Abstract
Polymerized porcine hemoglobin (pPolyHb), which was synthesized from chemically modified porcine hemoglobin, can carry and deliver oxygen to tissues in addition to restoring intravascular volume. Assessment of the inflammatory response generated in the host is a part of the overall safety evaluation of pPolyHb. In this study, we measured the levels of IL-1β, IL-10, and CD11b/CD18 in response to pPolyHb stimulation, both in vivo (in rats) and in vitro. Our results suggest that the levels of these indicators are not statistically changed by pPolyHb, indicating that pPolyHb is not immunotoxic to cells and animals in this respect.
Introduction
Hemoglobin-based oxygen carriers (HBOCs), which are synthesized from chemically modified human or bovine hemoglobin, are currently the most promising artificial resuscitation fluids (CitationBian and Chang 2015, CitationElmer et al. 2012, CitationXiong et al. 2012). HBOCs can expand intravascular volume and dramatically increase the solubility of dissolved oxygen in blood, and furthermore, they do not need cross-matching and can be prepared virus- and bacteria-free (CitationNucci and Abuchowski 1998, CitationCohn 1997). Development of the first generation of HBOCs was fraught with setbacks and failures because of toxicity issues (CitationElmer et al. 2012). Several studies of HBOCs raised questions about their safety, which has contributed to the rational design of a second generation of HBOCs with improved adverse-effect profiles. Polymerized porcine hemoglobin (pPolyHb), which is synthesized from chemically modified porcine hemoglobin, is a newer HBOC that maintains hemodynamic stability well. In an exchange transfusion rat model in which 120–140% of the estimated total blood volume was replaced by pPolyHb, this HBOC did not cause hypertension (CitationZhu et al. 2011a). Furthermore, pPolyHb was shown to increase tissue oxygen, reverse anerobic metabolism, and effectively sustain the lives of treated rats (CitationZhu et al. 2011b).
As part of the preclinical development of any HBOC, a thorough evaluation of its safety profile, including its immunotoxicity, is required. The objective of a previous immune safety study with pPolyHb was to evaluate its effects on the host inflammatory response through assessment of the level of three inflammatory indicators—complement C3a, IL-6, and TNF-β via rat models of hemorrhagic shock (HS) (CitationZhu et al. 2011b), since the course of HS exhibits a possible challenge to the immune system. Both in vitro and in vivo studies demonstrated that pPolyHb did not cause a significant inflammatory response (CitationZhu et al. 2011a, CitationGiannoudis 2003, CitationMannick et al. 2001). In addition, pPolyHb was shown to alleviate the existing inflammatory responses caused by HS. In this study, we continue the examination of pPolyHb's immunotoxicity through assessment of the levels of IL-1β, IL-10, and CD11b/CD18 expression on the surface of neutrophils in response to pPolyHb, both in vitro and in vivo.
Materials
Reagents
6% Hetastarch 200/0.5 in sodium chloride solution (HES) (Fresenius Kabi), Pentobarbital Sodium (Sigma), Hepalean 1000 U.S.P. units/ml (Organon), Sodium Chloride (Sigma), Potassium Chloride (Sigma), Sodium Phosphate Monobasic (Sigma), Disodium Hydrogen Phosphate (Sigma), Phosphate Buffered Saline (PBS) (Sigma), Sodium Hydroxide (Sigma), Phenol red (Sigma), Bovine Serum Albumin (BSA) (Sigma), Fetal Bovine Serum (FBS) (Gibco), RPMI Medium 1640 (Gibco), Mouse IL-1β Elisa Kit, Mouse IL-10 Elisa Kit, Rat IL-1β Elisa Kit, Rat IL-10 Elisa Kit (DAKEWE), Lysing buffer (BD Pharm Lyse), FITC Mouse Anti-Rat CD18 and PE Mouse Anti-Rat CD11b (BD Pharmingen), Flow Cytometer (Beckman Coulter).
Animals
Female KM mice aged 6–8 weeks, and male Sprague Dawley rats (Xian Jiaotong University, China) weighing 240 ± 20 g were used in the study. The experiments described in this study were performed in adherence to the guidelines of the National Institutes of Health on the use of experimental animals. Approval of the Animal Care Committee of Northwest University was obtained prior to initiating the experiments.
