Abstract
Quantum dots (QDs) have captured the fascination and attention of scientists due to their simultaneous targeting and imaging potential in drug delivery, in pharmaceutical and biomedical applications. In the present study, we have exhaustively reviewed various aspects of QDs, highlighting their pharmaceutical and biomedical applications, pharmacology, interactions, and toxicological manifestations. The eventual use of QDs is to dramatically improve clinical diagnostic tests for early detection of cancer. In recent years, QDs were introduced to cell biology as an alternative fluorescent probe.
Introduction
Currently, formulations developed by the pharmaceutical industry are in abundance, though their efficacy is limited because of decreased bioavailability due to low aqueous solubility and cell membrane permeability. Conventional formulations have some limitations like unacceptable release pattern of drugs, poor solubility, and toxicity (CitationUehara et al. 2009). Nanotechnology, a new system of drug delivery using various smart and intelligent nanocarriers having well-defined shapes and sizes, might resolve these limitations and help develop safe and effective nanomedicines (CitationDuncan and Vicent 2013). The solubility of the drug, its release at the site of disease, and reduced non-specific toxicity can be easily altered through formulations using nanotechnology. Quantum dots (QDs) are the semiconductors (group III–V and II–VI), pellucid nanoparticles having physical dimensions of 1–10 nm and are evident as fluorescence under a light source like laser. QDs discern themselves in offering many inherent photophysical properties that are enviable for the purposes imaging and targeted drug delivery. QDs are nanometer-sized radiant semiconductor crystals and have inimitable chemical and physical properties due to their size and highly squashed structure. This enable the synthesis of QDs for relevance in in vivo imaging including live-cell and whole-animal imaging, blood cancer assay, and cancer detection and treatment. QDs constitute the part of technological future having intriguing and useful properties. They have ability to emit light when any source of energy excites their electrons (CitationAbbasi et al. 2015, CitationProbst et al. 2013, CitationBera et al. 2010, CitationKhalid and Kontis 2008).
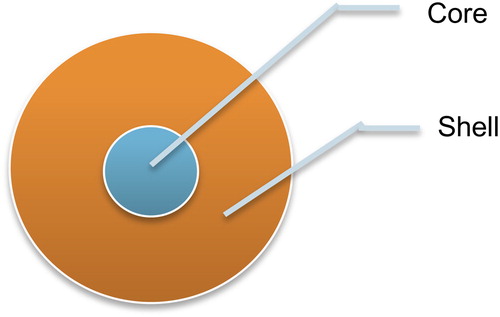
The term ‘Quantum’ implies a diminutive and discrete unit of any physical property (CitationKhalid and Kontis 2008). Energy is given to the electron of semiconductors to enliven them from ground state to excited state and they emanate radiations when they recede to ground state (CitationLeonard et al. 1993). The core-shell configuration () not only limits excitation and emission to the core, but also boosts the photoluminescence quantum yield (QY) of the core emission and shields the core from photobleaching (CitationMoriyama et al. 2005).
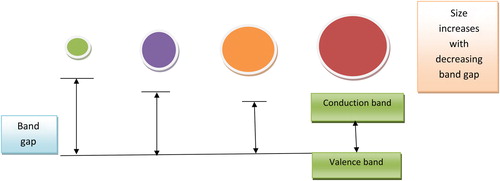
QDs can be designed to have emission peaks at diverse wavelengths by adjusting their size (CitationMisra 2008). The conductivity of these semiconductors lies between that of distinct molecules and bulk semiconductors (CitationMoriyama et al. 2005). The electron of these semiconductors is bound by the exciton Bohr radius, which is estimated by replacing the positively charged atomic core with the Bohr formula hole. The properties of semiconductors change by constraining the properties of the electron and the hole (CitationChakravarthy et al. 2011). On changing the size and shape of individual crystals, the conductive properties can be altered. The crystal size is inversely proportional to band gap (). The smaller the crystal size, the larger will be the band gap, and hence the greater will be difference in energy between the highest valence band and the lowest conduction band (CitationMaksym and Chakraborty 1990). Hence, the energy required to excite the dot will be more, and therefore, more energy is released when the crystal returns to resting state (CitationMinnich et al. 2009).
As crystal size grows smaller, there is a color shift from red to blue in the light emitted. QDs can be conjugated to biological molecules like proteins, targeting and imaging agents, oligonucleotides, and small molecules, which are used in the direct binding of QDs to areas of interest for biolabeling, biosensing, imaging, and targeting (CitationReed 1993, CitationAlivisatos 1996). Semiconductor QDs have attracted tremendous interest for biolabeling and bioimaging applications due to their considerable advantages over conventional organic dyes, such as high QY, size-tunable emission, photostability, and improved signal brightness. Recently, surface modification on QDs has led to the development of a new generation of probes with integrated functionalities of labeling and drug/gene delivery (CitationGuzelian et al. 1996). QDs are of great interest to fundamental studies but also have potential applications as biological probes, fluorescent biosensors, and biological imaging and labeling probes because of their unique optical properties, including broad absorption with narrow photoluminescence spectra, high QY, low photobleaching, and resistance to chemical degradation, in comparison with other classical fluorophores for light emitting diodes (LEDs) and solar cells (CitationReed 1993, CitationAlivisatos 1996).
Different aspects of QDs
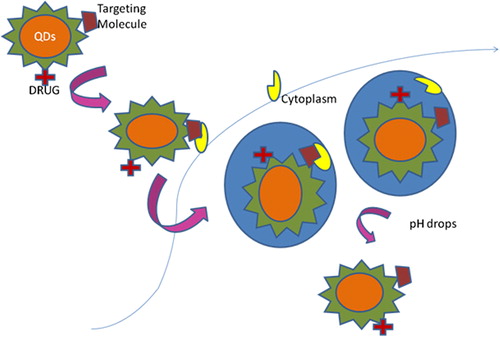
Current cancer therapy has some disadvantages like high toxicity, which have been overcome using the direct targeting property of QDs. QDs could selectively deliver drug to the target site, and by the imaging property, we could check whether the drug reached the target site or not () (CitationSavla et al. 2011).
