?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Acute spinal cord injury (ASCI) can lead to paraplegia or quadriplegia, the treatment of which has been a major problem. New therapeutic approaches in developing carbon nanotubes (CNT) functionalized with the Nafion nanocomposite, a sulfonated tetrafluoroethylene copolymer, have been shown to increase the length of selected neurites in vitro.
Objective
We hypothesized that the administration of the CNT/Nafion nanocomposite after experimental SCI will promote regeneration of axons into the lesion cavity and the functional recovery of the hind limbs in a rat model.
Methods
To evaluate this hypothesis through this experimental research paper, transection SCI was induced at the T9-T10 vertebral level in adult female rats. One week after transection, the epicenter of the lesion was injected with 25 lL of vehicle (saline), or 1 lg/mL, 10 lg/mL, or 100 lg/mL of CNT/Nafion nanocomposite. Behavioral analysis was carried out by assessing tail flick, chronic pain or mechanical allodynia, motor coordination, and the results of the rotarod test performed pre- and post-surgery, on days 3, 7, 14, 21 and 28, using the tail flick analysis, Noldus CatWalk gait analysis, open-field locomotor test, and Rotarod test. At 28 days post-injection, the rats were euthanized and spinal cord tissue was extracted.
Results
We found that post-SCI, administration of the CNT/Nafion nanocomposite resulted in decreased lesion volume, increased neurofilament-positive fibers and corticospinal tract fibers in the lesion, and no increase in reactive gliosis (P < 0.001). Additionally, post-SCI administration of CNT/Nafion nanocomposite induced a modest improvement in hind limb locomotor recovery without inducing hyperalgesia.
Conclusion
These data suggest that the CNT/Nafion nanocomposite may be an effective material to promote axonal repair and regeneration after SCI.
Introduction
Spinal cord injury (SCI) is one of the most prominent causes of severe disability worldwide, with lifelong devastating dysfunction, and high medical and social costs (CitationMcLean 2013). An estimated 2.5 million people live with SCI, with more than 130,000 new injuries reported each year (CitationDalbayrak et al. 2015, CitationMcLean 2013). Moreover, recent estimates suggest that there are over 250,000 persons living with SCI in the U.S. (CitationMoore et al. 2009).
Experimental and clinical studies have suggested that acute spinal cord injury (ASCI) occurs by primary and secondary mechanisms. Primary injury is the initial mechanical damage due to local deformation of the spine that causes direct compression and damage of neural elements and broken blood vessels (CitationKwon et al. 2004). The secondary mechanism includes a cascade of biochemical and cellular processes, such as electrolyte abnormalities, formation of free radicals, vascular ischemia, edema, post-traumatic inflammatory reaction, apoptosis or genetically programmed cell death, and other processes (CitationAmbrozaitis et al. 2006). Recently, studies have shown that tissue engineering may play a central role in bridging the structural lesion of the injured spinal cord and delivering agents that may facilitate functional recovery (CitationHulsebosch 2002). Among the studies, the benefits of the rat model are clear. The animals are inexpensive, easy to care for, can be studied in large numbers, early mortality is not costly, there are few surgical infections, and there are well-established functional analysis techniques. Repairing and restoring damaged axons and identifying an alternate route to guide the restoration of neuromuscular transmission are two major mechanisms of therapy to restore synaptic connections in SCI patients.
