Abstract
The circulatory persistence, distribution, and metabolism of hemoglobin-based oxygen carriers (HBOCs) is a major determinant of their safety and efficacy. In this communication, published data on the pharmacokinetics and routes of plasma elimination of HBOCs are summarized and evaluated. The circulating half-life of HBOCs is dose-dependent in both animals and humans. Half-life also increases with molecular weight in animals, at least up to the MDa range. The functional half-life of HBOCs is diminished by as much as 40% due to oxidation of the heme group relative to the overall rate of removal of hemoglobin (Hb) from plasma. Kidney excretion of HBOCs is greatly diminished compared to that of unmodified Hb, but the liver remains a primary site of catabolism. Both hepatocytes and Kupffer cells have been implicated in receptor-mediated HBOC uptake. Removal also occurs in the spleen and/or bone marrow and probably at dispersed sites in the endothelium as well. HBOCs extravasate into the lymph at a rate inversely proportional to their molecular weight and are taken up by monocyte/macrophage CD163 receptors, both as free Hb and in complexes with haptoglobin (Hp). The interactions with both Hp and the CD163 receptor are altered by Hb modification. However, monocyte/macrophage uptake may not be a quantitatively important route for the removal of clinically relevant doses of HBOCs. The relative contributions of different removal pathways have yet to be comprehensively determined, particularly in humans.
Introduction
Hemoglobin-based oxygen carriers (HBOCs) are being developed to facilitate oxygen transport to ischemic or hypoxic tissues (CitationKim and Greenburg 2013). Potential indications include resuscitation from hemorrhagic shock, cardiac bypass pump priming, patient support when blood is unavailable, perfusion of infarcted tissues, maintenance of organ viability for transplant, and facilitation of radiation and chemotherapy (CitationWinslow 1993). The active principles of HBOCs are mammalian origin or are recombinantly produced hemoglobins (Hbs), which have been modified to facilitate efficacy and minimize toxicity. The advantages of HBOCs include low risk of pathogen transmission, long-term storage stability, absence of need for immunologic matching to recipients, and enhanced ability to perfuse ischemic tissues (CitationMozzarelli et al. 2010). Although regulatory approval for human use has yet to be obtained due to safety concerns with the current generation of products, limited approval for veterinary indications has been granted and work continues on the development of improved formulations.
The distribution and metabolism of protein therapeutics strongly influences their efficacy and safety. As a consequence, the measurement of these parameters is both a regulatory requirement and a prudent means to understand whether a given formulation addresses the needs of specific clinical indications (CitationLin 2009, CitationShargel et al. 2012). For these reasons, the intravascular persistence of HBOCs and their routes of its elimination from circulation have been frequently studied. An overview of this topic is presented in the following text. Data on the pharmacokinetics of liposome-encapsulated Hb are not included, since a review of this subject was recently published (CitationTaguchi et al. 2011).
Considerations and limitations
One convention explicitly used in this review is the characterization of intravascular persistence in terms of a “half-life”, which is defined as that period of elapsed time after the completion of intravenous administration during which the HBOC concentration in plasma falls to one half that measured, or inferred, immediately after infusion. Although useful, this definition suffers from the fact that half-life is only rigorously defined mathematically for concentrations that decay in exponential fashion, that is, concentrations which decrease in a first-order manner. While published data for HBOC disappearance from plasma frequently appear exponential, this is not universally the case (CitationDeVenuto 1983, CitationSehgal et al. 1984, CitationBerbers et al. 1991, CitationBaek et al. 2012).
A second limitation is that most published studies have not measured blood volume concomitant with HBOC concentration. This can be problematic because the doses of infused HBOC solutions are often a significant fraction of the total blood volume, and, in addition, tend to further expand this volume due to oncotic effects (CitationFriedman and DeVenuto 1982, CitationBleeker et al. 1986, CitationBakker et al. 1986, CitationMigita et al. 1997, CitationConover et al. 1997a,b, CitationFischer et al. 1999, CitationCaron et al. 2001, CitationBuehler et al. 2007). This is evident in human clinical studies in which total Hb concentrations were raised less after HBOC administration than after infusion of the same total amount of Hb as packed red blood cells (CitationLaMuraglia et al. 2000). The reason is that packed red cells exhibit very little oncotic pressure, while that of HBOCs is often significant (CitationMigita et al. 1997). An important consequence of this initial blood volume expansion is that blood volume will decrease as the HBOC disappears from circulation. Thus, the concentration of HBOC in blood will decrease more slowly than the total dose of HBOC, resulting in an overestimate of the circulating half-life if half-life is calculated only on the basis of changes in concentration. A dramatic example of this was reported by Migita and coworkers who demonstrated that an apparent half-life of greater than 200 h in rats for a polyethylene glycol (PEG)-modified Hb based on plasma Hb concentrations was in fact approximately three hours when data were corrected for blood volume changes (CitationMigita et al. 1997). These and other scientists have also noted an apparent elevation in the circulating half-life of crosslinked and/or polymerized HBOCs due to blood volume changes (CitationBleeker et al. 1986, CitationBakker et al. 1986, CitationMigita et al. 1997).
A third complication is that many HBOCs are infused in exchange transfusion protocols, while blood is simultaneously withdrawn. This results in some removal of HBOC during infusion, making the determination of administered dose more difficult. It may also obscure initial distribution or elimination processes. However, such protocols are necessitated by the large fluid volumes required for certain indications. In some of these experiments, net doses were calculated as the difference between the infused dose and the amount recovered in the withdrawn blood. Alternatively, the initial HBOC concentration in the blood measured or extrapolated immediately after infusion is taken as the starting point for analyses. Since approximately half of all HBOC studies reporting circulatory persistence have utilized exchange transfusion protocols, data from these studies are included despite the inherent difficulties in their interpretation.
A final point is the distinction between the circulatory persistence of functional HBOC versus that of total HBOC, since Hbs can only transport oxygen when the heme iron is in the reduced (+ 2) oxidation state. Generally speaking, HBOC heme irons undergo oxidation to the inactive (+ 3) methemoglobin (metHb) form during storage and after intravenous infusion (CitationKramlova et al. 1976, CitationDeVenuto 1978, CitationSehgal et al. 1981, CitationBuehler et al. 2007, CitationBaek et al. 2012). Thus, the amount of functional HBOC is always less than the total plasma Hb content. The fraction of circulating reduced HBOC has not been consistently reported, but comparisons between functional and total HBOC content will be discussed on the basis of available data.
Plasma half-life—summary data and trends
Unmodified mammalian Hbs are rapidly excreted through the kidneys, resulting in short intravascular persistence and renal toxicity (CitationBaker and Dodds 1925). This is primarily due to rapid dissociation of the 64 kDa Hb tetramer into two 32 kDa αβ dimers which readily permeate the glomerular membrane. Bunn, Esham, and Bull demonstrated that kidney excretion is substantially reduced by chemical crosslinking of the dimers to form stabilized α2β2 tetramers (CitationBunn et al. 1969). As a result, a guiding principle in HBOC development has been to inhibit renal excretion by stabilization of the tetrameric conformation and/or incorporation of HBOCs into larger molecular complexes by polymerization, encapsulation, surface modification, or the utilization of naturally occurring high molecular weight heme proteins. In this review, intramolecularly stabilized Hb tetramers are denoted as crosslinked, while intermolecularly linked HBOCs are denoted as polymerized.
Building on the seminal work of Bunn and coworkers, a number of researchers have shown that crosslinking with a variety of reagents leads to increased intravascular persistence (CitationMok et al. 1975, CitationGreenburg et al. 1977, CitationGreenburg and Maffuid 1983, CitationBleeker et al. 1986, CitationSnyder et al. 1987, CitationHess et al. 1989, CitationKeipert et al. 1989a, CitationKeipert et al. 1989b, CitationUrbaitis et al. 1991, CitationDittmer et al. 1992, CitationBakker et al. 1993, CitationKeipert et al. 1993, CitationKeipert et al. 1994, CitationBush et al. 1994, CitationBucci et al. 1996, CitationMigita et al. 1997, CitationShip et al. 2005). Polymerization further increases half-life (CitationTam et al. 1978, CitationDeVenuto and Zegna 1983, CitationKeipert and Chang 1983, CitationSehgal et al. 1984, CitationKeipert and Chang 1987, CitationSnyder et al. 1987, CitationBerbers et al. 1991, CitationLenz et al. 1991, CitationBleeker et al. 1992, CitationHsia et al. 1993, CitationBakker et al. 1993, CitationAnderson et al. 1993, CitationMenu et al. 1994, CitationLee et al. 1995, CitationPearce et al. 2003, CitationWicks et al. 2003, CitationBonegio et al. 2006, CitationBuehler et al. 2007, CitationBuehler et al. 2010, CitationBaek et al. 2012, CitationElmer et al. 2012). Most reported values have not been corrected for blood volume changes, but the increased half-life of crosslinked and/or polymerized HBOCs relative to unmodified Hb is not an artifact of such changes because these modifications do not increase the oncotic pressure at a given Hb concentration (CitationDeVenuto 1983, CitationSehgal et al. 1984, CitationBerbers et al. 1991, CitationVandegriff et al. 1997). On the other hand, PEG modification markedly increases the oncotic pressure of Hbs (CitationVandegriff et al. 1997). For this reason, preclinical data obtained with PEG-modified Hbs are considered separately (CitationConover et al. 1997a,b, CitationMigita et al. 1997, CitationVandegriff et al. 1997, CitationConover et al. 1999, CitationVandegriff et al. 2003, CitationVandegriff et al. 2006). HBOC half-life has also been measured during human trials (CitationHughes et al. 1995, CitationSwan et al. 1995, CitationHughes et al. 1996, CitationPrzybelski et al. 1996, CitationViele et al. 1997, CitationStandl et al. 1998, CitationCarmichael et al. 2000, CitationO’Hara et al. 2001, CitationOlofsson et al. 2006, CitationVandegriff et al. 2006). In considering both preclinical and clinical data, several trends are apparent:
Infusion of increasing HBOC doses results in an apparent prolongation of half-life (CitationBleeker et al. 1986, CitationKeipert and Chang 1987, CitationHess et al. 1989, CitationKeipert et al. 1989a, CitationBleeker et al. 1992, CitationHughes et al. 1996, CitationPrzybelski et al. 1996, CitationViele et al. 1997, CitationCarmichael et al. 2000, CitationPearce et al. 2003) in both animals and humans ( and ). The effect is modest with unmodified Hbs, but more pronounced with crosslinked and polymerized HBOCs (CitationBleeker et al. 1986, CitationBleeker et al. 1992, CitationKeipert 1992). Bleeker and coworkers demonstrated that the apparent increase of half-life with increasing doses of unmodified Hb was greatly diminished, though not eliminated, when blood volume changes were considered (CitationBleeker et al. 1986). Unfortunately, blood volume change corrections have not been applied to dose-response series with crosslinked or polymerized HBOCs. The half-life of PEG-modified Hb in humans did not exhibit a dose dependence (CitationVandegriff et al. 2006, CitationOlofsson et al. 2006), albeit from data collected over a restricted dosing range. The reason for this difference is unclear.