Brief history of QDs
QDs were discovered in the 1980s by a Russian Physicist (CitationZhu et al. 2013). By applying a particle in sphere model, a relation between size and band gap was derived for semiconductor nanoparticles, approximate to wave function for bulk semiconductors. After that, enhanced excitonic optical non-linearity arising from state-filling of discrete levels due to quantum size effect was proposed (CitationTokihiro and Hanamura 1984). The third order optical non-linearity of GaAs QDs was studied (CitationOhno et al. 1996). In same scenario, collective excitation of QDs was reported (CitationNoglik and Pietro 1995). In 1990, efficient light emission from silicon was reported by Canham (CitationCanham 1990). In 2004, work on yield enhancement and photostability of QDs was done by Jaiswal (CitationJaiswal et al. 2004). In 2005, QD fluorescence quenching technique was used for optical DNA and oligonucleotide sensors (CitationJin et al. 2005). In 2007 the quenching dye was brought in close contact with the QDs (CitationKouwenhoven et al. 2001). In 2008, high photochemical stability of core/shell QDs was proposed as an alternative organic dye (CitationResch-Genger et al. 2008). In 2010, Biju et al. proposed functional group for bioreceptor immobilization and to anticipate toxicity issues (CitationBiju et al. 2010). After that, QDs have been used in drug delivery and targeting as well as for diagnostic and imaging purposes. Additionally, QDS are used for sensing of DNA and oligonucleotides (CitationMitchell et al. 1999).
Hierarchical development of quantum dot
The work on QDs originated in physics and then developed through technical and medical fields. Quantum transistors, oscillators, multispectral fluorescence imaging, development of filters, detectors, data analysis techniques, fluorescent dyes, and QD Light Emitting Devices (QLEDs) are some of the uses that have been achieved with QDs in physics. Technical optoelectronic devices like amplifiers and lasers use QDs (CitationCary 2009). The various unique and outstanding properties make QDs special and novel drug delivery materials in drug delivery and targeting () (CitationMinnich et al. 2009).
Characterization
The size, characterization, and structure of QD-doped samples have been determined by scanning transmission electron microscopy (STEM), X-ray fluorescence, and X-ray diffraction (CitationLipovskii et al. 1997). The optical characterization of QDs is done by UV–Visible and photoluminescence spectroscopy. The size of QDs is generally calculated using conventional techniques like scanning electron microscopy (SEM), transmission electron microscopy (TEM), and dynamic light scattering (DLS) (CitationYamashita et al. 2003). The size and composition of optically active QDs using photoluminescence, photoluminescence excitation, and Raman scattering spectroscopy are also reported (CitationGuzelian et al. 1996, CitationBanin et al. 1997). For monitoring the size of epitaxially prepared QDs, methods like TEM, atomic force microscopy (AFM), scanning tunneling microscopy, and magneto-tunneling experiments are also reported.
Other techniques for QD characterization include nuclear magnetic resonance spectroscopy (CitationRajh et al. 1993). The various characterization techniques have been summarized in .
Table I. Methods for characterization of quantum dots.
Physicochemical properties of QDs
The general physicochemical properties of QDs are summarized in and discussed below:
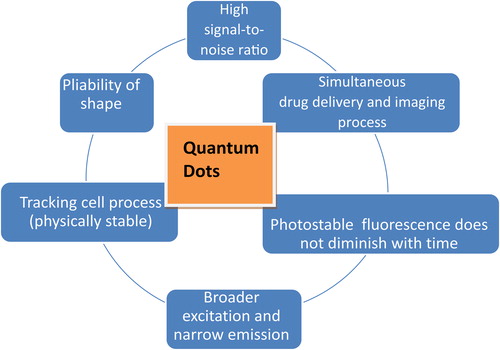
QDs are more resistant to degradation than other optical imaging probes and hence allow tracking of the cellular process for longer period of time (CitationDabbousi et al. 1997).
They have a longer-lasting photostability than traditional dyes, due to their inorganic composition and fluorescence intensity (CitationGhasemi et al. 2009).
QDs have high S/N ratio compared to organic dyes (CitationGhasemi et al. 2009).
QDs have broader excitation spectra and a narrow, sharply defined emission peak (CitationBera et al. 2010).
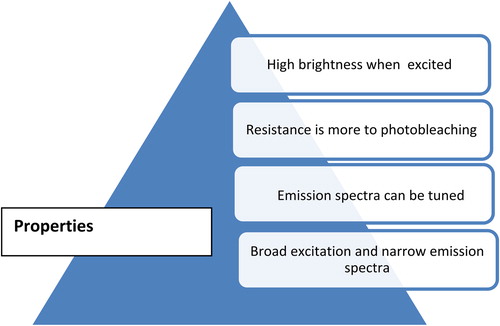
QDs are 10–20 times brighter than other organic dyes. QDs are stable fluorophores due to their inorganic composition, which reduces the effect of photobleaching when compared to organic dyes (CitationDubertret et al. 2002).
QDs have longer fluorescence and have significantly high photoresistance (CitationDubertret et al. 2002).
QDs have large Stokes shift and sharp emission spectra (CitationDubertret et al. 2002, CitationGhasemi et al. 2009).
QDs could be easily molded into any shape and coated with a variety of biomaterials (CitationKroutvar et al. 2004).
QDs are nanocrystals and provide better contrast with electron microscope as scattering increases (CitationHawrylak 1999).
QDs have novel optical and electronic properties due to quantum confinement of electrons and photons in the nanostructure. The phenomenon of QD confinement arises with the particle diameter being of the same magnitude as the wavelength of electron wave function (CitationHuang and Ren 2011). Quantum confinement results in a widening of band gap (gap between valence and conduction band), which increases when the size of the nanostructure is decreased. QDs of the same material with different sizes emit different colors () (CitationPhillips 2002).
Luminescence properties: QDs are semiconductor nanocrystals that possess unique optical properties including broad-range excitation, size-tunable narrow emission spectra, and high photo stability, giving them considerable value in various applications (CitationKroutvar et al. 2004). The size and composition of QDs can be varied to obtain the desired emission properties and make them amenable to simultaneous detection of multiple targets. These properties arise from interaction between electrons, holes, and the local environment. QDs absorb photons when excitation energy exceeds band gap, and after absorbing that energy, electrons jump from the ground state to the excited state (CitationMaksym and Chakraborty 1990). The energy associated with optical absorption is directly related with the electronic structure of material.
Excited electrons empty the space and leave a hole there. The electrons combine with the hole and relax to lower energy state and hence reach the ground state (CitationKulakovich et al. 2002). The excess energy results in recombination, and relaxation may be radiative (emit photon) or non-radiative (emit phonons) (CitationMaksym and Chakraborty 1990, CitationKroutvar et al. 2004). Radiative relaxation causes spontaneous luminescence from QDs.