Test solutions
pPolyHb (10.5 ± 0.5 g/dl polymerized porcine hemoglobin, methemoglobin < 5%, endotoxin < 1.0 EU/mL, osmolality 300–330 mOsm, pH 7.4 ± 0.05, average molecule of pPolyHb 600 ± 50 kD, 64 kD tetramer < 2%) was formulated in buffer consisting of Na+135–155 mmol/L, K+3.0–5.0 mmol/L, Ca2+1–3 mmol/L, Cl-140–160 mmol/L and stored at 4°C under nitrogen gas until use.
Methods
In vitro study
Neutrophil IL-1β/IL-10 measurement
Neutrophils were prepared as described by Haslett et al. (CitationHaslett et al. 1985). A volume of 5 ml of venous blood was collected in 15-ml polyethylene tubes, then Ficoll-Hypaque gradient centrifugation and erythrocyte lysis were used to prepare cells. Cells were treated with BSA (negative control), E.coli or Staphylococcus aureus (positive controls), or pPolyHb, for 12 h. Cell culture supernatants were pipetted, then centrifuged at 2800 g for 20 min. The supernatant fluids were transferred to a clean centrifuge tube and kept frozen at − 20°C until they were analyzed for cytokines by ELISA. IL-1β and IL-10 levels were detected using Rat IL-1β ELISA kits and Rat IL-10 ELISA kits (DAKEWE) according to manufacturer's protocol. Assays were performed in triplicate.
Neutrophil CD11b/CD18 measurement
Neutrophils were prepared as previously described. Cells were treated with BSA (negative control), E. coli (positive control), or pPolyHb, for 12 h. The cells were resuspended in PBS, and incubated with PE mouse anti-rat CD11b and FITC mouse anti-rat CD18 for 20 min at room temperature, then stored in 1% paraformaldehyde. The percentage of cells positive for CD11b/CD18 was determined by flow cytometry.
Macrophage IL-1β/IL-10 measurement
Mouse peritoneal macrophages were extracted directly from the abdominal cavity of mice. Mice were sacrificed and injected peritoneally with 5 ml of RMPI-1640 medium. Peritoneal fluid was aspirated 5 min later, then centrifuged at 1000 rpm for 5 min. Cells were cultivated for 2 h, then washed twice. Cells were treated with BSA (negative control), E. coli or Staphylococcus aureus (positive controls), or pPolyHb, for 12 h. Supernatants were isolated and IL-1β and IL-10 levels determined as described above for neutrophils.
In vivo study
IL-1β/IL-10 measurement and CD11b/CD18 neutrophil positive expression rate (%) assay in healthy rats
Twenty-one Sprague Dawley rats were randomized into seven groups: E.coli (positive control), pPolyHb (2 g/kg), pPolyHb (4 g/kg), saline (volume equal to that of pPolyHb for each concentration; negative control), Hetastarch (HES, volume equal to that of pPolyHb for each concentration; negative control). Before treatment, a blood sample was drawn from each rat. Serum was isolated and stored in small aliquots as pre-immune control serum. Equal volumes of pPolyHb (2 g/kg, 4 g/kg), saline, HES, or 2 × 1012 units E. coli were injected into rats intravenously. Serum was collected 12 and 24 h after injection and stored in small aliquots at − 80°C until cytokine analysis. IL-1β/IL-10 levels were detected using Rat IL-1β and Rat IL-10 Elisa Kit (DAKEWE) according to the manufacturer's protocol. Assays were performed in triplicate. In parallel, heparin-anti-coagulated blood was incubated with PE mouse anti-rat CD11b and FITC mouse anti-rat CD18 for 20 min at room temperature. The cells were washed twice in PBS to remove residual antibody and then resuspended in lysis buffer to remove erythrocytes, washed with PBS, and stored in 1% paraformaldehyde. The percentage of cells positive for CD11b/CD18 was detected by flow cytometry.
