deliv Lett. 2: 270–280.
- Garg T, Goyal AK. 2014a. Biomaterial-based scaffolds–current status and future directions. Expert Opin Drug Deliv. 11:767–789.
- Garg T, Goyal AK. 2014b. Liposomes: targeted and controlled delivery system. Drug Deliv Lett. 4:62–71.
- Garg T, Goyal AK. 2014c. Medicated chewing gum: patient compliance oral drug delivery system. Drug Deliv Lett. 4:72–78.
- Garg T, Goyal AK, Arora S, Murthy R. 2012a. Development, optimization & evaluation of porous chitosan scaffold formulation of gliclazide for the treatment of type-2 diabetes mellitus. Drug Deliv Lett. 2:251–261.
- Garg T, Kumar A, Rath G, Goyal AK. 2014a. Gastroretentive drug delivery systems for therapeutic management of peptic ulcer. Crit Rev Ther Drug Carrier Syst. 31:531–557.
- Garg T, Rath G, Goyal AK. 2014b. Ancient and advanced approaches for the treatment of an inflammatory autoimmune disease-psoriasis. Crit Rev Ther Drug Carrier Syst. 31:331–364.
- Garg T, Rath G, Goyal AK. 2014c. Biomaterials-based nanofiber scaffold: targeted and controlled carrier for cell and drug delivery. J Drug Target. 1–20.
- Garg T, Rath G, Goyal AK. 2014d. Comprehensive review on additives of topical dosage forms for drug delivery. Drug Deliv.
- Garg T, Singh O, Arora S, Murthy R. 2011a. Dendrimer—A novel scaffold for drug delivery. Int J Pharm Sci Rev Res. 7:211–220.
- Garg T, Singh O, Arora S, Murthy R. 2011b. Patented microencapsulation techniques and its application. J Pharm Res. 4:2097–2102.
- Garg T, Singh O, Arora S, Murthy R. 2012b. Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst. 29:1–63.
- Garg T, Singh S, Goyal AK. 2013. Stimuli-sensitive hydrogels: an excellent carrier for drug and cell delivery. Crit Rev Ther Drug Carrier Syst. 30:369–409.
- Gibaud S, Attivi D. 2012. Microemulsions for oral administration and their therapeutic applications. Expert Opin Drug Deliv. 9:937–951.
- Gillberg G, Lehtinen H, Friberg S. 1970. NMR and IR investigation of the conditions determining the stability of microemulsions. J Colloid Interface Sci. 33:40–53.
- Goyal AK, Rath G, Garg T. 2013a. Nanotechnological approaches for genetic immunization. DNA RNA Nanobiotechnol Med Diagnos Treatment Dis. 67–120.
- Goyal G, Garg T, Malik B, Chauhan G, Rath G, Goyal AK. 2013b. Development and characterization of niosomal gel for topical delivery of benzoyl peroxide. Drug Deliv.
- Goyal G, Garg T, Rath G, Goyal AK. 2014a. Current nanotechnological strategies for an effective delivery of drugs in treatment of periodontal disease. Crit Rev Ther Drug Carrier Syst. 31:89–119.
- Goyal G, Garg T, Rath G, Goyal AK. 2014b. Current nanotechnological strategies for treating glaucoma. Crit Rev Ther Drug Carrier Syst. 31:365–405.
- Hussain T, Garg T, Goyal AK, Rath G. 2014. Biomedical Applications of Nanofiber Scaffolds in Tissue Engineering. J Biomater Tissue Eng. 4:600–623.
- Jahanzad F, Chauhan G, Mustafa S, Saha B, Sajjadi S, Brooks BW. 2007. Composite polymer nanoparticles via transitional phase inversion emulsification and polymerisation. Macromol Symp.145–150.
- Johal HS, Garg T, Rath G, Goyal AK. 2014. Advanced topical drug delivery system for the management of vaginal candidiasis. Drug Deliv. 1–14.
- Joshi D, Garg T, Goyal AK, Rath G. 2014a. Advanced drug delivery approaches against periodontitis. Drug Deliv. 1–15.