Optical properties can be influenced by varying different aspects of the QDs, all of which can be controlled, including core size and core composition, shell composition, and surface coating (CitationPotasek et al. 2005). While all the aforementioned qualities influence QD emissions, the core size and composition have the most influence over the range of the emission spectra (CitationBailey and Nie 2003). Emission spectra for semiconductor nanoparticles are distinctive, containing narrow and symmetric peaks independent of the excitation energy, as long as the excitation energy is greater than that of the band gap energy (Grundman et al. 1999).
Pharmacology of QDs
The pharmacology, that is, absorption, distribution, metabolism, and excretion of QDs, is important in biomedical and pharmaceutical applications including drug delivery and targeting.
For QDs, the most important route of delivery at present appears to be systemic distribution through parenteral delivery, although occupational and environmental exposures via dermal and inhalation routes are also possible. QDs are absorbed at cellular level through receptor-mediated endocytic mechanism (CitationChithrani et al. 2006) The targeted QDs are incorporated in the cell by the endocytic pathway via mediated uptake mechanism, and QD targeting studies have shown that QDs with targeting functional groups can be accumulated in selected target tissues upon i.v. administration (CitationMichalet et al. 2005).
Regarding distribution, one of the first elements that parenterally delivered QD will encounter is the environment of the blood. Here, we have little to no information about blood–QD interactions. QDs are usually excreted in plasma proteins but interaction between QDs and plasma protein is still unknown, it is believed that the immune system can trigger this level (CitationMichalet et al. 2005).
The QD core does not appear to be involved in extensive enzymatic metabolism, but the shell and coating appear to degrade under photolytic and oxidative conditions and hence release toxic cadmium cores (CitationRzigalinski and Strobl 2009). Though QD shells and coatings appear to degrade under photolytic and oxidative conditions, we know little about the degradation products or their biological effects, which may regulate the release of toxic cores.
Excretion poses yet another pharmacological hurdle for QD research, as we have no comprehensive studies of QD removal via this route. Given the cadmium content of QDs, and the known renal toxicity of cadmium, the kidneys may be an important site for toxicological effects. Excretion will undoubtedly be regulated by size, nature of the coatings, and physico-chemistry. Several studies suggest that QDs of sizes less than approximately 5 nm can be easily removed by the kidneys (CitationRzigalinski and Strobl 2009).
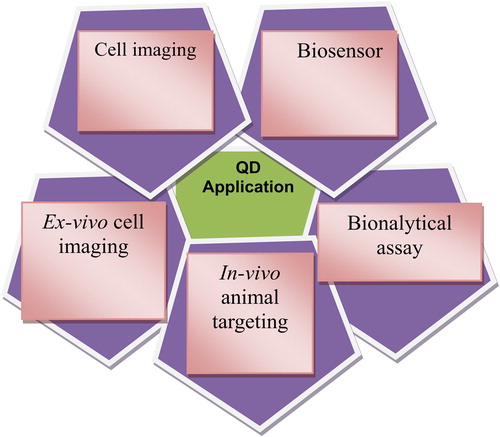
Applications of QDs
Currently, magnetic resonance imaging (MRI), optical, and nuclear imaging have been immense as key imaging techniques in biological systems (CitationCassidy and Radda 2005). They differ mostly in terms of sensitivity, resolution, complexity, acquisition time, and operational cost. However, these above mentioned techniques are complementary to each other. There are several reviews on the physical basis of these techniques, their instrumentation, and the issues that affect their performance (CitationZhou and El-Deiry 2009). Currently, a significant amount of research is aimed at using the unique optical properties of QDs in biological imaging. Much of optical bioimaging is based on traditional dyes (CitationSharma et al. 2006), but there are several drawbacks associated with their use. It is well known that cell autofluorescence in the visible spectrum leads to the following five effects: (i) the autofluorescence can mask signals from labeled organic dye molecules, (ii) instability of organic dye under photo-irradiation is well known in bioimaging which results in only short observation times, (iii) in general, conventional dye molecules have a narrow excitation window, which makes simultaneous excitation of multiple dyes difficult, (iv) dyes are sensitive to the environmental conditions, such as variation in pH, and (v) most of the organic dyes have a broad emission spectrum with a long tail at red wavelengths, which creates spectral cross talk between different detection channels and makes it difficult to quantitate the amounts of different probes (CitationSolanki et al. 2008). QDs, on the other hand, are of interest in biology for several reasons, including: (i) higher extinction coefficients, (ii) higher QY, (iii) less photobleaching, (iv) absorbance and emissions tunable with size, (v) generally broad excitation windows but narrow emission peaks, (vi) multiple QDs can be used in the same assay with minimal interference with each other, and (vii) toxicity may be less than that of conventional organic dyes (CitationJaiswal et al. 2004). QDs exhibit many applications in biomedical science. For example, they act as important agents for labeling cells, in tracking different particles, as imaging agents, in clinical application, in relation with neurosciences, as photodynamic therapy (PDT) agents, and for drug delivery. Some of the applications are discussed in . In addition to these, QDs also have other properties suitable for use in medical science (CitationBera et al. 2010, CitationOh et al. 2005).
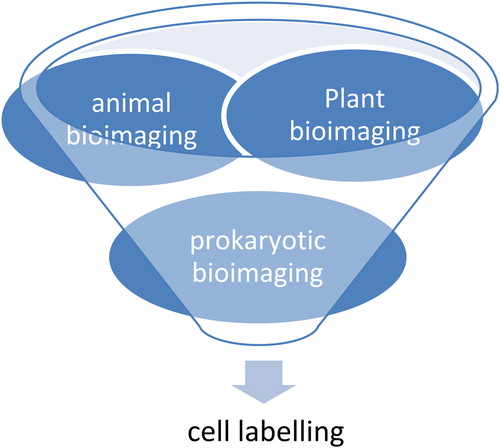
QDs for labeling cells
The optical properties of QDs, in particular the wavelength of their fluorescence, depend strongly on their size. Because of their reduced tendency to photobleach, colloidal QDs are interesting fluorescence probes for all types of labeling studies. As QDs have constant and unique optical properties, they are used in cell marking (CitationDerfus et al. 2004a, Citation2004b). QDs can concurrently tag multiple inter and intracellular components of live cells for time periods ranging from seconds to months. Different colors of QDs can label different cell components that can be easily visualized with fluorescent microscopy or in vivo (CitationPetta et al. 2005). For example, plant bioimaging: CdSe QDs bind typically to cellulose and lignin in the cell wall and hence give a fluorescent image of plant cells; animal bioimaging: biotinylated Cholera toxin B (CTxB) with QD–avidin conjugates for labeling of ganglion (CitationCheki et al. 2013); CHPNH2 QD nanogel has potential for use in long-term cell imaging; prokaryotic bioimaging: for measuring the bacterial cell, core magnetic beads that are anti E. coli 0157-coated and streptidine-coated are used. () (CitationAlgar et al. 2010).
