Abstract
Cell transplantation is a promising regenerative therapy for cartilage degeneration. However, obtaining sufficient numbers of cells for this purpose is a challenge, due a lack of autologous donor tissue and the difficulty of culturing chondrocytes in vitro. Tissue engineering strategies that induce or maintain chondrocytic phenotype may solve these problems by (1) broadening the range of available donor tissue, and (2) facilitating the expansion of these cells while controlling phenotypic drift. In this study, bone marrow-derived mesenchymal stem cells (MSCs) and cartilage-derived cells (CDCs) were cultured on composite hydrogels containing agarose and homogenized cartilage extracellular matrix (ECM). MSCs cultured on agarose-ECM scaffolds did not show significant signs of chondrogenic differentiation in the absence of additional cues. However, CDCs cultured on agarose-ECM scaffolds proliferated more rapidly than their ECM-free counterparts and MSCs, while retaining chondrocytic morphology. These results were corroborated via expression of cartilage marker genes: in autologous constructs, SOX 9 expression was upregulated by 12.6 ± 5.3-fold, and COL II was upregulated by 2.0 ± 0.3-fold. Agarose-ECM composite hydrogels are therefore useful for expanding partially differentiated CDCs for applications in regenerative medicine.
Introduction
Osteoarthritis is a multifactorial degenerative joint disorder involving articular cartilage dysfunction, resulting in loss of mobility and decreased quality of life (CitationLoeser et al. 2012). Osteoarthritis is a significant public health concern due to its prevalence: it is present in approximately 70% of the population older than 55 years of age (CitationElders 2000). Abnormal cartilage ECM metabolism is a hallmark of the condition (CitationAigner et al. 2007), thus chondrocyte activity is a primary target for therapeutic intervention (CitationRoach et al. 2007).
There are a variety of behavioral, surgical, pharmacological, and regenerative therapies currently in use to treat osteoarthritis, a review of which is beyond the scope of this article (CitationFelson et al. 2000). Of these, cell transplantation is a relatively recent approach that involves enriching the site of cartilage pathology with a population of therapeutic cells. These cells may assist in direct tissue remodeling or provide trophic and anti-inflammatory support (CitationNoth et al. 2008). There are two primary candidate cell types for this therapy: bone marrow-derived mesenchymal stem cells (MSCs) and cartilage-derived cells (CDCs). MSCs are readily collected in large numbers by routine biopsy, are proliferative and stable in laboratory culture, have immunomodulatory properties, and may be induced to differentiate toward cartilage (CitationPittenger et al. 1999, CitationGupta et al. 2012). In contrast, CDCs are relatively difficult to extract and grow, rapidly dedifferentiate in monolayer culture, and undergo replicative senescence in vivo, yet may be better predisposed to regenerate cartilage (CitationTew et al. 2008, CitationMartin and Buckwalter 2001).
Inspired by the architecture of the cartilage stem cell niche in vivo, tissue culture techniques have emerged to control cell behavior ex vivo (CitationPei et al. 2011). Biodegradable hydrogels formed of cross-linked polymers are one such way to provide a biomimetic three-dimensional environment for growing cells outside of the body (CitationDrury and Mooney 2003). For decades, naturally-derived polysaccharides, including agarose, have been used as the basis of hydrogel scaffolds for the purposes of cartilage tissue engineering (CitationBuschmann et al. 1992, CitationAwad et al. 2004), and have made their way into a variety of commercial surgical products (CitationSelmi et al. 2008, CitationFilardo et al. 2013). Agarose-based scaffolds are inexpensive, biologically inert, highly permeable, and can be cast in any desired shape (CitationRaghunath et al. 2007). Although the resulting constructs do not fully replicate the mechanical properties of native tissue, agarose provides a stable platform for in vitro growth and differentiation of stem cells toward cartilage (CitationAwad et al. 2004).