HS model and measurement of IL-1β/IL-10 and neutrophil CD11b/CD18 positive expression ratio (%)
Male Sprague Dawley rats were heparinized before HS through the venous catheter at 60 units heparin/100 g body weight. The test solution was warmed to the body temperature of 37°C. Rats (n = 12) were subjected to volume-controlled hemorrhage. Next, 55 ± 5% of estimated blood volume (EBV) was withdrawn via the right femoral artery in two steps at a rate of 0.5 ml/min. Rats were hemorrhaged over 90 min, after which they were resuscitated with pPolyHb or HES (volume equal to that of the lost blood), saline (volume triple that of the lost blood), or 2 g/kg pPolyHb plus saline (volume twice that of the lost blood) through the left jugular venous catheter at a rate of 0.5 ml/min. Mean arterial blood pressure (MAP), systolic blood pressure (SP), diastolic blood pressure (DP), heart rate (HR), and respiration rate were monitored every 5 min throughout the experiment. Blood samples were withdrawn before HS (base), at the end of shock, and at 1 h, 3 h, and 6 h after resuscitation. Plasma was collected by centrifuging at 5500 g for 5 min and stored in small aliquots at − 80°C until cytokine analysis. IL-1β and IL-10 levels were detected according to the manufacturer's protocol. Assays were performed in triplicate. In parallel, heparin-anti-coagulated blood was obtained and CD11b/CD18 expression determined as described above.
Statistical analysis
Data are presented as mean ± SD for replicate experiments. The differences between treatment groups were assessed by one-way ANOVA followed by unpaired Student's t-test. Statistical significance was defined as p < 0.05 to reject a null hypothesis. All statistical calculations were performed with JMP version 3.2 for the Macintosh (SAS Institute, Cary, NC).
Results
In vitro studies
Neutrophil IL-1β/IL-10 expression
Various observed toxicities have arisen during the preclinical and clinical development of the current generation of hemoglobin-based products. Pro-inflammatory activity is one such toxic property that may result in disseminated intravascular coagulation. Assessment of whether a new HBOC promotes release of inflammatory cytokines such as IL-1β and IL-10 is therefore a very important component of its safety evaluation. In our study, neutrophils were cultured and incubated with different amounts of pPolyHb or with Staphylococcus aureus, E.coli, and BSA for 12 h. IL-1β and IL-10 concentrations were then measured in the cell culture medium.
As shown in and , neutrophils released significantly higher amounts of IL-1β and IL-10 in the presence of Staphylococcus aureus and E.coli (2 × 108 units respectively) than the groups treated with BSA (1 μM) and BSA (10 μM), demonstrating that cells responded well to exogenous stimulation. The concentrations of IL-1β and IL-10 did not change significantly relative to BSA in the presence of either 1 μM or 10 μM of pPolyHb, indicating that pPolyHb did not induce IL-1β or IL-10 activation.
Figure 1. Concentrations of inflammatory mediators in neutrophils treated with different agents. Neutrophils were treated in vitro with different concentrations of BSA (negative control), Staphylococcus aureus or E. coli (positive controls), or pPolyHb, for 12 h. In and , IL-1β and IL-10 levels were detected using Rat IL-1β and Rat IL-10 Elisa Kits (DAKEWE) according to manufacturer's protocol. In , the percentage of CD11b/CD18-positive cells was determined by flow cytometric analysis of cells incubated with PE mouse anti-rat CD11b and FITC mouse anti-rat CD18 antibodies. Assays were performed in triplicate. (A) Comparison of IL-1β levels in neutrophils treated with different agents. **p < 0.01 compared with the E.coli group, ##p < 0.01 compared with the S.aureus group. (B) Comparison of IL-10 levels in neutrophils treated with different agents. *p < 0.05 compared with the E.coli group, ##p < 0.01 compared with the S.aureus group. (C) Percent of cells expressing CD11b/CD18 following treatment with different agents. **p < 0.01 compared with the E.coli group.