Tracking different particles
Single particle tracking (SPT) techniques were developed to explore the dynamics of biomolecules in live cells at single-molecule sensitivity and nanometer spatial resolution. Recent developments in QD surface coating and bioconjugation schemes have made them most suitable probes for live-cell applications (CitationSapmaz et al. 2006). QDs require intracellular delivery through the impermeable plasma membrane receptor, and the superior stability of QD fluorescence enables the possibility of improving quantitation of FISH (fluorescence in situ hybridization) analysis of human chromosomal changes (CitationCheki et al. 2013).
Imaging system
Due to their unique optical features, QDs are used for diagnostic purposes and have potential application in neuroscience manifestation. Antibody-functionalized QDs follow lateral diffusion of glycine receptor in a culture of primary spinal cord neurons (CitationBallou et al. 2004). Biocompatible water-soluble QD micelles demonstrate uptake and intracellular dispersion in cultured neurons (CitationChan et al. 2002). QDs–ligand interaction is used in DNA defection (caused by various DNA defects), other biomolecular and protein detection, and cellular labeling (CitationWalling et al. 2009, CitationMisra 2008).
Biomedical research
In vitro: Biomolecular tracking in cells, cellular imaging, and tissue staining.
In vivo: QD biodistribution, vascular imaging, QD tracking, and tumor imaging (CitationSchnee et al. 2012).
QDs in relation with neuroscience: QDs are used to track and complete molecular occurrence using fluorescence microscopy (CitationCheki et al. 2013). They are used for neural and ganglionic interactions, for instance in the tiny size of the synaptic cleft.
PDT with QDs
PDT is a treatment modality that uses a photosensitizer, usually a porphyrin-type pigment that preferentially localizes in target tissue, followed by exposure to visible light (CitationShiohara et al. 2004).
In combination with QDs, this photosensitizer is capable of absorbing light of a suitable wavelength and utilizing energy to stimulate oxygen to its singlet condition, which induces apoptosis of cancer cells. For example: CdSe QDs having silicon phthalocyanine photosensitizer (PC4) (CitationMichalet et al. 2005).
Drug delivery system
The use of QDs has negligible side effects as they can target the delivery system and can easily distinguish ailing cells from healthy cells by metal affinity-driven self-assembly between artificial polypeptides and the semiconductor core shell QDs (). Nanoparticles of QDs has long blood circulation time, protection, large drug-loading capacity, controlled drug release profile, and integration of multiple targeting ligands on surface (CitationCheki et al. 2013). Further, the improvement can be gained through carbon nanotubes (CNTs) for intracellular delivery of antisense oligonucleotides tagged with QDs (CitationMichalet et al. 2005).
QDs in clinical application
QDs are used as biomarkers for cancer detection in cancer cells. This is for diagnosis, forecasting of disease stage, and clinical management. QDs are 20 times brighter and 100 times more stable than traditional fluorescent reporters (CitationZrenner et al. 2002). QDs are dramatically better than existing methods for delivering a gene-silencing tool, known as siRNA, into cells (CitationBhattacharyya et al. 2002).
Tissue engineering
Tissue engineering is the study of the growth of new connective tissues or organs, from cells and collagenous scaffolds to produce a fully functional organ for implantation back into the donor host. Around 100 nm, features are present in natural bone surface. If the implant surface is smooth, the body rejects it, and the production of fibrous tissue on the surface of the implant can reduce bone–implant contact, thus reducing the inflammation at the site. If nanosized QDs are created on the surface of hip/knee prosthesis, the chances of rejection can be reduced by enhancing the osteoblast production (CitationJamieson et al. 2007).
Cancer therapy
Photodynamic cancer therapy is therapy in which cancer cells are destroyed with the generation of atomic oxygen, which is cytotoxic. QDs are porous nanoparticles which generate atomic oxygen and are taken up by cancer cells, hence only cancer cells are destroyed when exposed to laser light. (CitationGao et al. 2004). Unfortunately, the remaining molecules migrate to the skin and the eyes and make the patient very sensitive to the daylight exposure. This effect can last for up to 6 weeks. To avoid these side effects, the hydrophobic version of the dye molecule was enclosed inside porous nanoparticles (CitationSolanki et al. 2008). Dabbousi et al. have reported that the dye gets trapped in ormosil (silicate) nanoparticles, thus lowering the chances of leak and spread to other body parts. Hence, the oxygen-generating ability is not affected, and the pore size of about 1 nm freely allows for the outward diffusion of oxygen (CitationDabbousi et al. 1997).
Multicolor optical coding
This can be achieved by combining QDs of different fluorescent colors with polymeric microbeads (CitationChen et al. 2008).
Protein detection
The use of gold nanoparticles (GNPs) with surface-enhanced Raman scattering spectroscopy, and combining both techniques with QD nanoparticles, makes it easy for detection of protein (CitationChithrani et al. 2006).
Novel sensor for allergens and antigens
QDs are widely used as labeling probes because of their unique properties like high aspect ratio, substantial optical and electrical signal amplification, and unique coding capabilities (CitationStier et al. 1999). The transparency under visible light, and the high environmental and electrical stability are properties that make QDs suitable for use in sensing (CitationModani et al. 2013). Recently, an immunoassay for the detection of carbohydrate antigen has been developed. QDs have been conjugated with the antibody, and this immunosensor has high selectivity and sensitivity in the detection of antigen. (CitationMaiti and Bhattacharya 2013).
Fluorescent sensing platform for DNA detection
QDs combined with multiple-photon Raman lines have an excitation at the 325 nm wavelength, which is used as the characteristic fingerprint region. Hence, this combination is promising material for fingerprint signal characterization (CitationZhang and Hu 2010).
As promising antimicrobial agents
Several metal oxides like TiO2, MgO, and ZnO have been reported to present significant antimicrobial activity, and they are much safer and more heat-resistant than conventional organic antimicrobial agents. The ZnO QDs are observed to be effective with Bacillus subtilis and Escherichia coli (CitationStintz et al. 2000).
As transgenic vectors
Recent and ongoing work on surface modification of QDs has led to a new generation of probes for targeted drug delivery. CdSe QDs–amphipol technology is used both for intracellular as well as real-time imaging of delivery of siRNA into cancer cells with reduced cytotoxicity (CitationYoffe 2001) ().
Table II. Quantum dots delivery systems.