While agarose has a proven track record in cartilage tissue engineering, its utility in regenerative medicine applications may be enhanced by the addition of a complex cocktail of ECM components. There is a reciprocal relationship between cell phenotype and microenvironment, and the proteins and polysaccharides found within cartilage ECM modulate both tissue mechanics and cell microenvironments influencing phenotype (CitationMuzzarelli et al. 2012). While phenotype can be controlled using conditioned media or other techniques (CitationSomoza et al. 2014, CitationHwang et al. 2011), these practices are costly and unnatural. Tissue-derived biomolecules promote targeted differentiation in other musculoskeletal tissues: cell-free ECM extract induces proliferation and tenocytic differentiation of human adipose-derived stem cells (CitationYang et al. 2013), and similar behavior was observed using bovine muscle-derived fibroblasts cultured in standard flasks coated with a xenogeneic ECM extract (CitationRonning et al. 2013). Scaffolds comprised entirely of cartilage-derived ECM induce chondrocytic differentiation of adipose-derived stem cells without further growth factor supplementation (CitationCheng et al. 2008), but such quantities of donor material may be difficult to obtain.
In this study, a hybrid biomaterial was developed to reconcile the need for a stable three-dimensional scaffold (agarose) containing tissue-specific molecular cues (ECM extract) for cartilage tissue culture. The purpose of this investigation was to determine whether supplementation with natural ECM-derived soluble factors and modification of three-dimensional microenvironments using novel patient-derived scaffolds could (1) prime MSCs toward chondrogenic differentiation and/or (2) prevent dedifferentiation of CDCs during in vitro expansion. It was hypothesized that agarose-ECM composite hydrogels would promote a more chondrocytic phenotype of MSCs or CDCs prior to their use in cartilage cell therapy.
Materials and methods
Experimental design
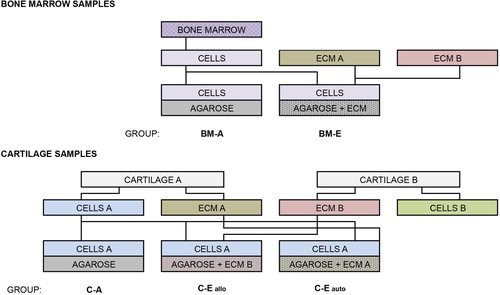
The aim of this study was to elucidate the influence of composite hydrogel scaffolds incorporating homogenized cartilage-derived ECM on the growth and differentiation of human progenitor cells in tissue culture (). In the first part of this investigation, four human bone marrow MSC lines were derived and cultivated in monolayers (BM-M), in agarose hydrogels (BM-A), or in agarose-ECM composite hydrogels (BM-E). It was hypothesized that MSCs cultured in three-dimensional hydrogels would adopt a more chondrocytic phenotype versus monolayer, and that this behavior would be more pronounced in hydrogels containing cartilage ECM. In the second part, two human CDC lines were grown in monolayers (C-M), in agarose hydrogels (C-A), in agarose composite hydrogels containing allogeneic cartilage ECM (C-Eallo), or in agarose composite hydrogels containing autologous cartilage ECM (C-Eauto). It was hypothesized that CDCs cultured in three-dimensional hydrogels would better retain phenotypic markers characteristic of mature chondrocytes, and that this effect would be more pronounced in composite hydrogels containing cartilage ECM. All samples were obtained from patients of mixed gender, more than 60 years of age, undergoing orthopedic surgeries at the Institute of Orthopedics and Traumatology in Riga, Latvia, with ethical approval from the Institutional Review Board.
Isolation of primary MSCs
Fifty mL of bone marrow was collected from the femoral head of four patients. MSCs were isolated using Ficoll-Paque PLUS (GE Healthcare), following the manufacturer's protocol. Briefly, bone marrow was diluted to a ratio of 1:3 using 0.9% sodium chloride solution supplemented with 100 U/mL sodium penicillin, 100 μg/mL streptomycin, 2.5 μg/mL amphotericin, and 50 U/mL heparin sodium. This solution was strained of clots, set over a 15 mL density gradient across four 50 mL centrifuge tubes, and spun for 22 min at 800 g without brakes. The interphase was collected and washed twice in four volumes of the above diluent solution, followed by centrifugation for 20 min at 600 g. The resulting cell pellet was resuspended in high-glucose GlutaMAX DMEM with 110 μg/mL sodium pyruvate (Gibco) plus 100 U/mL sodium penicillin, 100 μg/mL streptomycin, and 20% fetal bovine serum, before being plated in a T-75 tissue culture flask. The media were changed every 2–3 days and non-adherent cells were discarded. Cells were expanded to 80% confluence at 37°C, 5% CO2, and 90% humidity, lifted with 0.05% trypsin-EDTA, and frozen without passaging in media plus 10% dimethyl sulfoxide, until the start of the experiment.