Figure 1. Circulating half-life of HBOCs in rats as a function of dose: unmodified Hb (♦), crosslinked tetrameric Hb (■), NFPLP-crosslinked, glutaraldehyde-polymerized Hb (▲), glutaraldehyde-polymerized bovine Hb (x). Data from (CitationBleeker et al. 1986, CitationSnyder et al. 1987, CitationKeipert et al. 1989b, CitationBleeker et al. 1992, CitationKeipert et al. 1993, CitationKeipert et al. 1994, CitationMigita et al. 1997, CitationPearce et al. 2003).
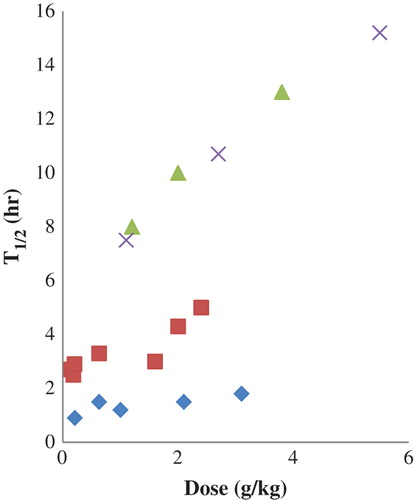
Figure 2. Circulating half-life of HBOCs in humans as a function of dose: crosslinked tetrameric human Hb (♦), raffinose-polymerized human Hb (■), glutaraldehyde-polymerized bovine Hb (▲), recombinant crosslinked tetrameric human Hb (x). Data from (CitationHughes et al. 1995, CitationHughes et al. 1996, CitationPrzybelski et al. 1996, CitationViele et al. 1997, CitationCarmichael et al. 2000).
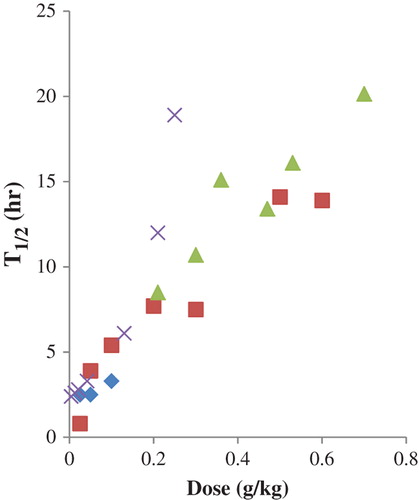
A second trend is that increasing the size of the HBOC complex is associated with an increase in half-life (CitationSehgal et al. 1984, CitationSnyder et al. 1987, CitationBerbers et al. 1991, CitationBleeker et al. 1992, CitationKeipert et al. 1992, CitationBakker et al. 1993, CitationHsia et al. 1993, CitationWicks et al. 2003, CitationBaek et al. 2012), an effect which does not depend on a particular modification chemistry (). The increase in half-life seems to approach a maximum as the molecular weight approaches one MDa. With bovine polymerized HBOCs, a 31-h half-life was observed with a 1.3 MDa average molecular weight product, compared to 19 and 15 h for 0.75 and 5.5 MDa products, respectively (CitationBaek et al. 2012). Sufficient comparative data have not been reported in humans to determine whether there is a dependency of circulatory persistence on the molecular weight of HBOCs similar to that observed in animal models, although one would be expected.
Figure 3. Circulating half-life of HBOCs in animals as a function of molecular weight: raffinose-polymerized human Hb—50% exchange transfusion in rats (♦), NFPLP-crosslinked and glutaraldehyde-polymerized human Hb—35% exchange transfusion in rats (■), NFPLP-crosslinked and glutaraldehyde-polymerized human Hb—50% exchange transfusion in rats (x), NFPLP-crosslinked and glutaraldehyde-polymerized human Hb—70% exchange transfusion in rats (▲), glutaraldehyde-polymerized bovine Hb—3 g/kg in guinea pigs (⋇). Data from (CitationBerbers et al. 1991, CitationBleeker et al. 1992, CitationHsia et al. 1993, CitationBaek et al. 2012).
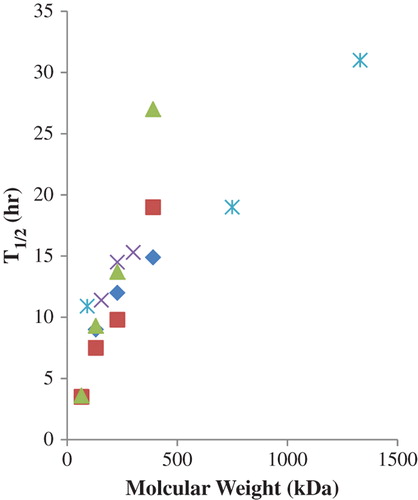
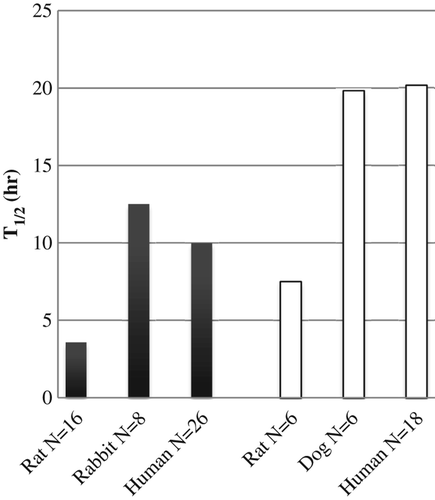
A third trend is the variation in circulatory persistence with species (CitationHess et al. 1989, CitationPearce et al. 2003). While the nature and relative paucity of comparable data do not permit a rigorous statistical analysis, two qualitative conclusions seem warranted. At similar doses with the same modified HBOC, the half-life in humans is approximately three-fold greater than that in rats and is comparable to that in intermediate sized mammals (). These results suggest that reasonable expectations of human circulatory persistence may be predictable for new HBOCs on the basis of animal data.
Figure 4. Comparison of intravascular half-life of two HBOCs in human and non-human species at 0.6–1.6 g/kg doses: crosslinked tetrameric Hb (■), glutaraldehyde-polymerized bovine Hb (□). Data from (CitationSnyder et al. 1987, CitationHess et al. 1989, CitationHughes et al. 1995, CitationMigita et al. 1997, CitationO’Hara et al. 2001, CitationPearce et al. 2003).
Functional half-life
As noted in “Considerations and limitations”, the concentration of functional HBOC is reduced by oxidation to the inactive metHb form during storage and circulation (CitationKramlova et al. 1976, CitationDeVenuto 1978, CitationTam et al. 1978, CitationSehgal et al. 1981, CitationSnyder et al. 1987, CitationBush et al. 1994, CitationLee et al. 1995, CitationCaron et al. 2000, CitationO’Hara et al. 2001, CitationSprung et al. 2002, CitationWicks et al. 2003, CitationOlofsson et al. 2006, CitationVandegriff et al. 2006, CitationBuehler et al. 2007, CitationBuehler et al. 2010, CitationBaek et al. 2012, CitationElmer et al. 2012). While the predominant oxidant during storage is oxygen, other oxidants are important intravenously, including hydrogen peroxide and nitric oxide (NO) (CitationBrooks 1932, CitationBuehler et al. 2007). Although NO is much less abundant than oxygen, the reaction with Hb is extremely rapid (CitationCassoly and Gibson 1975, CitationDoyle and Hoekstra 1981). On the other hand, several reductants are also present in blood that can reduce metHb (CitationAmes et al. 1981, CitationMcGown et al. 1990, CitationHarrington et al. 2000). Ascorbate is known to be a potent metHb reductant in the presence of red cells which can recycle the oxidized dehydroascorbate to ascorbate (CitationFrei et al. 1989, CitationMendiratta et al. 1998, CitationDorman et al. 2002, CitationDunne et al. 2006, CitationBuehler et al. 2007). Oxidation/reduction rates of Hbs vary with species, the presence of organic phosphates, pH, oxygen partial pressure, degree of Hb dissociation, extent of polymerization, and location of the heme group on alpha or beta subunits (CitationBrooks 1935, CitationMansouri and Winterhalter 1973, CitationHarvey and Kaneko 1976, CitationTomoda et al. 1976, CitationKikugawa et al. 1981, CitationZhang et al. 1991, CitationAlayash et al. 2001, CitationBaek et al. 2012). The functional state of HBOCs after infusion is therefore a product of a myriad of variables, including metHb content upon infusion, intrinsic reactivity with oxidants and reductants, and relative concentrations of these reactants (CitationFaivre-Fiorina et al. 1998). Due to this complexity, metHb formation rates after infusion cannot be predicted a priori. Not surprisingly, several researchers noted a poor correlation between oxidation rates in vitro and in vivo (CitationSnyder et al. 1987, CitationVandegriff et al. 2006, CitationBuehler et al. 2010).
A consistent finding from measurement of the in vivo oxidation of different HBOCs is that plasma metHb levels increase immediately after infusion due to the oxidized Hb contained in the preparation. Subsequently, if the metHb content is initially low, the percentage of oxidized Hb usually increases as the total plasma Hb concentration decreases, resulting in 20–60% of the plasma HBOC being in the met form after several half-lives (CitationTam et al. 1978, CitationLee et al. 1995, CitationConover et al. 1997a, CitationBuehler et al. 2007, CitationBuehler et al. 2010). This reduces the effective functional half-life by as much as 40% (), although this can be mitigated by the co-administration of reducing agents (CitationSehgal et al. 1981, CitationFaivre-Fiorina et al. 1998). The rate of metHb formation is also dose-dependent. When den Boer and coworkers (Citationden Boer et al. 1992) infused a polymerized Hb solution with 6% metHb into rats, plasma metHb content remained below 15% at exchange transfusion levels of 40% or 70% of blood volume, but after a 90% exchange metHb increased to 30%. The latter result probably reflects the removal of a substantial portion of the endogenous reduction capacity in blood during the high-volume exchange. Conversely, when fully oxidized Hb was infused, metHb levels decreased to 20% after 5% or 40% blood volume exchanges, but only 40% after a 70% exchange. A 40–80% reduction of high levels of oxidized HBOC was also observed by other authors in rats or guinea pigs, reinforcing the notion that mammalian blood exhibits substantial reducing potential (CitationSnyder et al. 1987; Citationden Boer et al. 1992, CitationFaivre et al. 1994). It should be noted that while the percentage of plasma metHb usually increases with time after HBOC infusion, the absolute concentration may increase, decrease, or stay relatively constant until the inevitable ultimate decrease (CitationTam et al. 1978, CitationSnyder et al. 1987, CitationBush et al. 1994, CitationLee et al. 1995, CitationO’Hara et al. 2001, CitationSprung et al. 2002, CitationWicks et al. 2003, CitationDunne et al. 2006, CitationOlofsson et al. 2006, CitationVandegriff et al. 2006, CitationBuehler et al. 2007, CitationBuehler et al. 2010, CitationBaek et al. 2012, CitationElmer et al. 2012), depending on dose, recipient species, and HBOC type.