Carbon nanotubes (CNTs) that are small in size, flexible, strong, inert, and have properties of electrical conductivity and ease of combination with various biological compounds are the best suited for medical treatment, because of the advantages offered by these features (CitationMalarkey and Parpura 2007, CitationClements et al. 2009). CNTs and their derivatives can be used as scaffolds for neuronal growth. Scaffolds may promote regeneration of injured neurons such as in SCI, and provide a marked improvement over traditional nerve grafts in their ability to overcome degenerative processes (CitationChang et al. 2008, CitationChauhan et al. 1999). Silva et al. designed a nanofiber scaffold composed of peptide amphiphile molecules which are capable of self-assembly to form a network (CitationSilva et al. 2004, CitationSilva 2006). The surface of nanofibers consists of the active peptide sequence isoleucine-lysine-valine-alanine-valine (IKVAV), designed to engage in cell signaling by functioning as ligands for cell surface receptors. Another study shows that CNT scaffolds can also be created using biocompatible polymers, such as poly-l-lactic acid (PLLA), poly-lactide-co-glycolide (PLGA), and polycaprolactone (PLC) (CitationYang et al. 2005). Guo et al. demonstrated that self-assembling peptide nanofiber scaffolds (SAPNS) could repair the injured spinal cord (CitationGuo et al. 2007). They isolated neural stem cells (NPCc) and Schwann cells (SCc), cultured them with the SAPNS, and then transplanted them into the transected dorsal column of the rat spinal cord. Thus, several studies have demonstrated the role of CNTs in treating SCI. On the other hand, a combination of these electron translation properties of CNT also formed the idea behind Nafion (CitationSheng et al. 2011). Nafion is the first in a class of synthetic polymers with ionic properties. It is a sulfonated tetrafluoroethylene-based tetrafluoroethylene copolymer (CitationLu and Tuszynski 2008). Nafion membranes have unique properties with respect to stability, solubility, and ionic conductivity, which make them suitable for a variety of applications. Owing to its polymeric structure, the biocompatibility of Nafion, with its good electron transport properties, is widely used in nanobiosensors (CitationRoman et al. 2011).
In this paper, we study the effects of the carbon nanotube/Nafion nanocomposite on functional recovery in injured spinal cord of male rats, with respect to all the properties mentioned.
Experimental materials and methods
Experimental groups and spinal cord injury
In this experimental study, thirty adult male Sprague- Dawley rats (200–250 g, 1.8 months old) were used. All animals in the experimental group were maintained on separate shelves in a 12-h light and dark cycle, and the temperature was controlled at 24 ± 2°C. The animals had adequate access to pellets at all times, except during testing. The experiments were performed at least a week after the rats were habituated to their pet home environment. The animals received a complete transection SCI induced by severing the spinal cord at the T9-T10 vertebral level with a Micro Feather ophthalmic scalpel (Feather Safety Razor Co., Osaka, Japan). After laminectomy, the spinal lesion was sutured by the contusion model. All surgical protocols were approved by the Institutional Animal Care and Use Committee of the Shahid Beheshti University at Tehran. The animals were randomly assigned to the following five experimental groups: (1) intact group that was left without interference; (2) uninjured controls receiving only laminectomy; (3) group with complete transection SCI; (4) intact group receiving nanodrugs (intact + nanodrug); and (5) group with complete transection SCI, receiving nanodrugs (SCI + nanodrugs). For behavioral studies, we considered 6 animals in each group (n = 6), and for the delayed administration experiment, the animals received the treatment 1 week following injury, with 25 μL of vehicle (saline), or 1 g/mL (n = 8), 10 μg/mL (n = 9), or 100 μg/mL (n = 9) of the SWNT-PEG solution, stereotaxically injected into the epicenter of the lesion site.
The total sample size was 6 for each group. The sample size was calculated based on the prior studies, considering 95% confidence level, 80% power, d = 1 and σ = 1.8., and the sampling formula was:
Postoperative animal care was conducted thrice daily. Animals received isotonic saline (4.3 mL SC BID), enrofloxacin (4.3 mg/kg SC QD), and carprofen (1.4 mg/kg SC BID) for 7 days post-SCI. Also, as an indicator of overall health, animals were weighed each day and body weight (in grams) was tracked.