Isolation of primary CDCs
Cartilage samples were obtained from the intervertebral disc or femoral head of two patients. Cartilage was liberally rinsed in phosphate-buffered saline, manually dissected from peripheral tissues, and minced using a scalpel. Half of the tissue was then set aside for subsequent extraction of ECM components. The remaining half was then digested with 1 mg/mL type-II collagenase (Sigma-Aldrich) in high-glucose GlutaMAX DMEM, with 110 μg/mL sodium pyruvate (Gibco) plus 100 U/mL sodium penicillin, 100 μg/mL streptomycin, and 2.5 μg/mL amphotericin, for either 4 h (intervertebral disc cartilage) or 18 h (femoral head cartilage), at 37°C. Digested solutions were then spun for 10 min at 100 g and resuspended in saline. This washing process was repeated twice more, before resuspending the pellet in 1:1 high- glucose GlutaMAX DMEM, with 110 μg/mL sodium pyruvate (Gibco) and F-12 Nutrient Mixture with GlutaMAX (Gibco), plus 100 U/mL sodium penicillin, 100 μg/mL streptomycin, 15% fetal bovine serum, and 50 μg/mL L-ascorbic acid. A cell count was performed, and 100,000 cells were cryopreserved in 300 μL of QIAzol lysis reagent (Qiagen) at − 80°C as a fresh cartilage control for gene expression analysis. The remaining cells were plated in T-25 tissue culture flasks and expanded at 37°C, 5% CO2, and 90% humidity. The cultures were left undisturbed for 7 days. The media were then changed every 2–3 days, discarding the non-adherent cells and the remaining extracellular matter. Once reaching 80% confluence, the cells were trypsinized and frozen until the start of the experiment.
Homogenization of ECM components
A portion of each cleaned and minced cartilage sample described above was subjected to four repeated freeze/thaw cycles between − 80°C and 37°C, and completely homogenized using a T10 ULTRA-TURRAX (IKA) in 10 mL of saline. Tissue homogenates were centrifuged twice for 10 min at 250 g, the wet mass of the extract was recorded, and the resulting ECM components were resuspended in saline.
Cultivation of hydrogel tissue constructs
Frozen cells (P0) were thawed and plated once all cell lines were derived, in order to prepare for tissue culture experiments (). These cells were expanded, as previously described, to 80% confluence, trypsinized, counted, and brought to the desired concentrations. A solution of 1 g/100 mL (1%) agarose was then prepared in distilled H2O in an Erlenmeyer flask by boiling until translucent. This solution was passed through a 0.45 μm sterile syringe filter and divided into three 50 mL conical tubes: two of which were supplemented with homogenized cartilage-derived ECM, as described above, from each source, formulating a final 1 g/100 mL solution of 90% agarose and 10% ECM. These melted hydrogels were further divided for cell seeding when the solution had cooled to approximately 40°C, at which point 500,000 cells of each cell type (P1) were added, the solution was thoroughly mixed, and 440 μL constructs were cast in 24-well flat-bottomed tissue culture plates. Scaffolds were set to polymerize for 15 min at 37°C and 90% humidity, after which each well was filled with 400 μL of chondrogenic media – a 1:1 mixture of high-glucose GlutaMAX DMEM with 110 μg/mL sodium pyruvate (Gibco) and F-12 Nutrient Mixture with GlutaMAX (Gibco), plus 100 U/mL sodium penicillin, 100 μg/mL streptomycin, 15% fetal bovine serum, and 50 μg/mL L-ascorbic acid. These constructs remained undisturbed for 18 h before being transferred to 6-well tissue culture plates using sterile forceps, and bathed in 3 mL of media each (). Monolayers of all 6 cell types (400,000 cells/well) were simultaneously grown as controls using the same chondrogenic media, half of which was changed every 2–3 days. Monolayers were maintained for 5 days, while experimental constructs were maintained for 19 days.