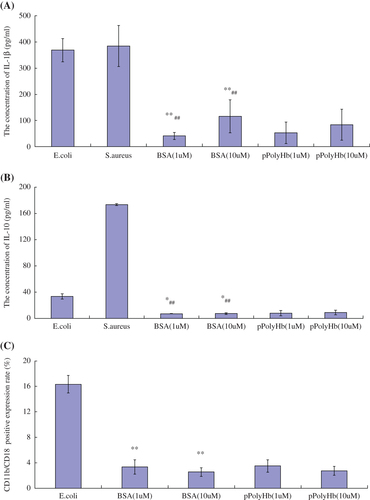
Neutrophil CD11b/CD18 expression
The β-2 integrin subunits CD11b and CD18 on the surface of the neutrophils and the intercellular adhesion molecule-1 (ICAM-1) on endothelial cells (ECs) are the main mediators of polymorphonuclear cell adhesion to ECs. In particular, the expression level of CD11b/CD18 is the main determinant of neutrophil adherence to ECs (CitationFabian et al. 1994, CitationZouki et al. 2001, CitationFernandez et al. 2001). The percentage of neutrophils expressing surface CD11b/CD18 in response to various treatments is shown in . The percentage of neutrophils expressing CD11b/CD18 following treatment with the positive control, E.coli, increased significantly compared with the BSA (1 μM) and BSA (10 μM) groups after 12 h of co-culture, indicating that the cells were responsive to stimulation. In contrast, the percentage of cells treated with either concentration of pPolyHb that exhibited CD11b/CD18 expression was similar to negative control cells (BSA), which implied that pPolyHb did not induce neutrophil activation in vitro.
Macrophage IL-1β/IL-10 expression
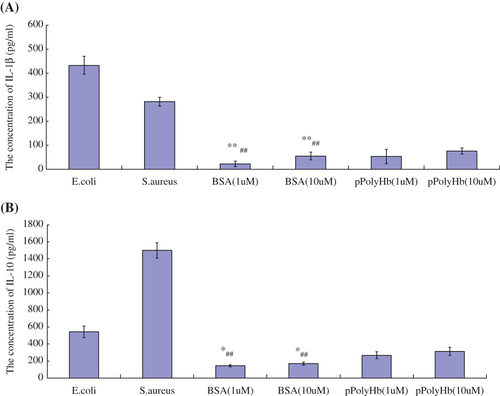
Macrophages were cultured and incubated with different amounts of pPolyHb or Staphylococcus aureus, E.coli, saline, or BSA for 12 h, and IL-1β and IL-10 concentrations were then measured from the cell culture medium. As shown in and , macrophages released significantly higher amounts of IL-1β and IL-10 in the presence of Staphylococcus aureus and E.coli (2 × 108 units of each) compared with the BSA (1 μM) and BSA (10 μM) groups, whereas the concentrations of IL-1β and IL-10 did not differ between negative controls (BSA) and pPolyHb. This result indicates that pPolyHb did not induce pro-inflammatory activation.
Figure 2. Concentrations of inflammatory mediators in macrophages treated with different agents. Macrophages were treated with different concentrations of BSA (negative control), Staphylococcus aureus or E. coli (positive controls), or pPolyHb, for 12 h. Assays were performed as described in , in triplicate. (A) Comparison of IL-1β levels in macrophages treated with different agents. **p < 0.01 compared with the E.coli group, ##p < 0.01 compared with the S.aureus group. (B) Comparison of IL-10 levels in macrophages treated with different agents. *p < 0.05 compared with the E.coli group, ##p < 0.01 compared with the S.aureus group.
In vivo study
IL-1β/IL-10 expression in healthy rats
Given that there was no immunotoxicity seen in an in vitro system, we continued our assessment in an animal model. For the study, 2 g/kg pPolyHb, 4 g/kg pPolyHb, or the same amount of HES or saline, were administrated intravenously to healthy rats. Next, 2 × 1012 units of E. coli was injected as the positive control. At 12 h and 24 h after administration, plasma was collected, and IL-1β and IL-10 levels were measured. As shown in and , the rats released significantly higher amounts of IL-1β and IL-10 in the presence of E. coli (2 × 1012 units/rat) compared with the negative controls (saline and HES groups) at 12 h, demonstrating that these cytokines were responsive to exogenous stimulation. In contrast, the concentrations of IL-1β and IL-10 remained low in the presence of pPolyHb, similar to rats treated with the negative controls, HES, and saline. These results suggest that pPolyHb administration does not activate an inflammatory response in healthy rats. The level of IL-1β and IL-10 returned to baseline at 24 h in all treatment conditions.