Preparation of nanocomposite
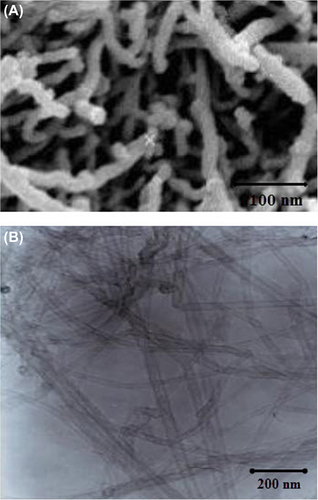
Nafion® perfluorinated resin solution (20 wt. %) in a mixture of lower aliphatic alcohols and water, toluene (≥ 99.5%), and thiophene (≥ 99.5%) were obtained from Sigma-Aldrich. Metallic multi-walled carbon nanotubes (MWCNTs) with high aspect ratio were used for this study. The CNTs were created at the Nanotechnology Research Center of Tehran University. The microstructure of the tubes, as investigated by Field Emission Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) (H987 Philips, Amsterdam, The Netherlands), showed a relatively wide distribution of diameters, ranging between 20 and 70 nm. Based on calculations for obtaining the nanocomposites, amounts of 0.015 g of Nafion solution and 0.01 g of MWCNTs were weighed and placed in an ultrasonic water bath for 20 min till Nafion dispersed into MWCNTs homogenously. Also, to prevent the agglomeration of particles, we used 10 μg of Triton x114 as surfactant. The amounts were calculated to give 0–5% MWCNT in Nafion on a dry weight basis. One ml of isopropyl alcohol was added to all mixtures to enable proper wetting and dispersion of the MWCNTs. The homogenous slurries were poured into Petri dishes and placed on a well-leveled flat surface to allow uniform solvent evaporation under ambient conditions for 3 h. The dishes were then placed in an oven at 40°C overnight. To confirm the size and diameter of the nanocomposites (then called nanodrugs), SEM and TEM images were taken. Nanodrug carriers were injected in the space between the vertebrae T9-10 with a Hamilton syringe [10 ml maximum, injection rate of 1 ml per minute in the cerebrospinal fluid (CSF)].
Behavioral outcome measures
In all groups studied, behavioral tests of tail flick, chronic pain or mechanical allodynia (with Von Frey filament (Stoelting, Inc., Wood Dale, IL)), motor coordination, and evaluation of animal muscle power (the Rotarod test) were assessed pre- and post-surgery on days 3, 7, 14, 21 and 28 using the tail flick analysis, Noldus CatWalk gait analysis (Noldus Information Technology, Leesburg, VA), open-field locomotor test, and the rotarod test.
Thermal hyperalgesia (heightened response to a noxious stimulus) was evaluated using the tail flick test, as previously described (CitationHurtado et al. 2011, CitationHamers et al. 2006). Briefly, a thermal stimulus was elicited by the radiant heat source from a tail flick unit (Columbia Instruments, Columbus, OH), and was focused on the rat's tail, 3–5 cm from the tip. Tail-flick latency was measured in 10 s by a timer connected to a photoelectric cell that stopped the timer and heat upon movement of the tail. The results of three trials were obtained for each animal evaluation session, and latencies were averaged.
Kinematic analysis was evaluated using the CatWalk gait analysis system (Noldus Information Technology, Leesburg, VA) (CitationHamers et al. 2006). In this gait analysis, the animals traverse a black glass walkway and a camera digitally records the paw prints. The gait is then analyzed frame by frame with the assistance of integrated software (Ethovision). One week preceding the injury, the animals were introduced to the procedure and baseline gait parameters were obtained 1 day pre-injury. Subsequent analysis was conducted once every week following the SCI for 28 days. Distances traveled by the animals were compared in all 5 groups at different days.
Mechanical allodynia (hypersensitivity to an originally non-noxious stimulus) was measured by determining the hind paw withdrawal threshold evoked by stimulation with a series of calibrated Von Frey filaments (Stoelting, Inc., Wood Dale, IL). After application of a stimulus of 2 g, 4 g, 6 g, 8 g, 15 g, 26 g, or 60 g to the plantar surface of the hind paw while the animal stood on a wire grid, the lowest filament receiving at least a 50% positive response of five trials was recorded as the conclusive sensitivity level using the up-down method, as previously described by Chaplan et al. (CitationChaplan et al. 1994). Only animals with plantar placement were evaluated.