Table I. Comparison of Total and Functional Half-Lives of HBOCs.a
One question which arises is whether HBOC oxidation facilitates plasma removal. Snyder et al., reported that the half-life of completely oxidized diaspirin-crosslinked hemoglobin (DCLHb) was the same as that of reduced DCLHb, although these results are confounded by the fact that 73% of the oxidized Hb was reduced after five hours (CitationSnyder et al. 1987). Vandegriff and coworkers argued on kinetic grounds that preferential clearance of the met form of PEG-conjugated Hb was unlikely because the overall rate of clearance of this HBOC from the circulation is significantly faster than the rate of metHb formation (CitationVandegriff et al. 2006). On the other hand, Buehler et al. suggested that the difference in plasma persistence between bovine Hbs polymerized in the oxygenated versus the deoxygenated states may be due to the fact that the in vivo oxidation rates are different (CitationBuehler et al. 2010); however, these two preparations also differed in a number of other physical and chemical properties. Overall, the extant literature is not definitive on this point, but suggestive that conversion of reduced Hb to the oxidized form is not a primary step in the Hb elimination process.
Overview of distribution and plasma elimination
Several laboratories have investigated the distribution and metabolism of HBOCs. General results will be summarized in this section, followed by detailed discussion of individual removal pathways in the next. One caution in interpreting these data is that the amount of HBOC present in an organ at any given time is a function of the difference between the rate of uptake and the rate of catabolism. Thus, if uptake rates are similar, but the catabolism rate is greater in one organ versus another, the amount of Hb removed by the latter may be similar even though the concentration of Hb at any particular time point will be lower. As a consequence, the relative amount of HBOC present may not quantitatively reflect the relative contributions of organs toward Hb removal.
Chromatographic analysis of HBOCs in plasma suggests that these proteins are not substantially degraded within the circulation (CitationBleeker et al. 1989, CitationHsia et al. 1993, CitationBush et al. 1994). Supporting this is the fact that the disappearance of radioactively labeled HBOCs from plasma corresponds closely to their rate of disappearance as determined by a spectrophotometric assay (CitationKeipert et al. 1993, CitationKeipert et al. 1994). In addition, when low doses of tritiated HBOC were recirculated through isolated, perfused rat liver, most of the radioactivity in the perfusate remained associated with intact Hb (CitationChow et al. 2008). On the other hand, low molecular weight metabolites rapidly appeared in the bile. Thus, the primary sites of HBOC catabolism are probably extravascular.
Three published studies investigated the plasma elimination and catabolism of human Hb intramolecularly crosslinked with 2-nor-2-formylpyridoxal 5’-phosphate (NFPLP) in rats (CitationBleeker et al. 1989, CitationKeipert and Triner 1989, CitationKeipert et al. 1989b). Using 99mTc labeling, Bleeker and coworkers found that the concentration of crosslinked HBOC in the kidney was greatly reduced relative to that seen with unmodified Hb two hours after high-dose exchange transfusion (CitationBleeker et al. 1989). Some HBOC accumulation was detected in the liver and spleen. However, scintigrams indicated that much of the protein was diffusely distributed throughout the body. One concern with these data is that technetium labeling demonstrably affected the kidney filtration of Hb, suggesting that the technique may influence the protein distribution.
Keipert and coworkers labeled this same HBOC with 3H in a manner which would not be expected to alter chemical or physical properties (CitationKeipert and Triner 1989, Keipert et al. 1989). Rats were monitored, and urine and feces collected, for up to nine days after the infusion of a relatively low (0.15 g/kg) dose. Organs were collected from exsanguinated and saline-flushed animals to quantify label accumulation. Nine hours after infusion, the highest concentration of label was detected in the kidneys, liver, and spleen, although the total amount of label in muscle was comparable to that in the liver and kidneys due to the large total muscle mass. Lesser amounts of label were detected in other organs and tissues. Label concentration rapidly decreased in the liver and kidney, suggesting that HBOC is actively catabolized in these organs. Radioactivity was found in urine several hours after infusion and throughout the monitoring period, albeit at a declining rate. A similar trend was seen in the feces. Ultimately, nearly 55% of the infused label was recovered in the urine and 28% in the feces. Ultrafiltration and chromatographic analyses demonstrated that radioactivity in the urine and feces was recovered predominantly in low molecular weight compounds. No intact HBOC could be detected in urine by spectrophotometric analysis.
Qualitatively similar results were obtained from studies of the distribution and elimination of DCLHb (CitationDittmer et al. 1992, CitationKeipert et al. 1993, CitationBush et al. 1994, CitationKeipert et al. 1994). After a 50% exchange transfusion into rats with 14C-labeled protein, Keipert and coworkers found the highest radioactivity in the kidney, spleen, bone marrow, and liver, 24 h after infusion (CitationKeipert et al. 1993, CitationKeipert et al. 1994). However, the highest overall label recovery was in the muscle and skin. Brain radioactivity was particularly low. Tissue and organ concentrations peaked 5 to 24 h after infusion and declined thereafter. Radioactivity appeared in the urine within an hour of infusion, peaking on the second day of collection, and at detectable, but decreasing, levels thereafter. Approximately 59% of the administered radioactivity was recovered in the urine and 9% in feces. Electrophoretic and chromatographic analyses of urine demonstrated the presence of intact DCLHb in early collected samples, suggesting that some crosslinked Hb crosses the glomerular membrane. Subsequently, intact Hb was undetectable in the urine 12 h after infusion. Dittmer et al., reported a more diffuse distribution of radioactivity after the infusion of 51Cr-labeled DCLHb into mice; however they also demonstrated some label elution from the protein (CitationDittmer et al. 1992), an artifact which was previously noted (CitationKeene and Jandl 1965). After the infusion of a 2 g/kg dose of DCLHb into swine, Bush and coworkers found increased iron concentrations in the kidney and liver, consistent with Hb catabolism in these organs (CitationBush et al. 1994). Serum iron levels increased to a maximum eight hours after infusion and decreased to baseline by 96 h, never exceeding the total serum iron binding capacity.
Collectively, studies of crosslinked Hb tetramers suggest that this class of HBOCs distributes into numerous tissues and extravascular spaces after infusion. Small amounts may also be filtered by the kidney, though such filtration is markedly reduced from that of unmodified Hb. These HBOCs are catabolized by the liver and probably other organs of the reticuloendothelial system (See Mechanisms of plasma elimination).
Polymerization of Hbs to form higher molecular weight entities alters their distribution and elimination patterns, although interpretation of these data is confounded by the fact that these preparations contain components with a variety of molecular weights, including unmodified Hb (CitationLenz et al. 1991, CitationKeipert et al. 1992, CitationAnderson et al. 1993, CitationHsia et al. 1993, CitationBaek et al. 2012). This difficulty was addressed by Hsia et al., who studied the distribution of human Hb polymers of different molecular weights synthesized by reaction with oxidized, ring-opened raffinose (CitationHsia et al. 1993). Differences were evaluated using both size-exclusion high-performance liquid chromatography and tritium-labeled polymer fractions separated and purified by preparative scale chromatography. After a 50% exchange transfusion into rats, kidney uptake decreased and liver uptake increased with increasing molecular weight, as measured ten hours after infusion. As expected, urinary excretion of the various polymerized Hb fractions was much lower than that of unmodified Hb. The similarity of plasma clearance rates for the different fractions determined by HPLC and labeled polymer clearance implies that polymers were chemically stable in plasma.
Anderson et al., radiolabeled unmodified human Hb with 3H-formaldehyde and purified glutaraldehyde-polymerized human Hb with 14C-formaldehyde (CitationAnderson et al. 1993). The latter contained less than 2% of unmodified Hb, with more than 95% of the material exhibiting an apparent molecular weight in excess of 440,000 Da. These labeled fractions were then spiked into an unfractionated mixture of polymerized Hb which was infused into dogs at a dose of 0.8 g/kg. Once again, there was greater kidney accumulation of the unmodified Hb and greater liver uptake of the polymerized Hb. There was also greater extravasation of the former into other tissues, as evidenced by a higher specific radioactivity in the heart than would be expected on the basis of vascular volume. In a comparison of different molecular weight average glutaraldehyde-polymerized bovine Hb preparations in guinea pigs, Baek and coworkers noted an inverse dependence of iron deposition in the kidneys relative to molecular weight, implying a decreasing Hb metabolism in this organ (CitationBaek et al. 2012). This was also corroborated by microscopic analysis for evidence of Hb deposition and heme oxygenase activity in the kidney, both of which are surrogate markers for the presence of intracellular Hb. Increases in iron content in the liver and spleen were noted for all three of the different molecular weight classes evaluated—91, 749, and 1330 kDa. The authors concluded that the 749 and 1330 kDa preparations were predominantly metabolized in the liver and spleen, while the 91 kDa Hb was also eliminated by the kidney. Lenz et al. found siderosis of Kupffer cells after the biopsy of chimpanzees partially exchange-transfused with a glutaraldehyde-polymerized human Hb, consistent with liver catabolism of this HBOC (CitationLenz et al. 1991). These authors observed renal excretion of approximately 7% of the administered dose of this preparation, which was expected due to the presence of 15% non-crosslinked Hb.
Mechanisms of plasma elimination
Haptoglobin and liver uptake of bound and free hemoglobin
Haptoglobin (Hp) binds unmodified Hb with near irreversible affinity, thereby incorporating it into high molecular weight complexes which prevent glomerular filtration (CitationLaurell and Nyman 1957, CitationKeene and Jandl 1965, CitationNagel and Gibson 1971). Infusion of preformed Hb–Hp complexes, or Hb at doses not exceeding the plasma Hp binding capacity, results in primary uptake by the liver (CitationMurray et al. 1961, CitationKeene and Jandl 1965, CitationBissell et al. 1972, CitationBunn 1972, CitationKino et al. 1980, CitationKino et al. 1987, CitationShip et al. 2005). At doses of Hb which exceed the limited plasma Hp binding capacity, the liver continues to be a significant organ for Hb removal, although substantial amounts are also excreted by the kidney (CitationKeene and Jandl 1965, CitationGoldfischer et al. 1970, CitationBissell et al. 1972, CitationBunn 1972, CitationGreenburg 1983, CitationKino et al. 1987). Within the liver, the relative contribution of hepatocytes versus resident macrophages (Kupffer cells) to Hb removal has been debated. After infusion of 0.15 g/kg of Hb into rats, histologic staining revealed the presence of Hb only in Kupffer cells (Goldfischer 1970). However, after the infusion of higher doses, Hb was found primarily in endocytic vacuoles in hepatocytes (Goldfischer 1970). Likewise, after the infusion of 59Fe-labeled Hb, 85–95% of the radioactivity associated with the liver was calculated to be in hepatocytes, regardless of association with Hp (CitationBissell et al. 1972). In vivo studies imply that Hb is taken up by hepatocytes in a receptor-mediated process (CitationKino et al. 1980, CitationKino et al. 1987), and that isolated hepatocytes can assimilate both Hp–Hb complexes and free Hb (CitationWeinstein and Segal 1984, CitationZuwała-Jagiello and Osada 1998). Other studies have also shown that Hp is not required for rapid in vivo Hb clearance or hepatocyte uptake (CitationHershko et al. 1972, CitationWeinstein and Segal 1984, CitationLim et al. 1998). On the other hand, an autoradiographic study of Hp–Hb uptake found that these complexes were primarily found in Kupffer cells (CitationWada et al. 1970), and Friedman and coworkers only detected Hb microscopically in Kupffer cells after the infusion of high doses of Hb (CitationFriedman et al. 1978).