- Joshi D, Garg T, Goyal AK, Rath G. 2014b. Development and Characterization of Novel Medicated Nanofibers against Periodontitis. Curr Drug Deliv.
- Kalia V, Garg T, Rath G, Goyal AK. 2014. Development and evaluation of a sublingual film of the antiemetic granisetron hydrochloride. Artif Cells Nanomed Biotechnol. 1–5.
- Kataria K, Sharma A, Garg T, Goyal AK, Rath G. 2014. Novel technology to improve drug loading in polymeric nanofibers. Drug Deliv Lett. 4:79–86.
- Kaur M, Garg T, Narang RK. 2014a. A review of emerging trends in the treatment of tuberculosis. Artif Cells Nanomed Biotechnol. 1–7.
- Kaur M, Garg T, Rath G, Goyal AK. 2014b. Current nanotechnological strategies for effective delivery of bioactive drug molecules in the treatment of tuberculosis. Crit Rev Ther Drug Carrier Syst. 31:49–88.
- Kaur M, Malik B, Garg T, Rath G, Goyal AK. 2014c. Development and characterization of guar gum nanoparticles for oral immunization against tuberculosis. Drug Deliv.
- Kaur N, Garg T, Goyal AK, Rath G. 2014d. Formulation, optimization and evaluation of curcumin-beta-cyclodextrin-loaded sponge for effective drug delivery in thermal burns chemotherapy. Drug Deliv. 1–10.
- Kaur P, Garg T, Rath G, Murthy RS, Goyal AK. 2014e. Development, optimization and evaluation of surfactant-based pulmonary nanolipid carrier system of paclitaxel for the management of drug resistance lung cancer using Box-Behnken design. Drug Deliv. 1–14.
- Kaur P, Garg T, Rath G, Murthy RS, Goyal AK. 2014f. Surfactant-based drug delivery systems for treating drug-resistant lung cancer. Drug Deliv. 1–12.
- Kaur P, Garg T, Vaidya B, Prakash A, Rath G, Goyal AK. 2014g. Brain delivery of intranasal in situ gel of nanoparticulated polymeric carriers containing antidepressant drug: behavioral and biochemical assessment. J Drug Target. 1–12.
- Kaur R, Garg T, Das Gupta U., Gupta P, Rath G, Goyal AK. 2014h. Preparation and characterization of spray-dried inhalable powders containing nanoaggregates for pulmonary delivery of anti-tubercular drugs. Artif Cells Nanomed Biotechnol. 1–6.
- Kaur R, Garg T, Malik B, Gupta UD, Gupta P, Rath G, Goyal AK. 2014i. Development and characterization of spray-dried porous nanoaggregates for pulmonary delivery of anti-tubercular drugs. Drug Deliv. 1–6.
- Kaur R, Garg T, Rath G, Goyal AK. 2014j. Advanced aerosol delivery devices for potential cure of acute and chronic diseases. Crit Rev Ther Drug Carrier Syst. 31:495–530.
- Kaur V, Garg T, Rath G, Goyal AK. 2014k. Therapeutic potential of nanocarrier for overcoming to P-glycoprotein. J Drug Target. 1–12.
- Malik R, Garg T, Goyal AK, Rath G. 2014. Polymeric nanofibers: targeted gastro-retentive drug delivery systems. J Drug Target. 1–16.
- Marwah H, Garg T, Goyal AK, Rath G. 2014. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 1–15.
- Modgill V, Garg T, Goyal AK, Rath G. 2014a. Permeability study of ciprofloxacin from ultra-thin nanofibrous film through various mucosal membranes. Artif Cells Nanomed Biotechnol. 1–6.
- Modgill V, Garg T, Goyal AK, Rath G. 2014b. Transmucosal Delivery of Linagliptin for the Treatment of Type- 2 Diabetes Mellitus by Ultra-Thin Nanofibers. Curr Drug Deliv.
- Morie A, Garg T, Goyal AK, Rath G. 2014. Nanofibers as novel drug carrier - an overview. Artif Cells Nanomed Biotechnol. 1–9.
- Narang AS, Delmarre D, Gao D. 2007. Stable drug encapsulation in micelles and microemulsions. Int J Pharm. 345:9–25.