Colony-forming unit assay
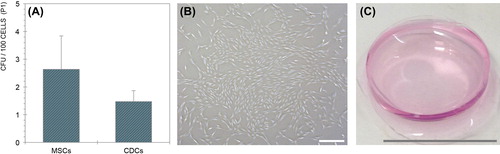
Cells from each sample (P1) were plated in 6-well Cell + tissue culture plates (Sarstedt) at 100–5,000 cells per cm2 in MSC media. After 7 days, the resulting colonies were stained using 0.05% crystal violet, and quantified by manual counting in all wells exhibiting linearity.
Cell quantitation in hydrogels
One replicate of each experimental group was documented every 2–3 days by brightfield microscopy at low magnification. Four representative images of each sample at random x-y-z coordinates were acquired, and cell counts were performed in ImageJ (National Institutes of Health, USA). Raw data was correlated with original density immediately following seeding, in order to obtain total cell counts per construct. Population doubling times were computed using initial and final cell numbers.
Microscopic imaging
Images of clonal cell colonies were obtained via phase contrast microscopy. Following the conclusion of the experiment, and prior to RNA isolation, a narrow transverse strip obtained from the center of each construct, constituting approximately 10% of the total volume of each sample, was dissected and immersed overnight in 10% buffered formalin. The following day, the fixative was changed out with PBS. Samples were stored at 4°C for 1 week before processing. Samples were then manually dissected and stained with hematoxylin or alcian blue.
Gene expression analysis
Following the experiment, hydrogel constructs assigned for gene expression analysis were sectioned into four equal pieces and individually lysed with 500 μL of QIAzol (Qiagen). These samples were briefly melted in a heated water bath and stored at − 70°C for 1 week prior to processing. Monolayer cells were lysed in 500 μL of QIAzol, and frozen. RNA was isolated by chloroform extraction, pelleted in isopropanol, washed in ethanol, and resuspended in water, according to the manufacturer's protocol. Further purification was conducted with a total RNA isolation spin kit (AppliChem). Five hundred μg of each RNA product was reverse transcribed using a RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific). Quantitative PCR () of each sample was then run in duplicate 20 μL reaction volumes using SYBR Green I Master Mix (Applied Biosystems). Gene expression was analyzed using the 2−ΔΔCt method (CitationLivak and Schmittgen 2001, CitationWiner et al. 1999), and reported as fold-change with respect to fresh cartilage explants.
Table I. Custom qPCR primers were used to evaluate the efficacy of chondrogenesis.
Statistics
All data is reported as mean ± standard error. Two-tailed paired Student's t-tests on cell counts were conducted between experimental groups at each time point and between final versus initial counts. Gene expression data was analyzed using two-way multivariate analyses of variance with repeated measures. Statistical significance (p < 0.05) is annotated graphically in figures using asterisks or comparison bars.
Results
MSCs and CDCs form monolayer colonies at similar rates
There was no statistically significant difference in the frequency of colony-forming units observed between MSCs and CDCs at P1 (p = 0.4496) (). Values averaged 2.63 ± 1.20 colonies per 100 cells for MSCs, and 1.48 ± 0.39 colonies per 100 cells for CDCs.
Cell proliferation is regulated by three-dimensional environment
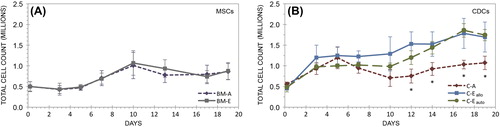
Over the 19-day course, MSCs proliferated in hydrogels, increasing their population size by 1.76-fold (p = 0.0002) in the BM-A group and 1.71-fold (p = 0.0038) in the BM-ECM group (). The population doubling time was 17.58 ± 4.48 days in the BM-A group and 16.79 ± 4.00 days in the BM-ECM group. These groups were not statistically distinguishable from one another at any time point. Similarly, all experimental groups incorporating CDCs underwent statistically significant increases in total cell number during the experiment (). Cells in the C-A group increased in number by 1.93-fold (p = 0.0010), in the C-Eallo group by 3.69-fold (p = 0.0002), and in the C-Eauto group by 3.60-fold (p < 0.0001). There were statistically significant increases in CDC count between the C-A group and both the C-Eallo and C-Eauto groups by day 12 (p = 0.0007 and p = 0.0076, respectively), and this difference persisted until the conclusion of the experiment. The population doubling time was 20.23 ± 2.44 days in the C-A group, 10.90 ± 3.30 days in the C-Eallo group, and 10.28 ± 0.29 days in the C-Eauto group. There were no significant differences in CDC count between the C-Eallo and C-Eauto groups at any time point. Final CDC density of C-Eallo and C-Eauto groups averaged 3900 cells/mm2. Final cell densities in the BM-A and C-A groups were not statistically different after 19 days (p = 0.2038).