Many, but not all, HBOCs are also bound by Hp, although the strength of binding varies, depending on the particular modification chemistry utilized (CitationBunn 1967, CitationLockhart and Smith 1975, CitationBenesch et al. 1976, CitationPanter et al. 1994, CitationShip et al. 2005, CitationSchaer et al. 2006, CitationBuehler et al. 2008, CitationBaek et al. 2012, CitationJia et al. 2013). These complexes are taken up by the liver, as are the much higher doses of free HBOC which are more representative of the anticipated clinical uses of these formulations (CitationBleeker et al. 1989, CitationKeipert et al. 1989b, CitationSmith et al. 1990, CitationDittmer et al. 1992, CitationHsia et al. 1993, CitationBush et al. 1994, CitationKeipert et al. 1994, CitationShip et al. 2005, CitationBaek et al. 2012). Only limited data are available concerning the specific liver cells involved with this uptake. After infusion of crosslinked Hb, light microscopy confirmed the presence of diaminobenzidene (DAB)-positive material within hepatocytes 15 min after exchange transfusion, presumably due to HBOC uptake (CitationBleeker et al. 1989). A small amount was detected in Kupffer cells. Smith and coworkers also detected Hb in both hepatocytes and Kupffer cells after the infusion of a different crosslinked Hb (CitationSmith et al. 1990).
In comparing the clearance of unmodified and crosslinked HBOC in isolated, perfused rat liver with that observed in vivo, Chow and coworkers determined that liver uptake could account for 30–50% of the total whole body clearance of the latter, demonstrating both the importance of the liver in HBOC metabolism and the significance of other pathways (CitationChow et al. 2008). Hp modestly facilitated the uptake of either Hb type, and the removal of the crosslinked Hb was slower than that of the unmodified counterpart. Breakdown of Hb into lower molecular weight metabolites excreted into bile began soon after liver uptake. Two caveats of this study are that it was performed with a low dose of Hb in mice. Similar comparisons at higher doses with other species would be of interest.
Kidney excretion
As summarized in the preceding rapid renal excretion of unmodified Hb is substantially mitigated by crosslinking and polymerization. Still, mild hemoglobinuria has been observed after the administration of modified HBOCs to both animals and humans (CitationKeipert and Chang 1983, CitationBakker et al. 1986, CitationBleeker et al. 1986, CitationLenz et al. 1991, CitationKeipert 1992, CitationKeipert et al. 1992, CitationAnderson et al. 1993, CitationKeipert et al. 1993, CitationGilbert et al. 1994, CitationKeipert et al. 1994, CitationLee et al. 1995, Conover et al. 1997, CitationSchubert et al. 2002, CitationWicks et al. 2003, CitationBonegio et al. 2006, CitationOlofsson et al. 2006, CitationBaek et al. 2012). While this hemoglobinuria has been widely attributed to the presence of residual unmodified Hb, intact tetramers were detected in urine as well (CitationKeipert et al. 1989b, CitationUrbaitis et al. 1991, CitationKeipert et al. 1992, CitationKeipert et al. 1993, CitationKeipert et al. 1994). This was somewhat surprising since it was historically believed that albumin, a protein only slightly larger than tetrameric Hb, undergoes little to no filtration by the kidney glomerulus (CitationPitts 1968, CitationGuyton 1976). However, recent work has demonstrated that albumin indeed passes through the glomerular membrane, after which it is reabsorbed in the renal tubules where it may be degraded and/or transcytosed intact back into the circulation (CitationComper and Russo 2009, CitationMenaka et al. 2009, CitationTojo and Kinugasa 2012, CitationTenten et al. 2013). Glomerular filtration is dependent on molecular size, charge, and shape, with reduced filtration favored by large size, negative charge, and globular conformation (CitationVenkatachalam and Rennke 1978, CitationGhitescu et al. 1992, CitationHaraldsson et al. 2008). Although albumin is a more elongated molecule than tetrameric Hb, it is much more negatively charged at physiologic pH and has a slightly greater molecular weight (CitationPitts 1968, CitationGros et al. 1978, CitationThomas et al. 1997, CitationTojo and Kinugasa 2012). In light of this, it is not surprising that some Hb tetramers are filtered. There is also evidence that, like unmodified Hb, some HBOCs passing through the glomerulus are reabsorbed by the kidney tubules, but the capacity of this system is limited (CitationLathem et al. 1960, CitationMiller 1960, CitationEricsson 1965a,Citationb, CitationFriedman and DeVenuto 1982, CitationBleeker et al. 1989, CitationLenz et al. 1991, CitationUrbaitis et al. 1991, CitationConover et al. 1997a,b, CitationGburek and Osada 2000, CitationGburek et al. 2002).
Extant data suggest that polymerization or conjugation of Hb into polymers of molecular weight greater than 100 kDa further reduces, and possibly, eliminates renal excretion, at least in animals with an intact glomerular filtration barrier (CitationBerbers et al. 1991, CitationKeipert 1992, CitationKeipert et al. 1992, CitationHsia et al. 1993, CitationLee et al. 1995, Conover et al. 1997, CitationWicks et al. 2003, CitationBonegio et al. 2006, CitationBaek et al. 2012). Thus, kidney excretion is considered to be a minor contributor to the removal of HBOCs from the intravascular circulation. On the other hand, studies performed with radioactively labeled HBOCs demonstrate substantial renal excretion of HBOC breakdown products, which probably derive from catabolism in various organs and tissues (CitationKeipert et al. 1989b, CitationKeipert et al. 1992, CitationAnderson et al. 1993, CitationKeipert et al. 1993, CitationKeipert et al. 1994).
CD163 and monocyte/macrophage phagocytosis
It has been known for decades that macrophages pinocytose Hb (CitationEhrenreich and Cohn 1968), but it was only in 2001 that a macrophage protein, CD 163, was identified as the receptor which mediates the endocytosis of Hp–Hb complexes (CitationKristiansen et al. 2001). CD 163 is only expressed in the monocyte/macrophage cell lineage, including tissue-resident macrophages such as Kupffer cells (CitationGraversen et al. 2002, CitationMoestrup and Møller 2004, CitationPolfliet et al. 2006). In addition to the high affinity binding of Hp–Hb complexes, CD163 was more recently shown to interact directly with Hb with lower affinity to effect receptor-mediated endocytosis in the absence of Hp (CitationSchaer et al. 2006). CD163 interacts with modified HBOCs to varying degrees, depending on the nature of the chemical derivatization (CitationSchaer et al. 2006, CitationBuehler et al. 2008). In light of these results, it is tempting to infer a significant role for the CD163-mediated uptake of HBOCs into macrophages as a primary route for Hb clearance from the circulation (CitationSchaer et al. 2006). This is consistent with the observation that Hb clearance was impaired in two patients with intravascular hemolysis concomitant with a treatment regimen for leukemia which reduced the number of CD163-expressing monocytes (CitationManiecki et al. 2008). However, Hb clearance was not markedly reduced in mice which were genetically depleted of CD163 compared to controls (CitationEtzerodt et al. 2013). Comparison of these seemingly conflicting results is confounded by species differences and possible indirect effects of the aggressive therapy required in the treatment of the human subjects; however, the recent observation that Hb clearance was little affected in dogs in which the circulating pool of CD163-expressing macrophages was increased with glucocorticoid stimulation again suggests that this pathway may not be primary in the clearance of HBOCs (CitationBoretti et al. 2014). A similar conclusion has been articulated by several of the authors involved with the characterization of the CD163–HBOC interaction (CitationSchaer et al. 2007). A possibly related set of observations is that the clearance of Hb and the clearance of particulate challenges by the reticuloendothelial system do not interfere with one another, suggesting different clearance pathways (CitationKeene and Jandl 1965, CitationGreenburg 1983, CitationKim et al. 1993).
Extravasation and distributed endocytosis
Plasma components extravasate through the endothelium of blood vessels by mechanisms which are dependent on capillary structure, disease state, and blood and lymph flows (CitationTakakura et al. 1998). Macromolecule extravasation is dependent on size, shape, charge, and hydrophilic/lipophilic balance (CitationGandhi and Bell 1992, CitationTakakura et al. 1998, CitationEl-Sayed et al. 2001, CitationLin 2009). Although the mechanisms of extravasation have been debated for decades, caveolae-mediated transcytosis through the endothelial cell interior of capillaries is believed to be an important pathway for albumin, along with passage through pericellular junctions between cells (CitationSzabó and Magyar 1982, CitationMalik et al. 1989, CitationSchnitzer et al. 1994, CitationSimionescu et al. 2002, CitationAird 2007, CitationLin 2009, CitationKumari et al. 2010). Measurements of radioiodinated albumin efflux indicate that 5–10% of plasma albumin extravasates per hour (CitationSzabó and Magyar 1982, CitationFleck 1985). It is therefore not surprising that HBOCs also extravasate (CitationKeipert et al. 1989a, CitationDittmer et al. 1992). Velky and coworkers noted that both unmodified and ATP-modified Hb leaked into the peritoneal cavity after intravenous infusion, with accumulation of the latter significantly inhibited by m-dansyl cadaverine, an endocytotic blocking agent (CitationVelky et al. 1987). Bleeker et al. detected crosslinked Hb in thoracic duct lymph (CitationBleeker et al. 1989). Other researchers have detected crosslinked Hb in the hilar, lung, and soft tissue lymph (CitationConhaim et al. 1998, CitationMatheson et al. 2000), and Burhop and Doyle reported that the appearance of genetically polymerized Hbs in rat thoracic lymph was inversely correlated with molecular weight (CitationBurhop and Doyle 2002).
An interesting question is the fate of extravasated Hb. Albumin has a plasma half-life of several hours, but an overall circulating life span of approximately three weeks, implying that it constantly recirculates between the intravascular and extravascular space (CitationSzabó and Magyar 1982, CitationFleck 1985). While the initial intravascular half-life of the comparably sized crosslinked Hb is similar to that of albumin, the overall circulatory persistence is much less, implying that Hb is subject to a catabolic process which does not operate on albumin. Similarly, IgG also exhibits an overall circulatory persistence which is much greater than like-sized HBOC polymers (170 kDa) (CitationMorell et al. 1970, CitationPeppard and Orlans 1980). On the other hand, other plasma proteins exhibit a more rapid catabolism which is qualitatively similar to that of HBOCs (CitationJarnum 1975, CitationBouma 1982). A large body of work has demonstrated that this difference is due to the fact that many plasma proteins are subject to catabolism in endothelial cells by endocytosis followed by lysosomal degradation, but that albumin and IgG are largely spared from this fate by a pH-specific binding to the FcRn receptor which redirects these proteins back into the circulation (CitationGhetie et al. 1996, CitationIsrael et al. 1996, CitationJunghans and Anderson 1996, CitationChaudhury et al. 2003). It is likely that HBOCs are also subject to this catabolic pathway, and, while this has not been specifically evaluated, several lines of evidence are consistent with this hypothesis:
HBOC clearance cannot be quantitatively explained by catabolism in the liver and monocytes/macrophages (CitationSchaer et al. 2007, CitationChow et al. 2008, CitationEtzerodt et al. 2013, CitationBoretti et al. 2014).