Excellent as magnetic resonance and fluorescence imaging (MRI–FI) nanoprobes
The limitations associated with both the techniques, (magnetic resonance [MR] and fluorescence imaging [FI]), can be effectively overcome by integrating MR and optical imaging functionalities into a single structure. For example, Gd-doped QDs are used for this purpose (CitationGulia and Kakkar 2013).
Surface modification of QDs
QDs are not very soluble in water, and the instability is due to surface non-radioactive transition from the conduction band and excitation energy, thereby decreasing the probability of electronic transition for transfer from excited state back to valence state (CitationDuan and Nie 2007). The modification results in excitation fluorescence quenching with decreased fluorescence intensity, and provides stability (CitationDayal et al. 2012). Recently, surface modifications have been developed for transferring hydrophobic ZnO QDs in water while preserving luminescent properties. Highly luminescent, transparent, chemically pure, and crystalline QDs using LP–PLA, without any aid of surfactants, have been reported. Surface modification of QDs with mercaptoacetic acid (MAA) resulted in major impact on luminescence properties of ZnO QDs as it strongly absorbs the QDs through its mercapto functional group, thereby modifying and reducing surface defects significantly, and improving the excitons’ emission peak and luminescence property by preventing reunion (CitationZheng et al. 2004).
Polymer-capped QDs
The stabilization of photophysical properties of the core can be achieved by using polymer shells or copolymers. These nanoprobes so formed have been used in cell imaging and in vivo applications.
Siloxane and poly (amido amine)-capped QDs
Organosilanes are mostly used for surface modification and stabilize QD nanocrystals for inhibiting decomposition in aqueous media. These highly luminescent ZnO QDs are used for imaging of Gram (+ ve) bacteria. The biocompatibility of QDs can be improved, thereby inhibiting cell growth (CitationMontini et al. 2009).
Doped QDs
Doping with elements is an effective approach to modify electronic, optical, and magnetic properties. Recently, the luminescence properties of doped QDs with rare earth elements like Yb, Ce, etc., have been reported. These doped QDs possess relevant properties like small size, luminescence, good magnetism, etc. (CitationLoss and DiVincenzo 1998).
Toxicity of QDs
Many QDs are cytotoxic to some extent. The cytotoxicity of QDs is mainly dependent on size, capping material used, dose, surface chemistry, coating bioactivity, and QD exposure route. The residual organic molecules can also induce a toxic impact in target cells/tissues (CitationDerfus et al. 2004a, Citation2004b). For example: Wistar rats were nasally exposed to 0.52 mg cd/m3 for 5 days (6 h/day). On histological examination, the clinical factors in blood, bronchoalveolar lavage (BAL) fluid, and lung tissue were examined after 3 days of exposure, and Cd-based QDs were detected. The Cd-based QDs were able to cause local neutrophil inflammation in lungs with no CNS toxicity (CitationRzigalinski and Strobl 2009). A large accumulation of QDs was observed in spleen due to the protective impact of the ZnS shell impeding the release of Cd ions from the inner side. The genotoxic impact of QDs in vivo, and the long-term toxicity of CdSe–ZnS QDs with surface coating in Drosophila melanogaster were studied (CitationBazzi 2008).
The coating significantly affects the lifespan of the treated group, and the in vivo degradation of QDs with the consequent release of Cadmium ion is the main reason for toxic effects, as coated QDs displayed decreased overall toxicity. The surface oxidation of QDs can lead to formation of reduced Cd that can be released from QDs, causing cell death (depending on processing condition and dose of QDs) (CitationJamieson et al. 2007). The CdSe-core QDs induce apoptosis. Chan et al. (2004) studied the mitochondrial membrane potential and cytochrome release in mitochondria in human neuroblastoma cells. The group III–IV QDs have been reported to display less cytotoxicity and appear to offer greater plausibility for use as an optical probe in vivo (CitationShiohara et al. 2004).
QDs have some shortcomings, which include: (i) high reaction rate, (ii) poorly controlled growth rate, (iii) long reaction time, and (iv) difficulties in high-throughput synthesis.
Hence, the toxicity of QDs is dependent on the type of core material used. Some of the morphological endpoint toxicities include pericardial, ocular, and yolk sac edema, nano-depleted yolk, spinal curvature, and tail malformation (Chan et al. 2004). Selenite exposure was found to result in high mortality of embryos/larvae.
Conjugation of QDs
In this review, we seek to explore the biomedical applications of QDs conjugated to CNTs, with a particular emphasis on their use as therapeutic platforms in oncology. CNTs and QDs are the two nanoparticles that have received considerable interest in view of their application for diagnosis and treatment of cancer. QDs are gaining momentum as imaging molecules, with applications in life science and clinical methods (CitationMoriyama et al. 2005). Clinically, they can be used for localization of cancer cells due to their nano size and their ability to penetrate individual cancer cells, and high-resolution imaging derived from their narrow emission bands when compared to those derived using organic dyes (CitationChico et al. 1998). CNTs are of interest to the medical community due to their unique properties such as their ability to deliver drugs to a site of action or convert optical energy into thermal energy (CitationCobden and Nygård 2002). By attaching antibodies that bind specifically to tumor cells, CNTs can navigate to malignant tumors (CitationSapmaz et al. 2006). Once at the tumor site, the CNTs enter the cancer cells through penetration or endocytosis, allowing drug release, and resulting in specific cancer cell death. Alternatively, CNTs can be exposed to near-infrared light in order to thermally destroy the cancer cells. The amphiphilic nature of CNTs allows them to penetrate the cell membrane, and their large surface area (in the order of 2600 m2/g) allows drugs to be loaded into the tube and released once inside the cancer cell (CitationBachtold et al. 2000). Many research laboratories, including our own, are investigating the conjugation of QDs to CNTs to allow localization of the cancer cells in the patient, by imaging with QDs, and subsequent killing of the cells via drug release or thermal treatment. This is an area of huge interest, and future research and therapy will focus on the multimodality of nanoparticles.
Dendrimers and QDs
Dendrimers are a class of polymers with a highly ordered structure (CitationLemon and Crooks 2000). The conjugation of QDs with dendrimers was aimed at developing a novel type of molecular imaging probes composed of aptamers (Apts), quantum dots (QDs), and poly-amidoamine (PAMAM) dendrimers, for targeting to tumor Cells. QDs have been widely studied due to their unique optical properties, and have become a novel functional platform in bioanalytical science and molecular imaging (CitationLi et al. 2010). However, some reports have shown that QDs exhibit cellular toxicity (CitationCary 2009). The challenge lies in finding methods to decrease their toxicity and enhance biocompatibility. In recent years, molecular imaging of tumors has become a research hotspot. Most probes for molecular imaging conjugate a targeting molecule to a reporter moiety (CitationLi et al. 2010).