CDCs retain chondrocytic morphology in hydrogels
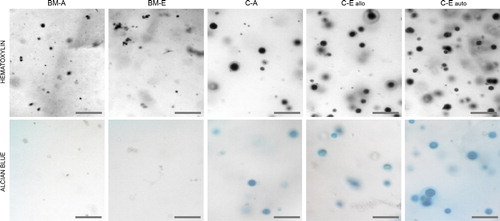
MSCs retained small, fibroblast-like morphologies in all hydrogels, and did not change significantly with ECM-supplementation (). In contrast with MSCs, CDCs in all hydrogels exhibited characteristically rounded morphologies with enlarged nuclear and cytoplasmic volumes. CDCs and their immediate surroundings stained positive for alcian blue, which was not observed in any of the MSC-seeded constructs. The only microscopic difference between the C-A group and the C-Eallo and C-Eauto groups was the moderately higher cell density in the ECM-supplemented groups.
Gene expression profiles are altered in hydrogels
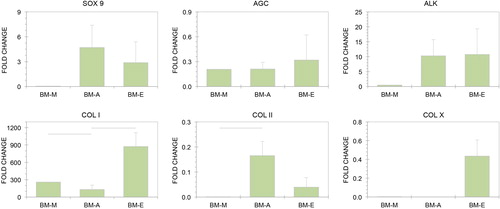
There were no significant differences between the BM-A and BM-E groups in the expression of SOX 9 (p = 0.6623), and AGC (p = 0.6821) or ALK (p = 0.5833), or between BM-M and either of these groups (). COL I was downregulated in the BM-A group compared to the BM-M group (p = 0.0118), but was expressed higher in the BM-E group versus the BM-A group (p = 0.0449). COL I expression was three orders of magnitude greater in the BM-E group than in fresh cartilage. COL II was upregulated in the BM-A group versus the BM-M group (p = 0.0497), but did not reach significance in the BM-E group. COL II expression was greater in fresh cartilage than in all experimental groups. COL X was upregulated only in the BM-E group compared to the BM-A group (p = 0.0858), but at less than half the rate of fresh cartilage.
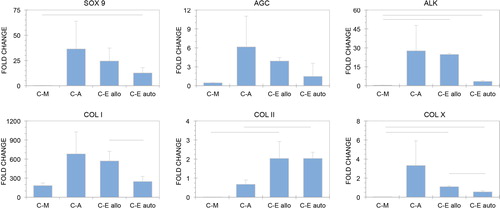
SOX 9 expression was significantly increased only in the C-Eauto group compared with the C-A group (p = 0.0424), and 12.6 ± 5.3-fold higher than in fresh cartilage (). The difference between the C-Eallo group and the C-A group only approached significance (p = 0.07430). The difference between the C-Eallo group and the C-Eauto group did not reach significance (p = 0.1142). AGN was downregulated in the C-M group versus fresh cartilage, but upregulated in all ECM-supplemented constructs. The differences did not reach significance, though there was a trend toward decreased expression in the C-Eallo and C-Eauto groups versus the C-A group (p = 0.1035 and p = 0.0874, respectively). ALK was expressed more highly in ECM-supplemented groups versus C-M (p = 0.0431 for the C-Eallo group and p = 0.0659 for the C-Eauto group). There was a non-significant trend toward downregulation of ALK in the C-Eauto group versus the C-Eallo group (p = 0.0727).
While COL I was more highly expressed in all CDC groups than in fresh cartilage, COL I expression was significantly lower in the C-Eauto group versus the C-Eallo group (p = 0.0076). COL II expression was increased in both ECM-supplemented experimental groups (upregulated by 2.03 ± 0.89 in the C-Eallo group and 2.04 ± 0.33 in the C-Eauto group). This trend only reached significance versus the C-M group in the C-Eallo group (p = 0.0032). However, COL II expression was significantly higher in the C-Eauto group than in the C-A group (p = 0.0476). There was no difference in COL II expression between the C-Eallo and C-Eauto groups. COL X was more highly expressed in the C-Eallo and C-Eauto groups than in the C-A group (p = 0.0016 and p = 0.0024, respectively), but was expressed at approximately the same level as in fresh cartilage. COL X was downregulated in the C-Eauto group versus the C-Eallo group (p = 0.0010). Expression of all target genes in the C-A group was highly variable compared with the other experimental groups.