In this study, the rotarod test was performed to assess the animal’s locomotor performance. The test device consists of a rotating wooden rod that rotates with speed, and the duration of locomotor balance of animals on the rod (up to 180 s) is recorded. This operation is repeated four times for each animal, and if there is imbalance, the animal is excluded. This test was repeated before and after the mice were injected, and the falling of animals was recorded with respect to time (seconds).
Statistical analysis
Data are shown as mean standard error of the mean (SEM), and transferred to Excel; after editing, data were analyzed using SPSS version 11.5 (SPSS Inc.s, Chicago, IL, USA). First, the Kolmogorov-Smirnov test was done to confirm normality of data. The study groups were compared in terms of the study variables using the independent samples t-test, the binominal test, and the Chi-square test. Moreover, we used the Repeated Measures Analysis of Variance (ANOVA) test for comparing changes in wound status across the seven assessment time-points. P values less than 0.05 were considered as significant.
Result
Confirmation of the size of CNT/Nafion nanocomposites
and show the SEM and TEM images of CNTs. illustrates the TEM image of the CNT/Nafion nanocomposite in various sizes, generated when the Nafion binds to the CNT surface, as seen in this TEM image of CNTs after being scraped from the nanocomposite surface. SEM and TEM show a relatively wide distribution of diameters, ranging between 20 and 70 nm.
Study of CNT/Nafion nanocomposite on thermal hyperalgesia threshold
There was no reflex in the animal’s tail and there was no response to thermal hyperalgesia, until the 28th day after the SCI. By injecting the CNT/Nafion nanocomposite, the animals’ responses to heat and pain were seen to be close to the base line (P < 0.001) (see ).
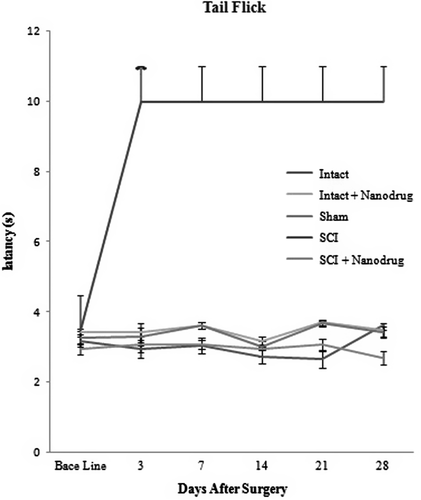
As can be seen in , in intact groups (Group 1), the administration of CNT/Nafion nanocomposite did not result in any change in the thermal hyperalgesia threshold, and a similar response was seen in the animals of the group left intact and administered nanodrugs alone (intact + Nanodrug) (P ≥ 0.05). However, the only change seen was in the SCI group (P < 0.001).
Study of the effect of CNT/Nafion nanocomposite on mechanical allodynia threshold
shows that animals with SCI, when compared with recipients of nanocomposites, have a lower response threshold to mechanical allodynia (Von Frey filaments). Pain in the right paw of animals in the SCI + nanodrugs group was significant after the seventh day of injection (P ≤ 0.05). The low mechanical allodynia threshold for pain in the left paw of the animals was more remarkable than that in the SCI + nanodrugs group (P ≤ 0.05). The difference between both groups was significant after 14 days (P ≤ 0.05). Laminectomized animals were also compared to the animals of the control group, and were found to have a lower response threshold to mechanical stimuli on days 3, 7, and 14, and this process was continued until day 21 (see ).
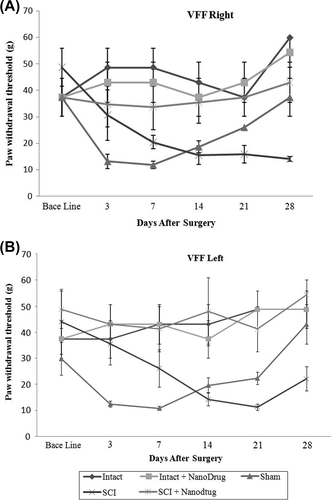
SCI groups compared with sham groups have a lower response threshold to mechanical stimuli (Von Frey filaments) (P ≤ 0.05). The difference can be seen in the threshold for pain in the right paw of animals, 14 days after injury (data not shown).