GNPs and QDs
Both GNPs and QDs have opto-electrical properties. Their interaction with surface plasmons can effect photoluminescent intensities of QDs. Photoionic interaction between QDs and GNPs in discrete structures is achieved by grouping CdSe–ZnS QDs with gold GNPs through DNA self- assembly (CitationKim et al. 2015, CitationSamanta et al. 2014).
Conclusion
In recent years, QDs have attracted tremendous attention as the most valuable and promising candidates in the areas of drug delivery, targeting, and imaging. Low toxicity, low cost, and good biocompatibility make them excellent candidates for in vivo bioimaging, gene/drug delivery, and cancer detection. This has created a powerful impact in various fields of disease diagnosis, intracellular tagging as photo sensitizers for treatment of cancer, biotechnology, and bioassays. Current advancement in the surface chemistry of QDs has expanded their use in biological applications, reduced their cytotoxicity, and rendered QDs a powerful device for the research of distinct cellular processes, like uptake, receptor trafficking, and intracellular delivery. Some of them (ZnO) have also promised significant breakthrough in the search for antibacterial agents, and the detection of antigens and allergens, due to their isoelectric point.
Future prospects
In future, QDs will be used to identify various categories of cancer with almost negligible side effects, identify the molecular mechanism of diseases, and the mechanism of action of new drugs. They can be used in intracellular and extracellular studies and for developing new methods for biochemical assay. Research into more luminescent hydrophilic QDs is ongoing since there is an urgent need for increasing QD efficiency and achieving better fluorescence. Research is also ongoing for a more selective and specific approach for cell and bimolecular labeling. More work is being carried out in studying the effect of interference of QDs with normal physiology. Research is going on into production of QDs with higher biosafety. The years ahead would see their potential applications in different fields such as molecular probes against various biological markers such as free antigens, cell surface markers/antigens, bacteria, viruses, and tissues. In our opinion, the multifunctional QDs will sparkle in targeted drug delivery and imaging, and due to these unique, extraordinary properties, additional QD conjugates will also be promising as nano medicines in biomedical applications.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abbasi E, Kafshdooz T, Bakhtiary M, Nikzamir N, Nikzamir N, Nikzamir M, et al. 2015. Biomedical and biological applications of quantum dots. Artificial Cells Nanomed Biotechnol. 23:1–7. doi:10.3109/21691401.2014.998826.
- Algar WR, Tavares AJ, Krull UJ. 2010. Beyond labels: A review of the application of quantum dots as integrated components of assays, bioprobes, and biosensors utilizing optical transduction. Anal Chim Acta. 673:1–25.
- Alivisatos AP. 1996. Semiconductor clusters, nanocrystals, and quantum dots. Science. 271:933–937.
- Bachtold A, Fuhrer MS, Plyasunov S, Forero M, Anderson EH, Zettl A, McEuen PL. 2000. Scanned probe microscopy of electronic transport in carbon nanotubes. Phys Rev Lett. 84:6082–6085.
- Bailey RE, Nie S. 2003. Alloyed semiconductor quantum dots: tuning the optical properties without changing the particle size. J Am Chem Soc. 125:7100–7106.
- Ballou B, Lagerholm BC, Ernst LA, Bruchez MP, Waggoner AS. 2004. Noninvasive imaging of quantum dots in mice. Bioconj Chem. 15:79–86.
- Banin U, Lee JC, Guzelian AA, Kadavanich AV, Alivisatos AP. 1997. Exchange interaction in InAs nanocrystal quantum dots. Superlattice Microst. 22:559–568.
- Bazzi R. 2008. Pharmacological iteractions between phenylbenzothiazoles and aryl hydrocarbon receptor (AhR). Thesis from University of Nottingham. (http://eprints.nottingham.ac.uk/10999/1/ranathesis.pdf).
- Bera D, Qian L, Tseng TK, Holloway PH. 2010. Quantum dots and their multimodal applications: A review. Materials. 3:2260–2345.
- Bhattacharya P, Ghosh S. 2002. Tunnel Injection In04 Ga0 0As/GaAs Quantum dots Lasers with 15GHz modulation bandwidth at room temperature. Appl Phys Lett. 80:3482–3484.
- Biju V, Mundayoor S, Omkumar RV, Aaas A, Ishikawa M. 2010. Bioconjugated quantum dots for cancer research: present status, prospects and remaining issues. Biotechnol Adv. 28:199–213.
- Canham L. 1990. Silicon quantum wire array fabrication by electrochemical and chemical dissolution of wafers. Apl Phys Lett. 57:1046–1048.
- Cary RB. 2009. Nanocrystal-Based Lateral Flow Microarrays and Low-Voltage Signal Detection Systems. Google Patents.
- Cassidy PJ, Radda GK. 2005. Molecular imaging perspectives. J Royal Soc Interface. 2:133–144.
- Chakravarthy KV, Davidson BA, Helinski JD, Ding H, Law WC, Yong KT, et al. 2011. Doxorubicin-conjugated quantum dots to target alveolar macrophages and inflammation. Nanomedicine. 7:88–96.
- Chan WC, Maxwell DJ, Gao X, Bailey RE, Han M, Nie S. 2002. Luminescent quantum dots for multiplexed biological detection and imaging. Curr Opin Biotech. 13:40–46.
- Cheki M, Moslehi M, Assadi M. 2013. Marvelous applications of quantum dots. Eur Rev Med Pharmacol Sci. 17:1141–1148.
- Chen K, Li ZB, Wang H, Cai W, Chen X. 2008. Dual-modality optical and positron emission tomography imaging of vascular endothelial growth factor receptor on tumor vasculature using quantum dots. Eur J Ncl Med Mol Imaging. 35:2235–2244.
- Chico L, Lopez Sancho MP, Munoz MC. 1998. Carbon-nanotube-based quantum dot. Phys Rev Lett. 81:1278–1281.
- Chithrani BD, Ghazani AA, Chan WC. 2006. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 6:662–668.
- Cobden DH, Nygard J. 2002. Shell filling in closed single-wall carbon nanotube quantum dots. Phys Rev Lett. 89:046803.
- Dabbousi BO, Rodriguez-Viejo J, Mikulec FV, Heine JR, Mattoussi H, Ober R, et al. 1997. (CdSe) ZnS core-shell quantum dots: synthesis and characterization of a size series of highly luminescent nanocrystallites. J Phys Chem B. 101:9463–9475.