Discussion
The first step in any cell transplantation protocol is obtaining a sufficient number of donor cells, and one way to achieve this is through in vitro expansion. Agarose hydrogels were selected as a foundation for this study due to their favorable three-dimensional structure for cartilage tissue engineering, and their technical simplicity (CitationZheng et al. 2011). It was hypothesized that ECM homogenate as a supplement would promote proliferation and differentiation of chondrocytic cells by means of physical or biochemical signals provided by the extract. MSCs and CDCs did not significantly differ in their growth rate or ability to form monolayer colonies, yet there were two distinct responses observed over the 19-day time course: the C-Eallo and C-Eauto groups proliferated approximately twice as much as the C-A, BM-A, and BM-ECM groups. Rapid proliferative activity was observed in the first few days following seeding, but this response stalled in the C-A, BM-A, and BM-ECM groups, while it continued in the C-Eallo and C-Eauto groups. This suggests that ECM-supplemented hydrogels are potentially useful in improving product yield of CDCs, but not MSCs, if cultured for at least 12 days.
A three-dimensional environment is more conducive to the development of a rounded cell morphology, and has been shown to restore chondrocytic phenotype in early-passage CDCs (CitationTew et al. 2008). The first assessment of this was undertaken using histological methods. The C-A, C-Eallo and C-Eauto groups all stained positive for alcian blue, indicative of new proteoglycan synthesis, and thus chondrogenesis (CitationTare et al. 2005). Cells within the BM-A and BM-ECM groups retained a small fibroblast-like morphology and did not stain positive for alcian blue. Therefore, microscopic evaluation produced evidence of differentiation in all CDCs, while MSCs appeared to become quiescent.
The MSC and CDC phenotype was further characterized using a panel of six gene expression markers: SOX 9, AGN, ALK, COL I, COL II, and COL X. Chondrocytic phenotype in tissue engineering has been defined as upregulation of SOX 9, AGN, and COL II, with downregulation of COL I (CitationKim et al. 2013). The serial monolayer culture of CDCs results in a loss of this phenotype, including a rapid increase in COL I through early passages (CitationTew et al. 2008), though there is evidence that this can be reversed (CitationBenya and Shaffer 1982). AGN loss is a hallmark of osteoarthritis, and its decreased production is associated with aging (CitationPollard et al. 2008). ALK is a marker of osteogenic differentiation (CitationOgawa et al. 2004), while COL X is present in hypertrophic cartilage (CitationWalker et al. 1995), thus the expression of these two genes should be limited in a healthy chondrocytic phenotype. Taken together, these six genes were used to describe the absence or development of a chondrocytic phenotype in all experimental groups.
Although MSCs did not adopt a chondrocytic phenotype, they experienced mild changes in the expression of certain marker genes. Indeed, SOX 9 was expressed in the BM-A and BM-ECM groups in greater amounts than in fresh cartilage. While this was insufficient to promote chondrogenesis, it contrasted with the BMM group, which expressed only trace amounts of SOX 9. The BM-A and BM-ECM groups only significantly differed in COL I expression, so there is no evidence corroborating a supporting role of cartilage ECM extract in MSC differentiation toward cartilage in the absence of additional soluble factors. This data corroborates a recent study indicating that cartilage-derived ECM alone is insufficient to induce chondrogenesis in human MSCs (CitationRowland et al. 2013).
CDCs underwent several prominent changes in gene expression. Most notably, the expression of SOX 9 and COL II was increased in hydrogel culture. COL I was expressed well above levels observed in fresh cartilage, but was lower in the C-Eauto group versus the C-A and C-Eallo groups. AGN, ALK, and COL X were expressed at baseline levels in the C-Eauto group, indicative of physiologically normal chondrogenesis. Overall, the chondrocytic phenotype was preserved using the novel composite hydrogel culture technique. Throughout, the C-A group demonstrated greater phenotypic heterogeneity than the C-Eallo and C-Eauto groups, indicating increased cellular homogeneity with ECM supplementation – a beneficial attribute for cell therapy. In addition, CDCs benefited not only from three-dimensional culture, but from autologous ECM supplementation in particular. However, when matched donor tissue is unavailable, a favorable phenotype can still be attained.