Kidney filtration of crosslinked and polymerized HBOCs is minimal (CitationBerbers et al. 1991, CitationKeipert 1992, CitationKeipert et al. 1992, CitationHsia et al. 1993, CitationLee et al. 1995, CitationConover et al. 1997a, CitationWicks et al. 2003, CitationBaek et al. 2012).
Hb has been detected inside endothelial cells (CitationFaivre-Fiorina et al. 1999).
The inhibition of peritoneal accumulation of Hb by the endocytotic inhibitor m-dansyl cadaverine implies that Hb is subject to endocytosis (CitationVelky et al. 1987).
It seems highly unlikely that a rescue receptor for Hb would have evolved, since several pathways for Hb removal have been favored by evolution (CitationMurray et al. 1961, CitationKeene and Jandl 1965, CitationGoldfischer et al. 1970, CitationBissell et al. 1972, CitationBunn 1972, CitationKino et al. 1980, CitationKino et al. 1987, CitationShip et al. 2005).
The wide detection of the Hb label throughout the body in distribution studies is consistent with a dispersed catabolism component (CitationDittmer et al. 1992, CitationKeipert et al. 1994, CitationBleeker et al. 1989).
FcRn is detected in many tissues, but particularly in endothelial cells, indicating that these are important sites of plasma protein catabolism (CitationBouma 1982, CitationGhetie et al. 1996, CitationBorvak et al. 1998).
One implication of this hypothesis is that HBOC circulatory half-life may be improved by association of the Hb with proteins or protein fragments that bind to the FcRn receptor. This strategy has been successfully employed to improve the circulatory persistence of therapeutic proteins by as much as 4-fold (CitationDall’Acqua et al. 2006, CitationAndersen and Sandlie 2009, CitationAndersen et al. 2011), and conjugation of Hb to albumin was reported to enhance intravascular half-life (CitationBonhard and Boysen 1982). However, the latter study employed non-specific crosslinking and was not directed to exploitation of the FcRn receptor. Thus, it is unclear whether the observed increase was due to FcRn binding or a simple increase in molecular weight. Similar experiments, coupled with measurement of the interaction of the complex with the FcRn receptor, would be of interest, both to confirm the role of distributed endocytosis in HBOC catabolism and as a possible route for enhancing therapeutic efficacy.
Immune system-mediated HBOC removal
Generation of neutralizing antibodies to therapeutic proteins may accelerate their clearance (CitationPorter 2001, CitationLin 2009). Human Hb A1 is only weakly antigenic, requiring co-administration with adjuvant to solicit a significant response (CitationChernoff 1953, CitationHeller et al. 1962, CitationRachmilewitz et al. 1963). It was found that crosslinked human Hb was not antigenic in rhesus monkeys or human patients after intravenous infusion (CitationEstep et al. 1992, CitationPatel et al. 1998). Hertzman and coworkers found that polymerization increased the antigenicity of heterologous Hbs (CitationHertzman et al. 1986), and a number of studies have further demonstrated that polymerized or conjugated heterologous Hbs stimulate antibody generation in several species, including humans (CitationCunnington et al. 1981, CitationMarks et al. 1987, CitationBleeker et al. 1995, CitationHamilton et al. 2001, CitationHamilton and Kickler 2007). However, the toxicologic consequences of such antibody generation appear to be modest, and no assessment has been reported on the effects of such antibodies on pharmacokinetics. Thus, any effect of antibody generation on the circulating half-life of HBOCs is currently unknown.
Summary
The circulatory persistence of HBOCs ranges from less than one hour to several days, depending on dose, species, and molecular weight. Half-lives of up to 20 h have been demonstrated in humans. Functional half-life is diminished relative to the overall circulatory persistence by as much as 40% due to oxidation of the HBOC heme. HBOCs are eliminated from the circulation and metabolized by several of the same pathways as unmodified Hb, but their relative importance varies depending on the physical and chemical characteristics of the given formulation (). There is also direct and indirect evidence that one or more additional pathways may be important, especially endocytosis by the endothelium. A complete accounting of the quantitative contribution of each of the possible pathways to HBOC removal from the circulation would be of interest to rigorously define the metabolism of these compounds.
Declaration of interest
Timothy Estep is a consultant for Omniox, Inc., an early stage biopharmaceutical company developing new medicines for hypoxic diseases. No financial, editorial, or writing support was received from Omniox for the production of this manuscript.
References
- Aird WC. 2007. Phenotypic heterogeneity of the endothelium I. Structure, function, and mechanisms. Circ Res. 100:158–173.
- Alayash AI, Summers AG, Wood F, Jia Y. 2001. Effects of glutaraldehyde polymerization on oxygen transport and redox properties of bovine hemoglobin. Arch Biochem Biophys. 391:225–234.
- Ames BN, Cathcart R, Schwiers E, Hochstein P. 1981. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc Natl Acad Sci USA. 78:6858–6862.
- Andersen JT, Pehrson R, Tolmachev V, Daba MB, Abrahmsén L, Ekblad C. 2011. Extending half-life by indirect targeting of the neonatal Fc receptor (FcRn) using a minimal albumin binding domain. J Biol Chem. 286:5234–5241.
- Andersen JT, Sandlie I. 2009. The versatile MHC class I-related FcRn protects IgG and albumin from degradation: implications for development of new diagnostics and therapeutics. Drug Metab Pharmacokinet. 24:318–332.
- Anderson PJ, Ning J, Biro GP. 1993. Clearance of differentially labelled infused hemoglobin and polymerized hemoglobin from dog plasma and accumulation in urine and selected tissues. In: Chang TMS, Ed. Blood substitutes and oxygen carriers. New York: Marcel Dekker, pp. 557–563.
- Baek JH, Zhou Y, Harris DR, Schaer DJ, Palmer AF, Buehler PW. 2012. Down selection of polymerized bovine hemoglobins for use as oxygen releasing therapeutics in a guinea pig model. Toxicol Sci. 127:567–581.
- Baker SL, Dodds EC. 1925. Obstruction of the renal tubules during the excretion of haemoglobin. Brit J Exp Path. 6:247–260.
- Bakker JC, Berbers GA, Bleeker WK, den Boer PJ, Biessels PT. 1993. Preparation and characterization of crosslinked and polymerized hemoglobin solutions. In: Chang TMS, Ed. Blood substitutes and oxygen carriers. New York: Marcel Dekker, pp. 67–75.
- Bakker JC, Bleeker WK, van der Plas J. 1986. Properties of hemoglobin interdimerically cross-linked with NFPLP. Prog Clin Biol. 211:49–55.
- Benesch RE, Ikeda S, Benesch R. 1976. Reaction of haptoglobin with hemoglobin covalently cross-linked between the αβ dimers. J Biol Chem. 251:465–470.
- Berbers GA, Bleeker WK, Stekkinger P, Agterberg J, Rigter G, Bakker JC. 1991. Biophysical characteristics of hemoglobin intramolecularly cross-linked and polymerized. J Lab Clin Med. 117:157–165.
- Bissell DM, Hammaker L, Schmid R. 1972. Hemoglobin and erythrocyte catabolism in rat liver: The separate roles of parenchymal and sinusoidal cells. Blood. 40:812–822.
- Bleeker WK, Berbers GAM, den Boer PJ, Agterberg J, Rigter G, Bakker JC. 1992. Effect of polymerization on clearance and degradation of free hemoglobin. Biomat Art Cells Immob Biotech. 20:747–750.
- Bleeker WK, van der Plas J, Agterberg J, Rigter G, Bakker JC. 1986. Prolonged vascular retention of a hemoglobin solution modified by cross-linking with 2-nor-2-formylpyridoxal 5’-phosphate. J Lab Clin Med. 108:448–455.
- Bleeker WK, van der Plas J, Feitsma RIJ, Agterberg J, Rigter G, De Vries-van Rossen A, et al. 1989. In vivo distribution and elimination of hemoglobin modified by intramolecular cross-linking with 2-nor-2-formylpyridoxal 5’-phosphate. J Lab Clin Med. 113:151–161.
- Bleeker WK, Zappeij LM, den Boer PJ, Agterberg JA, Rigter GM, Bakker JC. 1995. Evaluation of the immunogenicity of polymerized hemoglobin solutions in a rabbit model. Artif Cells Blood Substit Immobil Biotechnol. 23:461–468.
- Bonegio RG, Fuhro R, Ragno G, Valeri CR, Lieberthal W. 2006. A comparison of the acute hemodynamic and delayed effects of 50% exchange transfusion with two different cross-linked hemoglobin based oxygen carrying solutions and pentastarch. Artif Cells Blood Substit Immobil Biotechnol. 34:145–157.
- Bonhard K, Boysen U. 1982. Preparation of coupled hemoglobin molecules. US Patent. 4336248.
- Boretti FS, Baek JH, Palmer AF, Schaer DJ, Buehler PW. 2014. Modeling hemoglobin and hemoglobin:haptoglobin complex clearance in a non-rodent species-pharmacokinetic and therapeutic implications. Front Physiol. 5:385. Doi 10.3389/fphys.2014.00385. eCollection 2014.
- Borvak J, Richardson J, Medesan C, Antohe F, Radu C, Simionescu M, et al. 1998. Functional expression of the MHC class I-related receptor, FcRn, in endothelial cells of mice. Int Immunol. 10:1289–1298.
- Bouma JM. 1982. Some aspects of plasma protein metabolism as compared with intracellular protein breakdown. Acta Biol Med Ger. 41:53–60.
- Brooks J. 1932. The oxidation of haemoglobin to methaemoglobin by oxygen. Proc R Soc London B. 109:35–50.
- Brooks J. 1935. The oxidation of haemoglobin to methaemoglobin by oxygen II-the relation between the rate of oxidation and the partial pressure of oxygen. Proc R Soc London B. 118:560–577.
- Bucci E, Razynska A, Kwansa H, Matheson-Urbaitis B, O’Hearne M, Ulatowski JA, Koehler RC. 1996. Production and characteristics of an infusible oxygen-carrying fluid based on hemoglobin intramolecularly cross-linked with sebacic acid. J Lab Clin Med. 128:146–153.
- Buehler PW, D’Agnillo F, Hoffman V, Alayash AI. 2007. Effects of endogenous ascorbate on oxidation, oxygenation, and toxicokinetics of cell-free modified hemoglobin after exchange transfusion in rat and guinea pig. J Pharmacol Exp Ther. 323:49–60.
- Buehler PW, Vallelian F, Mikolajczyk MG, Schoedon G, Schweizer T, Alayash AI, Schaer DJ. 2008. Structural stabilization in tetrameric or polymeric hemoglobin determines its interaction with endogenous antioxidant pathways. Antioxid Redox Signal. 10:1449–1462.
- Buehler PW, Zhou Y, Cabrales P, Jia Y, Sun G, Harris DR, et al. 2010. Synthesis, biophysical properties and pharmacokinetics of ultrahigh molecular weight tense and relaxed state polymerized bovine hemoglobins. Biomaterials. 31:3723–3735.