Figure 3. Changes in the levels of inflammatory mediators in healthy rats injected with different agents. Sprague Dawley rats (n = 21) were randomized into seven groups: E.coli (2 × 1012 units; positive control), pPolyHb (2 g/kg), pPolyHb (4 g/kg), saline (equal volume to pPolyHb), and Hetastarch (HES, equal volume to pPolyHb) (negative control). Serum or blood was collected before the treatment (baseline) and at 12 h and 24 h after intravenous administration. IL-1β and IL-10 levels ( and ), and the percent of cells positive for CD11b/CD18 (), were determined as described in the methods. Assays were performed in triplicate. (A) Levels of IL-1β in healthy rats treated with various agents. **p < 0.01 compared with the E.coli group at 12 h. (B) Levels of IL-10 in healthy rats treated with various agents. **p < 0.01 compared with the E.coli group at 12 h. (C) Percent of CD11b/CD18-positive cells in healthy rats treated with different agents. **p < 0.01 compared with the group of E.coli at 12 h, *p < 0.05 compared with the E.coli group at 24 h.
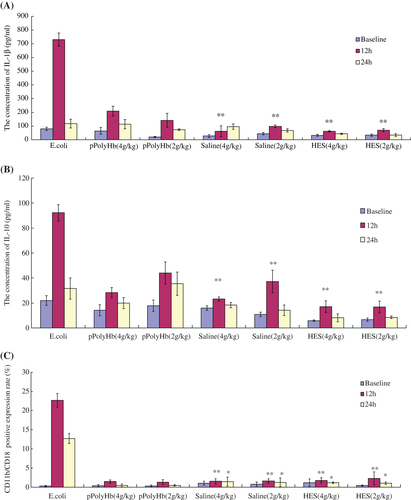
CD11b/CD18 expression in healthy rats
Expression of cell surface CD11b/CD18 on neutrophils from healthy rats is shown in . The rats treated with E. coli showed a significant increase in the percentage of neutrophils expressing CD11b/CD18 at 12 h after administration, and the percentage remained high at 24 h. In contrast, rats treated with pPolyHb exhibited no significant difference in CD11b/CD18 expression at either 12 h or 24 h in comparison to the HES and saline groups, demonstrating that pPolyHb did not induce neutrophil activation in vivo.
Assessment of IL-1β/IL-10 in rat model of HS
We then conducted similar experiments using a severe HS rat model. HS was induced by withdrawing 55 ± 5% of EBV via the right femoral artery over 90 min, and mean arterial pressure was maintained at 30–40 mmHg (CitationZhu et al. 2011a). Ninety minutes later, rats were resuscitated with pPolyHb or HES (volume equal to that of the lost blood), saline (volume triple that of the lost blood), or 2 g/kg pPolyHb plus two volumes of saline. Plasma was collected at the following time intervals: before HS (baseline), the end of shock, and at 1 h, 3 h, and 6 h after resuscitation. As shown in , IL-1β became elevated during HS and reached the highest levels at 1 h after resuscitation. Following the infusion of isometric pPolyHb, expression of this cytokine decreased significantly at 3 h versus 1 h after resuscitation. At 3 h, there was no difference between the four groups. For the group administered 2 g/kg pPolyHb plus saline, the expression of IL-1β significantly decreased at 6 h after resuscitation compared to the level at 1 h after resuscitation. In , the trend in the levels of IL-10 was similar to that of IL-1β. The concentration of IL-10 rose after HS and at 1 h compared to baseline, although there were no statistically significant differences between the pPolyHb groups and the groups with triple volume of saline. The concentration of both pPolyHb groups decreased significantly at 6 h after resuscitation relative to their levels at 1 h.