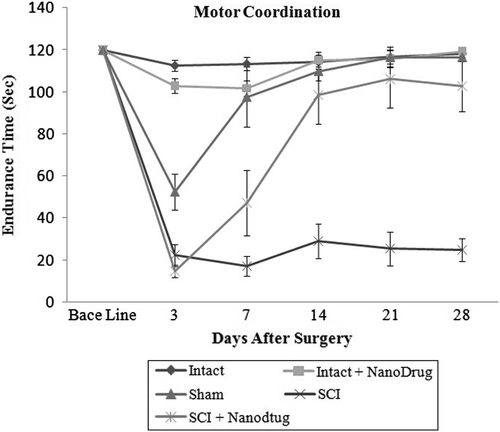
Study of the effect of CNT/Nafion nanocomposite on motor coordination
shows that the number of animals showing balanced mobility during the time they remain on the rotating rod is reduced after surgery in both groups, i.e., the laminectomy and SCI groups. However, in the sham group, this imbalance was seen to have reversed from the third day and the animal became normal after 14 days, while the reduction in balance was seen significantly in the SCI group, even for up to 28 days, in the animals left intact and animals subjected to laminectomy. Nanocomposite administration resulted in animals of the SCI groups showing improved locomotor performance, and the animals’ balance returned from day-7 onwards. This difference was significant on day-14 () (P ≤ 0.05). The intact group that received the CNT/Nafion nanocomposite did not show any change in motor coordination ability, and the response stimulation was similar to that of the control groups (See ) (P ≥ 0.05).
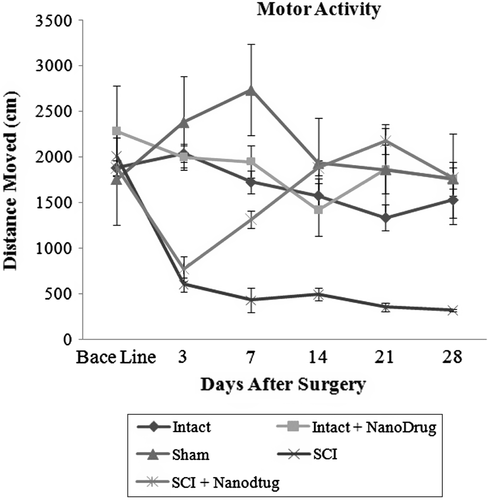
Study of CNT/Nafion nanocomposite on motor activity
SCI caused a significant reduction in the motor activity of the animal, which showed a significant difference in the distance traveled after the third day (P ≤ 0.05). However, in this study, no significant differences were seen in motor activity between intact groups and those that received nanodrugs (P ≥ 0.05). From day-3 to day-28 after surgery, motor activity of the SCI Group decreased. However, in the SCI + nanodrugs group, motor activity increased after the third day and was seen to be equivalent to the base line. Thus, the difference between both groups is significant after the seventh day (P ≥ 0.05). illustrates this test.
Discussion
This study was promoted by the hypothesis that the CNT/Nafion nanocomposite is useful for motor function in an animal model of SCI male Wistar rats. We have also tried to show the role of these nanodrugs in promoting neurite outgrowth in vitro (CitationChaplan et al. 1994), which would promote tissue repair and functional recovery after SCI in a rat model (CitationBunge and Pearse 2003). We report that acute post-SCI administration of the CNT/Nafion nanocomposite increased the numbers of neurofilament-positive fibers compared to the injured, non-treated group. Secondly, we evaluated behavioral outcome measures of delayed administration at the time-point of 3 weeks post-SCI. We found that the CNT/Nafion nanocomposite significantly increased tail flick, chronic pain or mechanical allodynia, motor coordination, and the animal's muscle power. Our results show that this nanodrug improved the motor coordination quality in animals after 14 days, locomotor coordination in the animals after 7 days, and motor activity after 7 days, but no change was seen in the hyperalgesia threshold. Our studies also show that this nanocomposite significantly increased the number of neurofilament-positive fibers and CST fibers in and around the lesion's epicenter, and did not induce reactive gliosis or overt toxicity; this result has not been reported in this paper. Additionally, we found that delayed post-SCI administration of CNT/Nafion nanocomposite at 100 μg/mL induced modest hind limb functional recovery, which was correlated with histological markers of tissue repair [histological results not reported]. Importantly, this is the first report to indicate that the CNT/Nafion nanocomposite promotes repair and regeneration of damaged SCI tissue in vivo.