- Bunn HF, Esham WT, Bull RW. 1969. The renal handling of hemoglobin I. Glomerular filtration. J Exp Med. 129:909–924.
- Bunn HF. 1967. Effect of sulfhydryl reagents on the binding of human hemoglobin to haptoglobin. J Lab Clin Med. 70:606–618.
- Bunn HF. 1972. Erythrocyte destruction and hemoglobin catabolism. Semin Hematol. 9:3–17.
- Burhop KE, Doyle MP. 2002. The development and preclinical testing of a second-generation recombinant hemoglobin solution, rHb2.0 for injection. In: Messmer K, Burhop KE, Hutter J, Eds. Microcirculatory effects of hemoglobin solutions. Basel: Karger, pp. 48–64.
- Bush S, Marshall T, Spicuzza J, Nelson D. 1994. Diaspirin crosslinked hemoglobin (DCLHb): Bioanalytical studies in swine. Art Cells Blood Subs Immob Biotech. 22:917–922.
- Carmichael FJL, Ali ACY, Campbell JA, Langlois SF, Biro GP, Willan AR, et al. 2000. A phase I study of oxidized raffinose cross-linked human hemoglobin. Crit Care Med. 28:2283–2292.
- Caron A, Mayer JC, Menu P, Alayash A, Marie PY, Vigneron C. 2001. Measurement of blood volume after haemodilution with haemoglobin-based oxygen carriers by a radiolabelled-albumin method. Transfus Med. 11:433–442.
- Caron A, Menu P, Faivre-Fiorina B, Labrude P, Alayash A, Vigneron C. 2000. Systemic and renal hemodynamics after moderate hemodilution with HBOCs in anesthetized rabbits. Am J Physiol Heart Circ Physiol. 278:H1974–1983.
- Cassoly R, Gibson Q. 1975. Conformation, co-operativity and ligand binding in human hemoglobin. J Mol Biol. 91:301–313.
- Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, Anderson CL. 2003. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med. 197:315–322.
- Chernoff AI. 1953. Immunologic studies of hemoglobins I. The production of antihemoglobin sera and their immunologic characteristics. Blood. 8:399–412.
- Chow ECY, Liu Lichuan, Ship N, Kluger RH, Pang KS. 2008. Role of haptoglobin on the uptake of native and β-chain [trimesoly-(lys82)β-(lys82)β] cross-linked human hemoglobins in isolated perfused rat livers. Drug Metabol Dis. 36:937–945.
- Comper WD, Russo LM. 2009. The glomerular filter: an imperfect barrier is required for perfect renal function. Curr Opin Nephrol Hypertens. 18:336–342.
- Conhaim RL, Cooler SC, McGrath AM, DeAngeles DA, Myers GA, Harms BA. 1998. Filtration of diaspirin crosslinked hemoglobin into lung and soft tissue lymph. Am J Respir Crit Care Med. 158: 1204–1212.
- Conover CD, Gilbert CW, Shum KL, Shorr RGL. 1997a. The impact of polyethylene glycol conjugation on bovine hemoglobin's circulatory half-life and renal effects in a rabbit top-loaded transfusion model. Artif Organs. 21:907–915.
- Conover CD, Linberg R, Gilbert CW, Shum KL, Shorr RG. 1997b. Effect of polyethylene glycol conjugated bovine hemoglobin in both top-load and exchange transfusion rat models. Artif Organs. 21: 1066–1075.
- Conover CD, Linberg R, Shum KL, Shorr RG. 1999. The ability of polyethylene glycol conjugated bovine hemoglobin (PEG-Hb) to adequately deliver oxygen in both exchange transfusion and top-loaded rat models. Art Cells Blood Subs Immob Biotech. 27: 93–107.
- Cunnington PG, Jenkins SN, Tam SC, Wong JTF. 1981. Oxygen-binding and immunological properties of complexes between dextran and animal haemoglobins. Biochem J. 193:261–266.
- Dall’Acqua WF, Kiener PA, Wu H. 2006. Properties of Human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J Biol Chem. 281:23514–23524.
- den Boer PJ, Bleeker WK, Rigter G, Agterberg J, Stekkinger P, Kannegieter LM, et al. 1992. Intravascular reduction of methemoglobin in plasma of the rat in vivo. Biomater Artif Cells Immobilization Biotechnol. 20:647–650.
- DeVenuto F, Zegna A. 1983. Preparation and evaluation of pyridoxylated-polymerized human hemoglobin. J Surg Res. 34:205–212.
- DeVenuto F. 1983. Modified hemoglobin solution as a resuscitation fluid. Vox Sang. 44:129–142.
- DeVenuto F. 1978. Stability of hemoglobin solution during extended storage. J Lab Clin Med. 92:946–954.
- Dittmer J, Ichikura T, Pivacek LE, Giorgio A, Prusty W, Valeri CR. 1992. Intravascular circulation and distribution of human 51Cr-DBBF stroma-free hemoglobin. Biomat Art Cells Immob Biotech. 20:751–755.
- Dorman SC, Kenny CF, Miller L, Hirsch RE, Harrington JP. 2002. Role of redox potential of hemoglobin-based oxygen carriers on methemoglobin reduction by plasma components. Artif Cells Blood Substit Immobil Biotechnol. 30:39–51.
- Doyle MP, Hoekstra JW. 1981. Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. J Inorg Biochem. 14:351–358.
- Dunne J, Caron A, Menu P, Alayash AI, Buehler PW, Wilson MT, et al. 2006. Ascorbate removes key precursors to oxidative damage by cell-free haemoglobin in vitro and in vivo. Biochem J. 399:513–524.
- Ehrenreich BA, Cohn ZA. 1968. Fate of hemoglobin pinocytosed by macrophages in vitro. J Cell Biol. 38:244–248.
- Elmer J, Palmer AF, Cabrales P. 2012. Oxygen delivery during extreme anemia with ultra-pure earthworm hemoglobin. Life Sci. 91:852–859.
- El-Sayed M, Kiani MF, Naimark MD, Hikal AH, Ghandehari H. 2001. Extravasation of poly(amidoamine) (PAMAM) dendrimers across microvascular network endothelium. Pharm Res. 18:23–28.
- Ericsson JL. 1965a. Transport and digestion of hemoglobin in the proximal tubule I. Light microscopy and cytochemistry of acid phosphatase. Lab Invest. 14:1–15.
- Ericsson JL. 1965b. Transport and digestion of hemoglobin in the proximal tubule II. Electron microscopy. Lab Invest. 14:16–39.
- Estep TN, Gonder J, Bornstein I, Aono F. 1992. Immunogenicity of diaspirin cross-linked human hemoglobin solutions. Biomater Artif Cells Immobilization Biotechnol. 20:603–609.
- Etzerodt A, Kjolby M, Nielsen MJ, Maniecki M, Svendsen P, Moestrup SK. 2013. Plasma clearance of hemoglobin and haptoglobin in mice and effect of CD163 gene targeting disruption. Antiox Redox Signal. 18:2254–2263.
- Faivre B, Menu P, Labrude P, Grandgeorge M, Vigneron C. 1994. Methemoglobin formation after administration of hemoglobin conjugated to carboxylate dextran in guinea pigs. Attempts to prevent the oxidation of hemoglobin. Artif Cells Blood Substit Immobil Biotechnol. 22:551–558.
- Faivre-Fiorina B, Caron A, Fassot C, Fries I, Menu P, Labrude P, Vigneron C. 1999. Presence of hemoglobin inside aortic endothelial cells after cell-free hemoglobin administration in guinea pigs. Am J Physiol. 276:H766–770.
- Faivre-Fiorina B, Caron A, Labrude P, Vigneron C. 1998. Les hémoglobines érythrocytaires, plasmatiques et substitutives face aux agents oxydants et réducteurs physiologiques. Ann Biol Clin. 56:545–556.
- Fischer SR, Burnet M, Traber DL, Prough DS, Kramer GC. 1999. Plasma volume expansion with solutions of hemoglobin, albumin, and Ringer lactate in sheep. Am J Physiol (Heart Circ Physiol). 276:H2194–2203.
- Fleck A. 1985. Computer models for metabolic studies on plasma proteins. Ann Clin Biochem. 22:33–49.
- Frei B, England L, Ames BN. 1989. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci. 86:6377–6381.
- Friedman HI, DeVenuto F, Lollini L, Mellick P, Zuck TF. 1978. Morphologic effects following massive exchange transfusions with a stroma-free hemoglobin solution I. Liver . Lab Invest. 39:167–177.
- Friedman HI, DeVenuto F. 1982. Morphological effects of transfusion with hemoglobin solutions. Crit Care Med. 10:288–293.
- Gandhi RR, Bell DR. 1992. Importance of charge on transvascular albumin transport in skin and skeletal muscle. Am J Physiol. 262: H999–1008.
- Gburek J, Osada J. 2000. Hemoglobin binding sites on renal brush-border membranes. Biochimie. 82:1135–1142.
- Gburek J, Verroust PJ, Willnow TE, Fyfe JC, Nowacki W, Jacobsen C, et al. 2002. Megalin and cubulin are endocytic receptors involved in renal clearance of hemoglobin. J Am Soc Nephrol. 13:423–430.
- Ghetie V, Hubbard JG, Kim JK, Tsen MF, Lee Y, Ward ES. 1996. Abnormally short serum half-lives of IgG in β2-microglobulin-deficient mice. Eur J Immunol. 26:690–696.
- Ghitescu L, Desjardins M, Bendayan M. 1992. Immunocytochemical study of glomerular permeability to anionic, neutral and cationic albumins. Kidney Int. 42:25–32.
- Gilbert C, Nho K, Johnson M, Linberg R, Shorr R. 1994. Hemoglobinuria in rats: a sensitive test of renal filtering and absorption of PEG-hemoglobin, a red blood cell substitute. Artif Cells Blood Substit Immobil Biotechnol. 22:535–541.
- Goldfischer S, Novikoff AB, Albala A, Biempica L. 1970. Hemoglobin uptake by rat hepatocytes and its breakdown within lysosomes. J Cell Biol. 44:513–529.
- Graversen JH, Madsen M, Moestrup SK. 2002. CD163: a signal receptor scavenging haptoglobin-hemoglobin complexes from plasma. Int J Biochem Cell Biol. 34:309–314.
- Greenburg AG, Maffuid PW. 1983. Modification of hemoglobin – ring opened dials. In: Bolin RB, Geyer RP, Nemo GJ, Eds. Advances in blood substitute research. New York: Alan R Liss. pp. 9–17.
- Greenburg AG, Schooley M, Peskin GW. 1977. Improved retention of stroma-free hemoglobin solution by chemical modification. J Trauma. 17:501–504.
- Greenburg AG. 1983. The effects of hemoglobin on reticulo-endothelial function. In: Bolin RB, Geyer RP, Nemo GJ, Eds. Advances in blood substitute research. New York: Alan R Liss. pp. 127–137.
- Gros G, Rollema HS, Jelkmann W, Gros H, Bauer C, Moll W. 1978. Net charge and oxygen affinity of human hemoglobin are independent of hemoglobin concentration. J Gen Physiol. 72:765–773.