- Dayal S, Kopidakis N, Rumbles G. 2012. Photoinduced electron transfer in composites of conjugated polymers and dendrimers with branched colloidal nanoparticles. Faraday Discuss. 155: 323–337.
- Derfus AM, Chan WC, Bhatia SN. 2004a. Intracellular delivery of quantum dots for live cell labeling and organelle tracking. Adv Mat. 16:961–966.
- Derfus AM, Chan WC, Bhatia SN. 2004b. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 4:11–18.
- Dixit SK, Goicochea NL, Daniel MC, Murali A, Bronstein IL, De M, et al. 2006. Quantum dots encapsulation in viral capsids. Nano Lett. 6:1993–1999.
- Duan H, Nie S. 2007. Cell-penetrating quantum dots based on multivalent and endosome-disrupting surface coatings. J Am Chem Soc. 129:3333–3338.
- Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. 2002. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 298:1759–1762
- Duncan R, Vicent MJ. 2013. Polymer therapeutics-prospects for 21st century: the end of the beginning. Adv Drug Deliv Rev. 65:60–70.
- Gao X, Cui Y, Levenson RM, Chung LWK, Nie S. 2004. In vivo cancer targeting and imaging with semiconductor quantum dots. Nature Biotech. 22:969–976.
- Ghasemi Y, Peymani P, Afifi S. 2009. Quantum dot: magic nanoparticle for imaging, detection and targeting. Acta Biomed. 80:156–165.
- Gulia S, Kakkar R. 2013. ZnO quantum dots for biomedical applications. Adv Mat Lett. 4:876–887.
- Guzelian AA, Banin U, Kadavanich AV, Peng X, Alivisatos AP. 1996. Colloidal chemical synthesis and characterization of InAs nanocrystal quantum dots. App Phys Lett. 69:1432–1434.
- Hawrylak P. 1999. Excitonic artificial atoms: engineering optical properties of quantum dots. Phys Rev B. 60:5597.
- Huang X, Ren J. 2011. Gold nanoparticles based chemiluminescent resonance energy transfer for immunoassay of alpha fetoprotein cancer marker. Anal Chim Acta. 686:115–120.
- Jaiswal JK, Goldman ER, Mattoussi H, Simon SM. 2004. Use of quantum dots for live cell imaging. Nature Methods. 1:73–78.
- Jamieson T, Bakhshi R, Petrova D, Pocock R, Imani M, Seifalian AM. 2007. Biological applications of quantum dots. Biomaterials. 28:4717–4732.
- Jia N, Lian Q, Tian Z, Duan X, Yin M, Jing L, et al. 2010. Decorating multi-walled carbon nanotubes with quantum dots for construction of multi-color fluorescent nanoprobes. Nanotechnology. 21:045606. doi:10.1088/0957–4484/21/4/045606.
- Jin WJ, Fernandezz-Arguelles MT, Costa-Fernandez JM, Pereriro R, Sanz-Medel A. 2005. Photoactivated luminescent CdSe quantum dots as sensitive cyanide probes in aqeous solutions. Chem Comm. 7:883–885.
- Khalid AH, Kontis K. 2008. Thermographic phosphors for high temperature measurements: principles, current state of the art and recent applications. Sensors. 8:5673–5744.
- Kim NY, Hong SH, Kang JW, Myoung N, Yim SY, Jung S, et al. 2015. Localized surface plasmon-enhanced green quantum dot light-emitting diodes using gold nanoparticles. RSC Adv. 5:19624–19629.
- Kouwenhoven LP, Austing D, Tarucha S. 2001. Few-electron quantum dots. Report Prog Phys. 64:701.
- Kroutvar M, Ducommun Y, Heiss D, Bichler M, Schuh D, Abstreiter G, Finley JJ. 2004. Optically programmable electrone spin memory using semiconductor quantum dots. Nature. 432:81–84.
- Kulakovich O, Strekal N, Yaroshevich A, Maskevich S, Gaponenko S, Nabiev I, et al. 2002. Enhanced luminescence of CdSe quantum dots on gold colloids. Nano Lett. 2:1449–1452.
- Lemon BI, Crooks RM. 2000. Preparation and characterization of dendrimer-encapsulated CdS semiconductor quantum dots. J Am Chem Soc. 122:12886–12887.
- Leonard D, Krishnamurthy M, Reaves CM, Denbaars SP, Petroff PM. 1993. Direct formation of quantum-sized dots from uniform coherent islands of InGaAs on GaAs surfaces. App Phys Lett. 63: 3203–3205.
- Li Z, Huang P, He R, Lin J, Yang S, Zhang X, et al. 2010. Aptamer-conjugated dendrimer-modified quantum dots for cancer cell targeting and imaging. Mat Lett. 64:375–378.
- Lipovskii A, Kolobkova E, Petrikov V, Kang I, Olkhovets A, Krauss T, et al. 1997. Synthesis and characterization of PbSe quantum dots in phosphate glass. App Phys Lett. 71:3406–3408.
- Loss D, DiVincenzo DP. 1998. Quantum computation with quantum dots. Phys Rev A. 57:120.
- Maiti A, Bhattacharyya S. 2013. Review: Quantum dots and application in medical science. Int J Chem Chem Eng. 3:37–42.
- Maksym P, Chakraborty T. 1990. Quantum dots in a magnetic field: Role of electron-electron interactions. Phys Rev Lett. 65: 108–111.
- Matan R, Haya LG. 2009. TAT-based drug delivery system-new direction in protein delivery for new hopes. Exp Opinion Drug Deliv. 6:453–463.
- Michalet X, Pinau FF, Bentolila LA, Tsay JM, Doose S, Li JJ, et al. 2005. Quantum dots for live cells, in vivo imaging and diagnostics. Science. 307:538–544.
- Minnich A, Dresselhaus M, Ren ZF, Chen G. 2009. Bulk nanostructured thermoelectric materials: current research and future prospects. Energy Environ Sci. 2:466–479.
- Misra RD. 2008. Quantum dots for tumor-targeted drug delivery and cell imaging. Nanomedicine (Lond). 3:271–274.
- Mitchell GP, Mirkin CA, Letsinger RL. 1999. Programmed assembly of DNA functionalized quantum dots. J Am Chem Soc. 121:8122–8123.
- Modani S, Kharwade M, Nijhawan M. 2013. Quantum dots: a novelty of medical field with multiple applications. Int J Curr Pharm Res. 5:55–59.