The research idea of engineering a biocompatible material to promote tissue repair and regeneration after SCI is well-established (CitationNovikova et al. 2003, CitationTabesh et al. 2009). Moreover, Malarkey et al. show the mechanism of action by which SWNT-PEG lead to enhancement of selected neurite outgrowth. These results point to an inhibitory action of SWNT-PEG on regulated endocytosis (CitationMalarkey et al. 2009). Panseri et al. studied the effectiveness of nanotubes made of biodegradable polymers (PLGA/PCL) in supporting regeneration of rat sciatic nerve in vivo (CitationPanseri et al. 2008); but in this study, we have shown that the behavior is better and more efficient with the use of the Nafion nanocomposite, which is useful for increased recovery of mechanical functional in SCI animals. Nafion forms a scaffold that directly connects injured nerves. However, this scaffold has not been evaluated in vivo. With regard to in vivo studies, fibrin scaffolds are modified to release growth factors, and when seeded with embryonic stem cell-derived neuronal progenitor cells, they have been shown to increase the survival and differentiation of neural progenitor cells following transplantation in a rat model of SCI (CitationSucapane et al. 2009, CitationRoman et al. 2011).
Additionally, nanomaterials can be manipulated to enhance growth-promoting properties (CitationAmbrozaitis et al. 2006) that when appropriately developed into regenerative scaffolds, may obviate the need for controversial and risky stem cell-based or tissue extract-based components. Furthermore, since CNTs are not biodegradable, they can impede the recovery of injured tissue for a prolonged period of time. Roman and et al. also demonstrated in this study that delayed post-SCI administration of SWNT-PEG induced tissue repair, axonal repair/regeneration, and functional recovery (CitationWouterlood et al. 1990, CitationRoman et al. 2011). It should be borne in mind that substantial regeneration/repair are much more difficult to achieve in a complete transection model than in models with more limited injury.
In conclusion, we report here that the CNT/Nafion nanocomposite improves axonal repair and regeneration in the lesion cavity and induces modest functional recovery, which suggests that this may be an effective nanomaterial to apply for promoting recovery after SCI. Future studies will explore the use of different concentrations of this nanocomposite and characterize and functionalize it with growth factors, as strategies to further promote repair and regeneration of damaged spinal cord tissue.
Acknowledgments
The authors greatly appreciate the financial support from Payame Noor University and Yazd University for conduction the present project.
Authors’ contributions
SRZ and SI designed and synthesized the nanocomposite, guided the contact angle analysis, and prepared the manuscript. SRZ participated in the SEM and TEM analysis and prepared the manuscript. ZZ and FL facilitated and enabled the animal studies and the SCI. All authors read and approved the final manuscript.
Funding
This research received no specific grant from any funding agency from the public, commercial, or not-for-profit sectors.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ambrozaitis KV, Kontautas E, Spakauskas B, Vaitkaitis D. 2006. [Pathophysiology of acute spinal cord injury]. Medicina (Kaunas). 42:255–261.
- Bunge MB, Pearse DD. 2003. Transplantation strategies to promote repair of the injured spinal cord. J Rehabil Res Dev. 40:55–62.
- Chang WC, Kliot M, Sretavan DW. 2008. Microtechnology and nanotechnology in nerve repair. Neurol Res. 30:1053–1062.
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. 1994. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 53:55–63.