- Guyton AC. 1976. Textbook of medical physiology. 5th ed. Philadelphia: WB Saunders.
- Hamilton RG, Kelly N, Gawryl MS, Rentko VT. 2001. Absence of immunopathology associated with repeated IV administration of bovine Hb-based oxygen carrier in dogs. Transfusion. 41:219–225.
- Hamilton RG, Kickler TS. 2007. Bovine hemoglobin (glutamer-250, Hemopure)-specific immunoglobulin G antibody cross-reacts with human hemoglobin but does not lyse red blood cells in vitro. Transfusion. 47:723–728.
- Haraldsson B, Nyström J, Deen WM. 2008. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 88: 451–487.
- Harrington JP, Gonzalez Y, Hirsch RE. 2000. Redox concerns in the use of acellular hemoglobin-based therapeutic oxygen carriers of plasma components. Artif Cells Blood Sustit Immobil Biotechnol. 28:477–492.
- Harvey JW, Kaneko JJ. 1976. Oxidation of human and animal haemoglobin with ascorbate, acetylphenylhydrazine, nitrite, and hydrogen peroxide. Br J Haematol. 32:193–203.
- Heller P, Yakulis VJ, Josephson AM. 1962. Immunologic studies of human hemoglobins. J Lab Clin Med. 59:401–411.
- Hershko C, Cook JD, Finch CA. 1972. Storage iron kinetics. II. The uptake of hemoglobin by hepatic parenchymal cells. J Lab Clin Med. 80:624–634.
- Hertzman CM, Keipert PE, Chang TM. 1986. Serum antibody titers in rats receiving repeated small subcutaneous injections of hemoglobin or polyhemoglobin: a preliminary report. Int J Artif Organs. 9:179–182.
- Hess JR, Fadare SO, Tolentino LSL, Bangal NR, Winslow RM. 1989. The intravascular persistence of crosslinked human hemoglobin. In: Brewer GJ, Ed. The red cell: seventh Ann Arbor conference. New York: Alan R. Liss. pp. 351–360.
- Hsia JC, Song DL, Er SS, Wong LTL, Keipert PE, Gomez CL, et al. 1993. Pharmacokinetic studies in the rat on a o-raffinose polymerized human hemoglobin. In: Chang TMS, Ed. Blood substitutes and oxygen carriers. New York: Marcel Dekker. pp. 383–391.
- Hughes GS, Antal EJ, Locker PK, Francom SF, Adams WJ, Jacobs EE. 1996. Physiology and pharmacokinetics of a novel hemoglobin-based oxygen carrier in humans. Crit Care Med. 24:756–764.
- Hughes GS, Francom SF, Antal EJ, Adams WJ, Locker PK, Yancey EP, Jacobs EE. 1995. Hematologic effects of a novel hemoglobin-based oxygen carrier in normal male and female subjects. J Lab Clin Med. 126:444–451.
- Israel EJ, Wilsker DF, Hayes KC, Schoenfeld D, Simister NE. 1996. Increased clearance of IgG in mice that lack β2-microglobulin: possible protective role of FcRn. Immunology. 89:573–578.
- Jarnum S. 1975. Turnover of plasma proteins. J Clin Pathol Suppl (Assoc Clin Pathol). 6:13–21.
- Jia Y, Wood F, Buehler PW, Alayash AI. 2013. Haptoglobin preferentially binds β but not α subunits cross-linked hemoglobin tetramers with minimal effects on ligand and redox reactions. PloS One. 8:e59841. Doi:10.1371/journal.pone.0059841.
- Junghans RP, Anderson CL. 1996. The protection receptor for IgG catabolism is the β2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci USA. 93:5512–5516.
- Keene WR, Jandl CH. 1965. The sites of hemoglobin catabolism. Blood. 26:705–719.
- Keipert PE, Adeniran AJ, Kwong S, Benesch RE. 1989a. Functional properties of a new crosslinked hemoglobin designed for use as a red cell substitute. Transfusion. 29:768–773.
- Keipert PE, Chang TM. 1987. Effects of partial and total isovolemic exchange transfusion in fully conscious rats using pyridoxylated polyhemoglobin solution as a colloid oxygen-delivering replacement fluid. Vox Sang. 53:7–14.
- Keipert PE, Chang TM. 1983. In vivo assessment of pyridoxylated crosslinked polyhemoglobin as an artificial red cell substitute in rats. Trans Am Soc Artif Intern Organs. 29:329–333.
- Keipert PE, Gomez CL, Gonzales A, MacDonald VW, Hess JR, Winslow RM. 1994. Diaspirin cross-linked hemoglobin: Tissue distribution and long-term excretion after exchange transfusion. J Lab Clin Med. 123:701–711.
- Keipert PE, Gomez CL, Gonzales A, MacDonald VW, Winslow RM. 1992. The role of the kidneys in the excretion of chemically modified hemoglobins. Biomater Artif Cells, Immob Biotech. 20:737–745.
- Keipert PE, Gonzales A, Gomez CL, Macdonald VW, Hess JR, Winslow RM. 1993. Acute changes in systemic blood pressure and urine output of conscious rats following exchange transfusion with diaspirin-crosslinked hemoglobin solution. Transfusion. 33:701–708.
- Keipert PE, Triner L. 1989. Catabolism and excretion of crosslinked hemoglobin. In: Brewer GJ, Ed. The red cell: seventh Ann Arbor conference. New York: Alan R Liss. pp. 383–405.
- Keipert PE, Verosky M, Triner L. 1989b. Plasma retention and metabolic fate of hemoglobin modified with an interdimeric covalent cross link. ASAIO Transactions. 35:153–159.
- Keipert PE. 1992. Properties of chemically cross-linked hemoglobin solutions designed as temporary oxygen carriers. Adv Exp Med Biol. 317:453–464.
- Kikugawa K, Sasahara T, Sasaki T, Kurechi T. 1981. Factors influencing the autoxidation of hemoglobin A. Chem Pharm Bull. 29:1382–1389.
- Kim HW, Clancy T, Chen F, Greenburg AG. 1993. Hepatic reticuloendothelial function following resuscitation with hemoglobin solutions. In: Chang TMS, Ed. Blood substitutes and oxygen carriers New York: Marcel Dekker Inc. pp. 564–566.
- Kim HW, Greenburg AG, Eds. 2013. Hemoglobin-based oxygen carriers as red cell substitutes and oxygen therapeutics. Heidelberg: Springer.
- Kino K, Mizumoto K, Watanabe J, Tsunoo H. 1987. Immunohistochemical studies on hemoglobin-haptoglobin and hemoglobin catabolism sites. J Histochem Cytochem. 35:381–386.
- Kino K, Tsunoo H, Higa Y. 1980. Takami M, Hamaguchi H, Nakajima H. Hemoglobin-haptoglobin receptor in rat liver plasma membrane . J Biol Chem. 255:9616–9620.
- Kramlova M, Pristoupil TI, Ulrych S, Hrkal Z. 1976. Stroma-free haemoglobin solution for infusion: changes during storage. Haematologia. 10:365–371.
- Kristiansen M, Graversen JH, Jacobsen JC, Sonne O, Hoffman HJ, Law SK, Moestrup SK. 2001. Identification of the haemoglobin scavenger receptor. Nature. 409:198–201.
- Kumari S, Swetha MG, Mayor S. 2010. Endocytosis unplugged: multiple ways to enter the cell. Cell Res. 20:256–275.
- LaMuraglia GM, O’Hara PJ, Baker WH, Naslund TC, Norris EJ, Li J, Vandermeersch E. 2000. The reduction of the allogenic transfusion requirement in aortic surgery with a hemoglobin-based solution. J Vasc Surg. 31:299–308.
- Lathem W, Davis BB, Zweig PH, Dew R. 1960. The demonstration and localization of renal tubular reabsorption of hemoglobin by stop flow analysis. J Clin Invest. 39:840–845.
- Laurell CB, Nyman M. 1957. Studies on the serum haptoglobin level in hemoglobinemia and its influence on renal excretion of hemoglobin. Blood. 12:493–506.
- Lee R, Neya K, Svizzero TA, Vlahakes GJ. 1995. Limitations of the efficacy of hemoglobin-based oxygen-carrying solutions. J Appl Physiol. 79:236–242.
- Lenz G, Junger H, Schneider M, Kothe N, Lissner R, Prince AM. 1991. Elimination of pyridoxated polyhemoglobin after partial exchange transfusion in chimpanzees. Biomat Art Cells Immob Biotech. 19:699–707.
- Lim SK, Kim H, Lim SK, bin Ali A, Lim YK, Wang Y, et al. 1998. Increased susceptibility in HP knockout mice during acute hemolysis. Blood. 92:1870–1877.
- Lin JH. 2009. Pharmacokinetics of biotech drugs: peptides, proteins and monoclonal antibodies. Curr Drug Metab. 10:661–691.
- Lockhart WL, Smith DB. 1975. Cross-linking of hemoglobin, haptoglobin, and hemoglobin-haptoglobin complex with bifunctional imidoesters. Can J Biochem. 53:861–867.
- Malik AB, Lynch JJ, Cooper JA. 1989. Endothelial barrier function. J Invest Dermatol. 93:62S–67S.
- Maniecki MB, Hasle H, Friis-Hansen L, Lausen B, Nielsen OJ, Bendix K, et al. 2008. Impaired CD163-mediated hemoglobin-scavenging and severe toxic symptoms in patients treated with gemtuzumab ozogamicin. Blood. 112:1510–1514.
- Mansouri A, Winterhalter KH. 1973. Nonequivalence of chains in hemoglobin oxidation. Biochemistry. 12:4946–4949.
- Marks DH, Brown DR, Ottinger WE, Atassi MZ. 1987. Antibody response to transfusion with pyridoxalated polymerized hemoglobin solution. Mil Med. 152:473–477.
- Matheson B, Razynska A, Kwansa H, Bucci E. 2000. Appearance of dissociable and cross-linked hemoglobins in the renal hilar lymph. J Lab Clin Med. 135:459–464.
- McGown EL, Lyons MF, Marini MA, Zegna A. 1990. Reduction of extracellular methemoglobin by erythrocytes. Biochim Biophys Acta. 1036:202–206.
- Menaka S, Wang Y, Hack BK, Chang A, Jensen M, Bao L, Quigg RJ. 2009. Renal FcRn reclaims albumin but facilitates elimination of IgG. J Am Soc Nephrol. 20:1941–1952.
- Mendiratta S, Qu ZC, May JM. 1998. Erythrocyte ascorbate recycling: antioxidant effects in blood. Free Radic Biol Med. 24:789–797.
- Menu P, Faivre B, Labrude P, Riffard P, Grandgeorge M, Vigneron C. 1994. Human hemoglobin conjugated to carboxylate dextran as a potential red blood cell substitute-II−pharmacotoxicological evaluation. Artif Cells Blood Substit Immobil Biotechnol. 22:543–549.
- Migita R, Gonzales A, Gonzales ML, Vandegriff KD, Winslow RM. 1997. Blood volume and cardiac index in rats after exchange transfusion with hemoglobin-based oxygen carriers. J Appl Physiol. 82:1995–2002.