- Montini G, Zucchetta P, Tomasi L, Talenti E, Rigamonti W, Picco G, et al. 2009. Value of Imaging studies after first fabrile urinary tract infections in young children. Padiatrics. 123:e239–e246.
- Moriyama S, Fuse T, Suzuki M, Aoyagi Y, Ishibashi K. 2005. Four-electron shell structures and an interacting two-electron system in carbon-nanotube quantum dots. Phys Rev Lett. 94:186806.
- Noglik H, Pietro WJ. 1995. Surface functionalization of cadmium sulfide quantum confined semiconductor nanoclusters. 2. Formation of a “Quantum Dot” condensation polymer. Chem Mater. 7: 1333–1336.
- Oh E, Hong MY, Lee D, Nam SH, Yoon HC, Kim HS. 2005. Inhibition assay of biomolecules based on fluorescence resonance energy transfer (FRET) between quantum dots and gold nanoparticles. J Am Chem Soc. 127:3270–3271
- Ohno H, Shen A, Matsukura F, Oiwa A, Endo A, Katsumoto S. Iye Y. 1996. (Ga, Mn)As: a new diluted magnetic semiconductor based on GaAs. App phys Lett. 69:363–365.
- Petta J, Johnson AC, Taylor JM, Laird EA, Yacoby A, Lukin MD, et al. 2005. Coherent manipulation of coupled electron spins in semiconductor quantum dots. Science. 309:2180–2184
- Phillips J. 2002. Evaluation of the fundamental properties of quantum dot infrared detectors. J App Phys. 91:4590–4594.
- Potasek M, Gersten B, Zaitsev A. 2005. Organic-inorganic nanostructured composites with large optical nonlinearity for optical applications. Frontiers in Optics, Optical Society of America. 16–25.
- Probst CE, Zrazhevskiy P, Bagalkot V, Gao X. 2013. Quantum dots as platform for nanoparticle drug delivery vehicle design. Adv Drug Deliv Rev. 65:703–718.
- Rajh T, Micic OI, Nozik OJ. 1993. Synthesis and characterization of surface-modified colloidal cadmium telluride quantum dots. J Phys Chem. 97:11999–12003.
- Reed MA 1993. Quantum dots. Sci Am. 268:118–123.
- Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T. 2008. Quantum dots versus organic dyes as fluorescent labels. Nature Methods. 5:763–775.
- Rzigalinski BA, Strobl JS 2009. Cadmium-containing nanoparticles: perspectives on pharmacology and toxicology of quantum dots. Toxicol Appl Pharmacol. 238:280–288.
- Samanta A, Zhou Y, Zou S, Yan H, Liu Y. 2014. Fluorescent quenching of quantum dots by gold nanoparticles: a potential long range spectroscopic ruler. Nano Lett. 14:5052–5057.
- Sapmaz S, Jarillo-Herrero P, Kouwenhoven LP, S J vander Zant H. 2006. Quantum dots in carbon nanotubes. Semicond Sci Technol. 21: S52–S63.
- Savla R, Taratula O, Garbuzenko O, Minko T. 2011. Tumor targeted quantum dot-mucin 1 aptamer-doxorubicin conjugate for imaging and treatment of cancer. J Control Rel. 153:16–22.
- Schnee VP, Woodka MD, Pinkham D. 2012. Quantum dot material for the detection of explosive-related chemicals. SPIE Defense, Security, and Sensing, International Society for Optics and Photonics. XVII. 83571J. doi: 10.1117/12.921403.
- Sharma, P, Brown S, Walter G, Santra S, Moudgil B. 2006. Nanoparticles for bioimaging. Adv Colloid Interface Sci. 123–126:471–485.
- Shiohara A, Hoshino A, Hanaki K, Suzuki K, Yamamoto K. 2004. On the cyto-toxicity caused by quantum dots. Microbiol Immunol. 48:669–675.
- Solanki A, Kim JD, Lee KB. 2008. Nanotechnology for regenerative medicine: nanomaterials for stem cell imaging. Nanomedicine (Lond). 3:567–578.
- Stier O, Grundmann M, Bimberg D. 1999. Electronic and optical properties of strained quantum dots modeled by 8-band k⋅ p theory. Phys Rev B. 59:5688.
- Stintz A, Liu GT, Li H, Lester LF, Malloy KJ. 2000. Low-threshold current density 1.3-μm InAs quantum-dot lasers with the dots-in-a-well (DWELL) structure. Photon Technol Lett. 12:591–593.
- Tokihiro T, Hanamura E. 1984. Multi-polariton scattering via excitonic molecules. Solid State Commun. 52:771–774.
- Uehara T, Ishii D, Uemura T, Suzuki H, Kanei T, Takagi K, et al. 2009. gamma-Glutamyl PAMAM dendrimer as versatile precursor for dendrimer-based targeting devices. Bioconj Chem. 21:175–181
- Walling MA, Novak JA, Shepard JRE. 2009. Quantum dots for live cell and in vivo imaging. Int J Mol Sci. 10:441–491.
- Yamashita K, Nakate T, Okimoto K, Ohike A, Tokunaga Y, Ibuki R, et al. 2003. Establishment of new preparation method for solid dispersion form of tacrolimus. Int J Pharm. 267:79–91.
- Yoffe AD. 2001. Semiconductor quantum dots and related systems: electronic, optical, luminescence and related properties of low dimensional systems. Adv Phys. 50:1–208.
- Yuan Q, Hein S, Misra RDK. 2010. New generation of chitosan encapsulated ZnO quantum dots loaded with drug colon synthesis characterization and in vitro drug delivery response. Acta Biomaterialia. 6:2732–2739.
- Yum K, Na S, Xiang Y, Wang N, Yu M. 2009. Mechanochemical delivery and dynamic tracking of fluorescent QDs in cytoplasm and nucleus of living cells. Nano Lett. 9:2193–2198.
- Zhang CY, Hu J. 2010. Single quantum dot-based nanosensor for multiple DNA detection. Anal Chem. 82:1921–1927.
- Zheng J, Zhang C, Dickson RM. 2004. Highly fluorescent, water-soluble, size-tunable gold quantum dots. Phys Rev Lett. 93:077402.
- Zhou L, El-Deiry WS. 2009. Multispectral fluorescence imaging. J Nucl Med. 50:1563–1566.
- Zhu JJ, Li JJ, Huang HP, Cheng FF. 2013. Quantum dots for DNA biosensing. Springer. 165:341–346.
- Zrenner A, Beham E, Stuffer S, Findeis F, Bichler M, Abstreiter G. 2002. Coherent properties of a two-level system based on a quantum-dot photodiode. Nature. 418:612–614.