- Pabreja S, Garg T, Rath G, Goyal AK. 2014. Mucosal vaccination against tuberculosis using Ag85A-loaded immunostimulating complexes. Artif Cells Nanomed Biotechnol. 1–8.
- Rohilla R, Garg T, Bariwal J, Goyal AK, Rath G. 2014a. Development, optimization and characterization of glycyrrhetinic acid-chitosan nanoparticles of atorvastatin for liver targeting. Drug Deliv. 1–8.
- Rohilla R, Garg T, Goyal AK, Rath G. 2014b. Herbal and polymeric approaches for liver-targeting drug delivery: novel strategies and their significance. Drug Deliv. 1–17.
- Sadtler V, Galindo-Alvarez JM, Marie–Bégué E. 2012. Low Energy Emulsification Methods for Nanoparticles Synthesis.
- Schott H. 1985. Surfactant systems: their chemistry, pharmacy and biology. By D. Attwood and A. T. Florence. Chapman & Hall, London EC4P 4EE, United Kingdom. 1983. 794 pp. J Pharm Sci. 74:1140–1141.
- Schulman JH, Stoeckenius W, Prince LM. 1959. Mechanism of formation and structure of micro emulsions by electron microscopy. J Phys Chem. 63:1677–1680.
- Shalviri A, Sharma AC, Patel D, Sayani A. 2011. Low-surfactant microemulsions for enhanced topical delivery of poorly soluble drugs. J Pharm Pharm Sci. 14:315–324.
- Sharma A, Garg T, Aman A, Panchal K, Sharma R, Kumar S, Markandeywar T. 2014a. Nanogel-an advanced drug delivery tool: Current and future. Artif Cells Nanomed Biotechnol. 1–13.
- Sharma R, Garg T, Goyal AK, Rath G. 2014b. Development, optimization and evaluation of polymeric electrospun nanofiber: A tool for local delivery of fluconazole for management of vaginal candidiasis. Artif Cells Nanomed Biotechnol. 1–8.
- Sharma R, Singh H, Joshi M, Sharma A, Garg T, Goyal AK, Rath G. 2014c. Recent advances in polymeric electrospun nanofibers for drug delivery. Crit Rev Ther Drug Carrier Syst. 31:187–217.
- Shi FF, Bulkowski M, Hsieh KC. 2007. Synthesis of indium nanoclusters and formation of thin film contacts on plastic substrates for organic and flexible electronics applications. Nanotechnology. 18:265301.
- Shinoda K, Kunieda H. 1971. Conditions to produce so-called microemulsions–factors to increase mutual solubility of oil and water by solubilizer.
- Shinoda K, Kunieda H, Arai T, Saijo H. 1984. Principles of attaining very large solubilization (microemulsion): inclusive understanding of the solubilization of oil and water in aqueous and hydrocarbon media. J Phys Chem. 88:5126–5129.
- Singh B, Garg T, Goyal AK, Rath G. 2014a. Recent advancements in the cardiovascular drug carriers. Artif Cells Nanomed Biotechnol. 1–10.
- Singh H, Sharma R, Joshi M, Garg T, Goyal AK, Rath G. 2014b. Transmucosal delivery of Docetaxel by mucoadhesive polymeric nanofibers. Artif Cells Nanomed Biotechnol.
- Singh K, Arora N, Garg T. 2012a. RFID: A Trustable Security Tool in Pharmaceutical Industry. Am J Pharm Tech Res. 2:113–127.
- Singh K, Arora N, Garg T. 2012b. Superbug: Antimicrobial resistance due to NDM-1. Int J Institut Pharm Life sci. 2:58–66.
- Singh O, Garg T, Rath G, Goyal AK. 2014c. Microbicides for the treatment of sexually transmitted HIVinfections. J Pharm. 1–18.
- Xu SX, Li YC, Liu X, Mao LJ, Zhang H, Zheng XD. 2012. In vitro and in vivo antifungal activity of a water-dilutable cassia oil microemulsion against Geotrichum citri-aurantii. J Sci Food Agric. 92: 2668–2671.