- Miller F. 1960. Hemoglobin absorption by the cells of the proximal convoluted tubule in the mouse kidney. J Biophys Biochem Cytol. 8:689–718.
- Moestrup SK, Møller HJ. 2004. CD163: a regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann Med. 36:347–354.
- Mok W, Chen DE, Mazur A. 1975. Cross-linked hemoglobins as potential plasma protein extenders. Fed Proc. 34:1458–1460.
- Morell A, Terry WD, Waldman TA. 1970. Metabolic properties of IgG subclasses in man. J Clin Invest. 49:673–680.
- Mozzarelli A, Ronda L, Faggiano S, Bettati S, Bruno S. 2010. Haemoglobin-based oxygen carriers: research and reality towards an alternative to blood transfusion. Blood Transfus. 8 Suppl 3:s59–68.
- Murray RK, Connell GE, Pert JH. 1961. The role of haptoglobin in the clearance and distribution of extracorpuscular hemoglobin. Blood. 17:45–53.
- Nagel RL, Gibson QH. 1971. The binding of hemoglobin to haptoglobin and its relation to subunit dissociation of hemoglobin. J Biol Chem. 246:69–73.
- O’Hara JF, Colburn WA, Tetzlaff JE, Novick AC, Angermeier KW, Schubert A. 2001. Hemoglobin and methemoglobin concentrations after large-dose infusions of diaspirin cross-linked hemoglobin. Anesth Analg. 92:44–48.
- Olofsson C, Ahl T, Johansson T, Larsson S, Nellgård P, Ponzer S, et al. 2006. A multicenter clinical study of the safety and activity of maleimide-polyethylene glycol-modified hemoglobin (Hemospan) in patients undergoing major orthopedic surgery. Anesthesiology. 105:1153–1163.
- Panter SS, Vandegriff KD, Yan PO, Regan RF. 1994. Assessment of hemoglobin-dependent neurotoxicity: Alpha-alpha crosslinked hemoglobin. Artif Cells Blood Substit Immobil Biotechnol. 22:399–413.
- Patel MJ, Webb EJ, Shelbourn TE, Mattia-Goldberg C, George AJT, Zhang F, et al. 1998. Absence of immunogenicity of diaspirin cross-linked hemoglobin in humans. Blood. 91:710–716.
- Pearce LB, Rentko VT, Moon-Massat PF, Gawryl MS. 2003. Comparative pharmacokinetics of a hemoglobin-based oxygen carrier. Acad Emerg Med. 10:557–558.
- Peppard JV, Orlans E. 1980. The biological half-lives of four rat immunoglobulin isotypes. Immunology. 40:683–686.
- Pitts RF. 1968. Physiology of the kidney and body fluids. 2nd ed. Chicago: Year Book Medical Publishers.
- Polfliet MM, Fabriek BO, Daniëls WP, Dijkstra CD, van den Berg TK. 2006. The rat macrophage scavenger receptor CD163: expression, regulation and role in inflammatory mediator production. Immunobiology. 211:419–425.
- Porter S. 2001. Human immune response to recombinant human proteins. J Pharm Sci. 90:1–11.
- Przybelski RJ, Daily EK, Kisicki JC, Mattia-Goldbery C, Bounds MJ, Colburn WA. 1996. Phase I study of the safety and pharmacologic effects of diaspirin cross-linked hemoglobin solution. Crit Care Med. 24:1993–2000.
- Rachmilewitz EA, Izak G, Nelken D. 1963. Studies on hemoglobin I. Antigenic properties of human, canine and rabbit hemoglobin solutions . Blood. 22:566–579.
- Schaer DJ, Alayash AI, Buehler PW. 2007. Gating the radical hemoglobin to macrophages: the anti-inflammatory role of CD163, a scavenger receptor. Antiox Redox Signal. 9:991–999.
- Schaer DJ, Schaer CA, Buehler PW, Boykins RA, Schoedon G, Alayash AI, Schaffner A. 2006. CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood. 107:373–380.
- Schnitzer JE, Oh P, Pinney E, Allard J. 1994. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 127:1217–1232.
- Schubert A, O’Hara JF, Przybelski RJ, Tetzlaff JE, Marks KE, Mascha E, Novick AC. 2002. Effect of diaspirin crosslinked hemoglobin (DCLHb HemAssist™) during high blood loss surgery on selected indices of organ function. Artif Cells Blood Substit Immobil Biotechnol. 30:259–283.
- Sehgal LR, Gould SA, Rosen AL, Sehgal HL, Moss GS. 1984. Polymerized pyridoxylated hemoglobin: A red cell substitute with normal oxygen capacity. Surgery. 95:433–438.
- Sehgal LR, Sehgal HL, Rosen AL, Gould SA, Rice CL, Moss GS. 1981. Control of methemoglobin formation in stroma-free hemoglobin solutions. J Surg Res. 31:13–17.
- Shargel L, Wu-Pong S, Andrew Y. 2012. Applied biopharmaceutics & pharmacokinetics. 6th ed. New York: McGraw-Hill.
- Ship NJ, Toprak A, Lai RP, Tseng E, Klugar R, Pang KS. 2005. Binding of acellular, native and cross-linked human hemoglobins to haptoglobin: enhanced distribution and clearance in the rat. Am J Physiol Gastointest Liver Physiol. 288:1301–1309.
- Simionescu M, Gafencu A, Antohe F. 2002. Transcytosis of plasma macromolecules in endothelial cells: a cell biological survey. Microsc Res Tech. 1:269–288.
- Smith DC, Schuschereba ST, Hess JR, McKinney L, Bunch D, Bowman PD. 1990. Liver and kidney injury after administration of hemoglobin cross-linked with bis(3,5-dibromosalicyl) fumarate. Biomater Artif Cells Artif Org. 18:251–261.
- Snyder SR, Welty EV, Walder RY, Williams LA, Walder JA. 1987. HbXL99α: A hemoglobin derivative that is cross-linked between the α subunits is useful as a blood substitute. Proc Natl Acad Sci USA. 84:7280–7284.
- Sprung J, Kindscher JD, Wahr JA, Levy JH, Monk TG, Moritz MW, O’Hara PJ. 2002. The use of bovine hemoglobin glutamer-250 (Hemopure®) in surgical patients: results of a multicenter, randomized, single-blinded trial. Anesth Analg. 94:799–808.
- Standl T, Burmeister MA, Horn EP, Wilhelm S, Knoefel T, Schulte am Esch J. 1998. Bovine haemoglobin-based oxygen carrier for patients undergoing haemodilution before liver recession. Br J Anaesth. 80:189–194.
- Swan SK, Halstenson CE, Collins AJ, Colburn WA, Blue J, Przybelski RJ. 1995. Pharmacologic profile of diaspirin cross-linked hemoglobin in hemodialysis patients. Am J Kidney Dis. 26:918–923.
- Szabó G, Magyar Z. 1982. Extravascular circulation of plasma proteins. Acta Physiol Acad Sci Hung. 60:65–74.
- Taguchi K, Maruyama T, Otagiri M. 2011. Pharmacokinetic properties of hemoglobin vesicles as a substitute for red blood cells. Drug Metab Rev. 43:362–373.
- Takakura Y, Mahato RI, Hashida M. 1998. Extravasation of macromolecules. Adv Drug Deliv Rev. 34:93–108.
- Tam SC, Blumenstein J, Wong JTF. 1978. Blood replacement in dogs by dextran-hemoglobin. Can J Biochem. 56:981–984.
- Tenten V, Menzel S, Kunter U, Sicking EM, van Roeyen CR, Sanden SK, et al. 2013. Albumin is recycled from the primary urine by tubular transcytosis. J am Soc Nehprol. 24:1966–1980.
- Thomas M, Matheson-Urbaitis B, Kwansa H, Bucci E, Fronticelli C. 1997. Introduction of negative charges to a crosslinked hemoglobin: lack of effect on plasma half life. Artif Cells Blood Substit Immobil Biotechnol. 25:309–314.
- Tojo A, Kinugasa S. 2012. Mechanisms of glomerular albumin filtration and tubular reabsorption. Int J Nephrol. 2012:481520. Doi: 10.1155/2012/481520.
- Tomoda A, Matsukawa S, Takeshita M, Yoneyama Y. 1976. Effect of organic phosphates on methemoglobin reduction by ascorbic acid. J Biol Chem. 251:7494–7498.
- Urbaitis BK, Razynska A, Corteza Q, Fronticelli C, Bucci E. 1991. Intravascular retention and renal handling of purified natural and intramolecularly cross-linked hemoglobins. J Lab Clin Med. 117: 115–121.
- Vandegriff KD, Malavalli A, Minn C, Jiang E, Lohman J, Young MA, et al. 2006. Oxidation and haem loss kinetics of poly(ethylene glycol)-conjugated haemoglobin (MP4): dissociation between in vitro and in vivo oxidation rates. Biochem J. 399:463–471.
- Vandegriff KD, Malavalli A, Woodridge J, Lohman J, Winslow RM. 2003. MP4, a new nonvasoactive PEG-Hb conjugate. Transfusion. 43:509–516.
- Vandegriff KD, McCarthy M, Rohlfs RJ, Winslow RM. 1997. Colloid osmotic properties of modified hemoglobins: chemically cross-linked versus polyethylene glycol surface-conjugated. Biophys Chem. 69:23–30.
- Velky TS, Lee ES, Maffuid PW, Robinson GT, Yang JC, Greenburg AG. 1987. Peritoneal accumulation of infused stroma-free hemoglobin. Potential toxicity of an oxygen-carrying substitute. Arch Surg. 122:355–357.
- Venkatachalam MA, Rennke HG. 1978. The structural and molecular basis of glomerular filtration. Circ Res. 43:337–347.
- Viele MK, Weiskopf RB, Fisher D. 1997. Recombinant human hemoglobin does not affect renal function in humans: Analysis of safety and pharmacokinetics. Anesthesiology. 86:848–858.
- Wada T, Oara H, Watanabe K, Kinoshita H, Yachi A. 1970. Autoradiographic study on the site of uptake of the haptoglobin-hemoglobin complex. J Reticuloendothel Soc. 8:185–193.
- Weinstein MB, Segal HL. 1984. Uptake of free hemoglobin by rat liver parenchymal cells. Biochem Biophys Res Commun. 123:489–496.
- Wicks D, Wong LT, Sandhu R, Stewart RK, Biro GP. 2003. The intravascular persistence and methemoglobin formation of hemolink (hemoglobin raffimer) in dogs. Artif Cells Blood Substit Biotechnol. 31:1–17.
- Winslow RM. 1993. Potential clinical applications for blood substitutes. In: Chang TMS, Ed. Blood substitutes and oxygen carriers, New York: Marcel Dekker. pp. 44–56.
- Zhang L, Levy A, Rifkind JM. 1991. Autoxidation of hemoglobin enhanced by dissociation into dimers. J Biol Chem. 266: 24698–24701.
- Zuwała-Jagiełło J, Osada J. 1998. Internalization study using EDTA-prepared hepatocytes for receptor-mediated endocytosis of haemoglobin-haptoglobin complex. Int J Biochem Cell Biol. 30: 923–931.