Figure 4. Changes in the levels of inflammatory mediators in rat HS model. Sprague Dawley rats (n = 12) underwent volume-controlled hemorrhage. They were resuscitated 90 min later, with pPolyHb or HES (volume equal to that of lost blood), saline (volume triple that of the lost blood), or 2 g/kg pPolyHb plus two volumes of saline. Blood samples were withdrawn before HS (baseline), at the end of shock, and at 1 h, 3 h, and 6 h after resuscitation. IL-1β, IL-10 levels ( and ) and the percent of cells positive for CD11b/CD18 () were detected as in . Assays were performed in triplicate. (A) Levels of IL-1β in rat HS model resuscitated with different fluids. *p < 0.05 compared with the isovolume pPolyHb group at 1 h after resuscitation, #p < 0.05 compared with the group of 2 g/kg pPolyHb + 2 volumes of saline at 1 h after resuscitation. (B) Levels of IL-10 in HS rats resuscitated with different fluids. *p < 0.05 compared with the isovolume pPolyHb group at 1 h after resuscitation, #p < 0.05 compared with the group of 2 g/kg pPolyHb + 2 volumes of saline at 1 h after resuscitation. (C) Percent of CD11b/CD18-positive cells in HS rats resuscitated with different fluids. *p < 0.05, **p < 0.01 compared with the isovolume pPolyHb group at the end of HS; #p < 0.05, ##p < 0.01compared with the group of 2 g/kg pPolyHb + 2 volumes of saline at the end of HS.
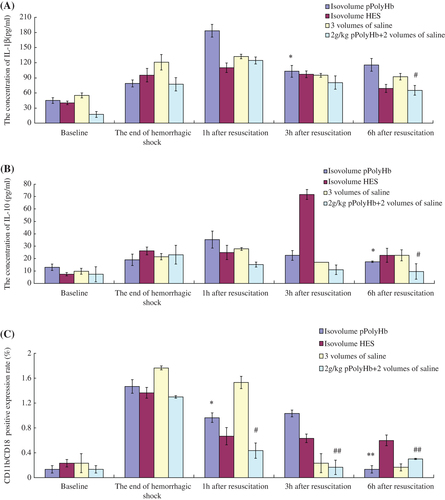
CD11b/CD18 expression in rat model of HS
Expression of cell surface CD11b/CD18 is shown in . The percentage of CD11b/CD18-positive cells rose during HS in the groups administered isometric pPolyHb and 2 g/kg pPolyHb + 2 volumes of saline (1.47% and 1.32% at the end of HS, compared to 0.13% and 0.129% at baseline). The percentage of CD11b/CD18-positive cells in both groups decreased significantly at 1 h after resuscitation, and was near to baseline for both groups at 6 h after resuscitation, indicating that administration of pPolyHb may alleviate the expression of CD11b/CD18 via neutrophil priming during HS.
Discussion
A large number of studies of HBOCs have raised questions about their safety. The similarity of the serious adverse event profiles among these products has raised questions regarding the possibility of common underlying mechanism(s) of toxicity (CitationSilverman and Weiskopf 2009).
Polymerized Porcine Hemoglobin (pPolyHb) is a newer HBOC that was developed as a potential red blood substitute for clinical applications. pPolyHb has shown good potential for tissue oxygenation and offered survival benefit in rat models (CitationZhu et al. 2011b). However, pPolyHb's effects on host immune responses are not well described. In this study, we assessed three indicators—CD11b/CD18, IL-1β, and IL-10— in cultured cells and animal models in response to pPolyHb in order to evaluate its immune safety.
Inflammation is the body's response to alien invaders and is designed to get rid of foreign bodies and allow healing of damaged tissue. Neutrophil activation is an important component of the inflammatory response, and plays an important role in early inflammation and development (CitationHill 1998, CitationTakahashi et al. 2001). White blood cells, macrophages, and mast cells that are involved in the inflammatory response work together in smooth muscle and blood vessels, causing inflammation (CitationNathan 2002). The β-2 integrin subunits CD11b and CD18 on the surface of neutrophils, and intercellular adhesion molecule-1 (ICAM-1) on ECs, are the main adhesion molecules that mediate neutrophil adhesion with ECs (CitationYan et al. 2004). Macrophages are also an important part of the immune system, and play an immunomodulatory role in maintaining the body's immune system at a steady state by secreting IL-1, IL-10, and other cytokines (CitationFujimoto and Yamamoto 1994). Cytokine IL-1 has broad immunomodulatory effects: by binding with its corresponding receptor, it is able to mediate inflammation (CitationLam et al. 2010), induce activation of ECs, stimulate neutrophils to release inflammatory proteins and mediators, and is directly involved in the inflammation process (CitationWest et al. 1994). IL-10, a pleiotropic cytokine, inhibits the secretion of a number of cytokines including IL-1 and TNF by effector cells such as NK cells (CitationZhai et al. 1997, CitationFlohe et al. 1999).