There are several factors that may have influenced the results of this investigation. Primary MSC and CDC lines in this study were obtained from patients suffering from preexisting musculoskeletal problems including osteoarthritis, potentially influencing baseline phenotype. However, a previous study determined that age and osteoarthritic status are not correlated with decreased chondrogenic differentiation of MSCs in micromass culture (CitationScharstuhl et al. 2007). Results obtained via our method may also be dependent on donor species, as agarose hydrogels are not universally useful in chondrocytic differentiation of MSCs across the animal kingdom (CitationWatts et al. 2013).
In future studies, it would be interesting to characterize the biomechanical properties of hydrogel constructs resulting from cell-mediated remodeling over time. Furthermore, mechanical stimulation of these constructs, such as the application of cyclic compression, may improve chondrogenesis (CitationMauck et al. 2000). As a translational application, a sophisticated new generation of hydrogels promises to better address the clinical need for injectable biomaterials in the treatment of osteoarthritis (CitationLin et al. 2014). ECM extracts such as what is described here may improve the efficacy of such techniques.
Agarose-ECM composite hydrogels are inexpensive and accessible tools for the expansion and differentiation of human CDCs. The ECM extract increases the relatively low proliferative capacity of CDCs and promotes retention of a chondrocytic phenotype during in vitro culture. However, further optimization and characterization is likely necessary before this technique finds its way into clinical practice.
Acknowledgements
The authors would like to thank Dmitrijs Zulenkovs for useful discussions, and Martins Boroduskis for technical assistance. DWY acknowledges support from the U.S. Fulbright Student Program.
Declarations of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aigner T, Söder S, Gebhard PM, Mcalinden A, Haag J. 2007. Mechanisms of disease: Role of chondrocytes in the pathogenesis of osteoarthritis—structure, chaos and senescence. Nat Clin Pract Rheumatol. 3:391–399.
- Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. 2004. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 25:3211–3222.
- Benya PD, Shaffer JD. 1982. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 30:215–224.
- Buschmann MD, Gluzband YA, Grodzinsky AJ, Kimura JH, Hunziker EB. 1992. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 10:745–758.
- Cheng NC, Estes BT, Awad HA, Guilak F. 2008. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng Part A. 15:231–241.
- Drury JL, Mooney DJ. 2003. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials. 24:4337–4351.
- Elders MJ. 2000. The increasing impact of arthritis on public health. J Rheumatol Suppl. 60:6–8.
- Felson DT, Lawrence RC, Hochberg MC, McAlindon T, Dieppe PA, Minor MA, et al. 2000. Osteoarthritis: New insights. Part 2: Treatment approaches. Ann Intern Med. 133:726–737.
- Filardo G, Kon E, Roffi A, Di Martino A, Marcacci M. 2013. Scaffold-based repair for cartilage healing: a systematic review and technical note. Arthroscopy. 29:174–186.
- Gupta PK, Das AK, Chullikana A, Majumdar AS. 2012. Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res Ther. 3:25.
- Hwang NS, Im SG, Wu PB, Bichara DA, Zhao X, Randolph MA, et al. 2011. Chondrogenic priming adipose-mesenchymal stem cells for cartilage tissue regeneration. Pharm Res. 28:1395–1405.
- Kim IL, Khetan S, Baker BM, Chen CS, Burdick JA. 2013. Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues. Biomaterials. 34:5571–5580.
- Lin H, Cheng AWM, Alexander PG, Beck AM, Tuan RS. 2014. Cartilage tissue engineering application of injectable gelatin hydrogel with in situ visible-light-activated gelation capability in both air and aqueous solution. Tissue Eng Part A. 20:2402–2411.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 25:402–408.
- Loeser RF, Goldring SR, Scanzello CR, Goldring MB. 2012. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 64:1697–1707.
- Martin JA, Buckwalter JA. 2001. Telomere erosion and senescence in human articular cartilage chondrocytes. J Gerontol A Biol Sci Med Sci. 56:B172–179.
- Mauck RL, Soltz MA, Wang CC, Wong DD, Chao P-HG, Valhmu WB, et al. 2000. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 122:252–260.