- Chauhan NB, Figlewicz HM, Khan T. 1999. Carbon filaments direct the growth of postlesional plastic axons after spinal cord injury. Int J Dev Neurosci. 17:255–264.
- Clements IP, Kim YT, English AW, Lu X, Chung A, Bellamkonda RV. 2009. Thin-film enhanced nerve guidance channels for peripheral nerve repair. Biomaterials. 30:3834–3846.
- Dalbayrak S, Yaman O, Yilmaz T. 2015. Current and future surgery strategies for spinal cord injuries. World J Orthop. 6:34–41.
- Guo J, Su H, Zeng Y, Liang YX, Wong WM, Ellis-Behnke RG, et al. 2007. Reknitting the injured spinal cord by self-assembling peptide nanofiber scaffold. Nanomedicine. 3:311–321.
- Hamers FP, Koopmans GC, Joosten EA. 2006. CatWalk-assisted gait analysis in the assessment of spinal cord injury. J Neurotrauma. 23:537–548.
- Hulsebosch CE. 2002. Recent advances in pathophysiology and treatment of spinal cord injury. Adv Physiol Educ. 26:238–255.
- Hurtado A, Cregg JM, Wang HB, Wendell DF, Oudega M, Gilbert RJ, Mcdonald JW. 2011. Robust CNS regeneration after complete spinal cord transection using aligned poly-L-lactic acid microfibers. Biomaterials. 32:6068–6079.
- Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR. 2004. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 4:451–464.
- Lu P, Tuszynski MH. 2008. Growth factors and combinatorial therapies for CNS regeneration. Exp Neurol. 209:313–320.
- Malarkey EB, Fisher KA, Bekyarova E, Liu W, Haddon RC, Parpura V. 2009. Conductive single-walled carbon nanotube substrates modulate neuronal growth. Nano Lett. 9:264–268.
- Malarkey EB, Parpura V. 2007. Applications of carbon nanotubes in neurobiology. Neurodegener Dis. 4:292–299.
- McLean AN. 2013. The spinal cord-injured patient in the medical ward. Clin Med. 13:549–552.
- Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL. 2009. KLF family members regulate intrinsic axon regeneration ability. Science. 326:298–301.
- Novikova LN, Novikov LN, Kellerth JO. 2003. Biopolymers and biodegradable smart implants for tissue regeneration after spinal cord injury. Curr Opin Neurol. 16:711–715.
- Panseri S, Cunha C, Lowery J, Del Carro U, Taraballi F, Amadio S, et al. 2008. Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections. BMC Biotechnol. 8:39.
- Roman JA, Niedzielko TL, Haddon RC, Parpura V, Floyd CL. 2011. Single-walled carbon nanotubes chemically functionalized with polyethylene glycol promote tissue repair in a rat model of spinal cord injury. J Neurotrauma. 28:2349–2362.
- Sheng L, Dajing C, Yuquan C. 2011. A surface acoustic wave humidity sensor with high sensitivity based on electrospun MWCNT/Nafion nanofiber films. Nanotechnology. 22:265504.
- Silva GA. 2006. Neuroscience nanotechnology: progress, opportunities and challenges. Nat Rev Neurosci. 7:65–74.
- Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. 2004. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 303:1352–1355.
- Sucapane A, Cellot G, Prato M, Giugliano M, Parpura V, Ballerini L. 2009. Interactions between cultured neurons and carbon nanotubes: a nanoneuroscience vignette. J Nanoneurosci. 1:10–16.
- Tabesh H, Amoabediny G, Nik NS, Heydari M, Yosefifard M, Siadat SO, Mottaghy K. 2009. The role of biodegradable engineered scaffolds seeded with Schwann cells for spinal cord regeneration. Neurochem Int. 54:73–83.
- Wouterlood FG, Goede PH, Groenewegen HJ. 1990. The in situ detectability of the neuroanatomical tracer Phaseolus vulgaris- leucoagglutinin (PHA-L). J Chem Neuroanat. 3:11–18.
- Yang F, Murugan R, Wang S, Ramakrishna S. 2005. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 26:2603–2610.