In this study, the results measuring the expression of inflammatory mediators in neutrophils in vitro demonstrated that the two positive bacterial strains promote the expression of IL-1β, IL-10 and CD11b/CD18. In contrast, the negative control and pPolyHb did not induce expression of these indicators. Likewise, in macrophages, the negative control and pPolyHb treatment groups had lower expression of IL-1β and IL-10 than the groups stimulated with the two positive control bacterial strains. Therefore, pPolyHb did not promote an inflammatory response in vitro. Moreover, in the healthy rat model, the two positive bacterial stains provoked significantly more expression of the inflammatory cytokines IL-1β and IL-10, and stimulated more expression of surface protein CD11b/CD18, than either the negative control or pPolyHb. Therefore, we conclude that pPolyHb does not induce an inflammatory response either in vitro or in the healthy rat model.
The controlled HS and resuscitation model was also used to evaluate the safety of pPolyHb administration. HS is characterized by hemodynamic instability with consecutive impairment of organ perfusion and cellular oxygenation (CitationPeitzman et al. 1995). It is possible to stimulate a systemic inflammatory response with the activation of immune cells and systemic liberation of pro-inflammatory and anti-inflammatory mediators (CitationProbst et al. 2009). Our results showed that inflammatory factors IL-1β and IL-10 rose to the highest level at 1 h after resuscitation. This may be because resuscitation fluid needs a period of time to exert its impact and the response time for the expression of different cytokines is varied. After infusion of different recovery solutions, especially after infusion of isovolume pPolyHb and 2 g/kg pPolyHb plus 2 volumes of saline, the expression of IL-1β and IL-10 attenuated to some extent at 3 h after resuscitation. Similarly, the expression of CD11b/CD18 rose at the end of HS, and in all the groups, decreased and returned to near baseline levels at 6 h after resuscitation. Therefore, the infusion of pPolyHb did not induce or aggravate the inflammatory response caused by HS.
Conclusion
We examined the inflammatory responses of pPolyHb in both cultured cells and animal models. The results demonstrated that pPolyHb did not cause a significant inflammatory response. In addition, it may not be able to exacerbate the existing inflammatory response caused by HS. These results provide support for future investigation of pPolyHb in clinical trials.
Acknowledgement
We acknowledge with thanks the grants from National High-tech R & D Program (863 Program) (grant nos. 2012AA021902), National Natural Science Foundation of China (grant nos. 81102367), China Scholarship Council of the Ministry of Education, as well as grants from Shaanxi Science and Technology Department (grant nos. 2011KTCL03-23, 2011 K12-03-10).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bian YZ, Chang TMS. 2015. A novel nanobiotherapeutic poly-[hemoglobin-superoxide dismutase-catalase-carbonic anhydrase] with no cardiac toxicity for the resuscitation of a rat model with 90 minutes of sustained severe hemorrhagic shock with loss of 2/3 blood volume. Artif cells Nanomed Biotechnol. 43: 1–9.
- Cohn SM. 1997. Is blood obsolete? Trauma. 42:730–732.
- Elmer J, Alam HB, Wilcox SR. 2012. Hemoglobin-based oxygen carriers for hemorrhagic shock. Resuscitation. 83:285–292.
- Fabian TC, Croce MA, Stewart RM, Dockter ME, Proctor KG. 1994. Neutrophil CD18 expression and blockade after traumatic shock and endotoxin challenge. Ann Surg. 220:552–563.
- Fernandez PC, Zouki C, Whittal R, Chan JS, Davidge ST, Filep JG. 2001. Matrix metalloproteinases regulate neutrophil-endothelial cell adhesion through generation of endothelin-1 [1–32]. FASEB J. 15:2230–2240.