- Muzzarelli RA, Greco F, Busilacchi A, Sollazzo V, Gigante A. 2012. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: A review. Carbohydr Polym. 89:723–739.
- Noth U, Steinert AF, Tuan RS. 2008. Technology insight: Adult mesenchymal stem cells for osteoarthritis therapy. Nat Clin Pract Rheumatol. 4:371–380.
- Ogawa R, Mizuno H, Watanabe A, Migita M, Shimada T, Hyakusoku H. 2004. Osteogenic and chondrogenic differentiation by adipose-derived stem cells harvested from GFP transgenic mice. Biochem Biophys Res Commun. 313:871–877.
- Pei M, Li JT, Shoukry M, Zhang Y. 2011. A review of decellularized stem cell matrix: a novel cell expansion system for cartilage tissue engineering. Eur Cell Mater. 22:333–343; discussion 343.
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. 1999. Multilineage potential of adult human mesenchymal stem cells. Science. 284:143–147.
- Pollard TC, Gwilym SE, Carr AJ. 2008. The assessment of early osteoarthritis. J Bone Joint Surg Br. 90:411–421.
- Raghunath J, Rollo J, Sales KM, Butler PE, Seifalian AM. 2007. Biomaterials and scaffold design: Key to tissue-engineering cartilage. Biotechnol Appl Biochem. 46:73–84.
- Roach HI, Aigner T, Soder S, Haag J, Welkerling H. 2007. Pathobiology of osteoarthritis: pathomechanisms and potential therapeutic targets. Curr Drug Targets. 8:271–282.
- Ronning SB, Pedersen ME, Andersen PV, Hollung K. 2013. The combination of glycosaminoglycans and fibrous proteins improves cell proliferation and early differentiation of bovine primary skeletal muscle cells. Differentiation. 86:13–22.
- Rowland CR, Lennon DP, Caplan AI, Guilak F. 2013. The effects of crosslinking of scaffolds engineered from cartilage ECM on the chondrogenic differentiation of MSCs. Biomaterials. 34:5802–5812.
- Scharstuhl A, Schewe B, Benz K, Gaissmaier C, Buhring HJ, Stoop R. 2007. Chondrogenic potential of human adult mesenchymal stem cells is independent of age or osteoarthritis etiology. Stem Cells. 25:3244–3251.
- Selmi TA, Verdonk P, Chambat P, Dubrana F, Potel JF, Barnouin L, Neyret P. 2008. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: Outcome at two years. J Bone Joint Surg Br. 90:597–604.
- Somoza RA, Welter JF, Correa D, Caplan AI. 2014. Chondrogenic differentiation of mesenchymal stem cells: Challenges and unfulfilled expectations. Tissue Eng Part B Rev. 20:596–608.
- Tare RS, Howard D, Pound JC, Roach HI, Oreffo RO. 2005. Tissue engineering strategies for cartilage generation—micromass and three dimensional cultures using human chondrocytes and a continuous cell line. Biochem Biophys Res Commun. 333:609–621.
- Tew SR, Murdoch AD, Rauchenberg RP, Hardingham TE. 2008. Cellular methods in cartilage research: Primary human chondrocytes in culture and chondrogenesis in human bone marrow stem cells. Methods. 45:2–9.
- Walker GD, Fischer M, Gannon J, Thompson Jr RC, Oegema Jr TR. 1995. Expression of type-X collagen in osteoarthritis. J Orthop Res. 13:4–12.
- Watts AE, Ackerman-Yost JC, Nixon AJ. 2013. A comparison of three-dimensional culture systems to evaluate in vitro chondrogenesis of equine bone marrow-derived mesenchymal stem cells. Tissue Eng Part A. 19:2275–2283.
- Winer J, Jung CK, Shackel I, Williams PM. 1999. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 270:41–49.
- Yang G, Rothrauff BB, Lin H, Gottardi R, Alexander PG, Tuan RS. 2013. Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix. Biomaterials. 34:9295–9306.
- Zheng X, Yang F, Wang S, Lu S, Zhang W, Liu S, et al. 2011. Fabrication and cell affinity of biomimetic structured PLGA/ articular cartilage ECM composite scaffold. J Mater Sci Mater Med. 22:693–704.