- Flohe S, Dominguez Fernandez E, Ackermann M, Hirsch T, Börgermann J, Schade FU. 1999. Endotoxin tolerance in rats: expression of TNF-α, IL-6, IL-10, VCAM-1 and HSP70 in lung and liver during endotoxin shock. Cytokine. 11:796–804.
- Fujimoto J, Yamamoto T. 1994. A mouse gene encoding a novel receptor-type protein-tyrosine kinase, is preferentially expressed in the brain. Oncogene. 9:693–698.
- Giannoudis PV. 2003. Current concepts of the inflammatory response. Injury. 34:397–404.
- Haslett C, Guthrie LA, Kopaniak MM, Johnston RB, Henson PM. 1985. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 119:101.
- Hill GE. 1998. Cardiopulmonary bypass-induced inflammation: is it important?. J Cardiothorac Vasc Anesth. 12:21.
- Lam PD, Mandal PK, Hak SY, Hwang S-G. 2010. Study of the molecular mechanism of anti-inflammatory activity of bee venom in lipopolysaccharide stimulated RAW 264.7 macrophages. Trop J Pharm Res. 9.
- Mannick JA, Rodrick ML, Lederer JA. 2001. The immunologic response to injury. Am Coll Surg. 193:237–244.
- Nucci ML, Abuchowski A. 1998. The search for blood substitutes. Scientific America. 2:72–77.
- Nathan C. 2002. Points of control in inflammation. Nature. 420: 846–852.
- Peitzman AB, Harbrecht BG, Udekwu AO, Billiar TR, Kelly E, Simmons RL. 1995. Hemorrhagic shock. Curr Probl Surg. 32:925–1002.
- Probst C, Zelle BA, Sittaro NA, Lohse R, Krettek C, Pape HC. 2009. Late death after multiple severe trauma: when does it occur and what are the causes?. J Trauma Acute Care Surg. 66:1212–1217.
- Silverman TA, Weiskopf RB. 2009. Hemoglobin‐based oxygen carriers: current status and future directions. Transfusion. 49:2495–2515.
- Takahashi T, Hato F, Yamane T, Fukumasu H, Suzuki K, Ogita S, et al. 2001. Activation of human neutrophil by cytokine-activated endothelial cells. Circ Res. 88:422–429.
- West MA, Li MH, Seatter SC, Bubrick MP. 1994. Pre-exposure to hypoxia or septic stimuli differentially regulates endotoxin release of tumor necrosis factor, interleukin-6, interleukin-1, prostaglandin E2, nitric oxide, and superoxide by macrophages. J Trauma. 37:82–89.
- Xiong Y, Steffen A, Andreas K, Müller S, Sternberg N, Georgieva R, Bäumler H. 2012. Hemoglobin-based oxygen carrier microparticles: synthesis, properties, and in vitro and in vivo investigations. Biomacromolecules. 13:3292–3300.
- Yan SR, Sapru K, lssekutz AC. 2004. The CD11b/CD18 integrins modulate neutrophil caspase activation and survival following TNF—alpha or endotoxin induced tram endothelial migration. Immunol Cell Biol. 82:435–446.
- Zhai QH, Futrell N, Chen FJ. 1997. Gene expression of IL-10 in relationship to TNF-α, IL-1β and IL-2 in the rat brain following middle cerebral artery occlusion. J Neurol Sci. 152:119–124.
- Zhu HL, Yan KP, Dang XJ, Dai P, Luo C, Ma J, et al. 2011a. Pharmacodynamic study of polymerized porcine hemoglobin (pPolyHb) in a rat model of exchange transfusion. Artif Cells Blood Substit Immobil Biotechnol. 39:119–126.
- Zhu HL, Yan KP, Dang XJ, Huang H, Chen E, Chen B, et al. 2011b. Immune safety evaluation of polymerized porcine hemoglobin (pPolyHb): a potential red blood cell substitute. Artif Cells Blood Substit Immobil Biotechnol. 39:398–405.
- Zouki C, Haas B, Chan JSD, Potempa LA, Filep JG. 2001. Loss of pentameric symmetry of C-reactive protein is associated with promotion of neutrophil-endothelial cell adhesion. J Immunol. 167:5355–5361.

