Abstract
Objective To investigated the antioxidant protection effects of vitamin C (Vc) on the hemoglobin-based oxygen carriers (HBOCs) solution. Methods Three influential variables were studied including the molar ratio of Vc and hemoglobin, pH and temperature. The HBOCs molecules were characterized through spectral analysis, circular dichroism spectra, the oxygen binding curves and the molecular weights. Results The optimized conditions were molar ratios of above 3, pH of 7.5–8.0 and temperature of 4 °C. HBOCs characterization showed no differences before and after Vc addition. Conclusion Antioxidant effects in HBOCs solution could be presented by Vc, indicating the potential application of Vc in HBOCs development.
Introduction
During the past decades, blood transfusion has played an important role in the treatment for the clinical emergency, accidental trauma and so on (Allen et al. Citation2014, Vermeijden et al. Citation2015). Due to a series of disadvantages of blood transfusion such as supply shortages, risk of infectious diseases, virus infections and limited transportation, the blood substitutes have been always considered to be an effective technical approach to overcome the shortages of the blood transfusion (Chen et al. Citation2009). Up to now, several kinds of blood substitutes have been developed, among which the hemoglobin-based oxygen carriers (HBOCs) have attracted the most attention because of its special oxygen affinity similar with natural red blood cells, favorable biocompatibility and physiological metabolic pathways (Greenburg Citation2009). As a class of chemical or genetic derivatives of natural hemoglobin, HBOCs have been proved to be effective in the treatment of hemorrhagic shock (Ortiz et al. Citation2014), isolated organ preservation (Fontes et al. Citation2014), acute myocardial infarction and many other hypoxic diseases (Rempf et al. Citation2014).
So far, the main obstacles in the development of HBOCs were considered that its side effects showed in the clinical trials (Jahr et al. Citation2007). It was reported that vasoconstriction, nephrotoxicity, myocardical infarction were the main reflections of toxic side effects of HBOCs (Buehler et al. Citation2010, Silverman and Weiskopf Citation2009). Oxidation reactions of HBOCs were considered as one of the main reasons to its side effects. After extracted from the red blood cells, the oxidation process of HBOCs was accelerated due to the absence of the protective reductase systems with superoxide dismutase (SOD), catalase and methemoglobin (MetHb) reductase inclusion, leading to the formation of free radicals and MetHb, which has been listed in the necessarily detected qualities of HBOCs by Food and Drug Administration.
To restrain the oxidant of HBOCs, reduced agents and reductase with antioxidant properties were usually used for MetHb prohibition and free radicals scavenging. As an easy-get and cheap natural product, vitamin C (Vc) is a well-known reductant and has been well established in a series of biological systems including the blood systems in vivo (Karamian et al. Citation2015). In this study, with the human cord blood hemoglobin used as samples, the protective effects of Vc on HBOCs solution were investigated in detail. After optimization of the antioxidant conditions include mole ratios, pH values and temperatures, the effects of Vc on the construction and functions of HBOCs were also researched for the purposes of further application of Vc in the development of HBOCs.
Experiment section
Materials
The human cord blood was kindly supplied by Sichuan New-Life Stem Cell Biotech Co. Ltd (Chengdu, China). The HBOCs solution was prepared as described previously (Li et al. Citation2012). Vc was purchased from Kelong Chemical Company (Chengdu, China). Other reagents were purchased in A.R. grade and without further pretreatment before used.
Instruments
UV/visible spectrophotometer (Ultrspec 6300™, Amersham Bioscience, Sweden); HEMOX™-ANALYZER (TCS Scientific Corp.); circular dichroism (CD) analyzer (J-815, JASCO, Japan); higher performance liquid chromatography (HPLC) with 2489 UV-detector (e2695, Waters); experimental pH meter (FE20, Mettler-Toledo Instruments, China); electronic balance (BSA124S, Sartorius Science Technology Instruments, China); blood cell analyzer (BC-3200, Mindray, Shanghai, China); electric thermostatic water bath (XMTD-6000, Shanghai Yuejin Company, Shanghai, China); RO/BIO Water System (Cascada™, PALL).
Methods
Effects of mole ratio between Vc and hemoglobin on antioxidant protection
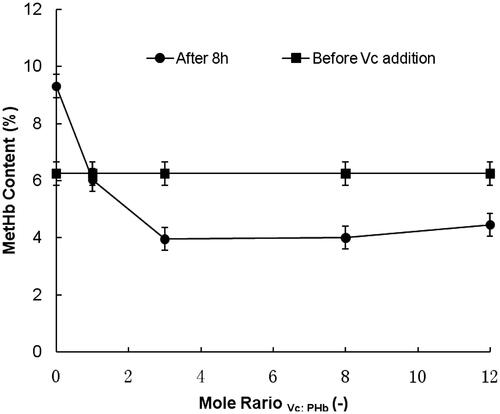
A series of HBOCs solution (with 6 g/dL Hb concentration and initial MetHb content of 6.25%) containing different Vc contents were prepared in 0.2 mol/L phosphate buffer saline (pH 7.5 PBS). By changing the Vc addition amounts, the mole ratios in HBOCs solutions between Vc and hemoglobin were adjusted to be 0, 1, 3, 8 and 12, respectively. The antioxidant reaction was started with the Vc addition at the temperature of 4 °C. After duration of 8 h, the MetHb contents in HBOCs solutions were tested and the results were illustrated by the profiles of mole ratios (Vc:HBOCs) versus MetHb contents.
Effects of pH values on antioxidant protection in HBOCs solution
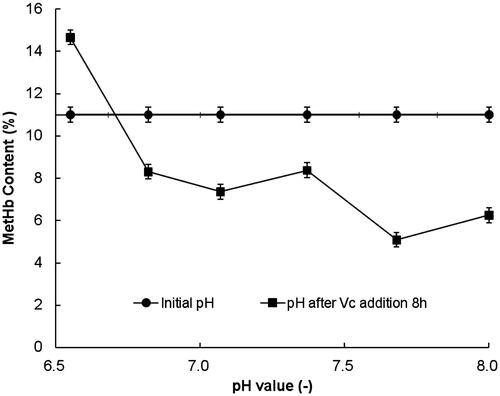
According to the methods mentioned in the section “Effects of mole ratio between Vc and hemoglobin on antioxidant protection”“, a series of HBOCs solution (with 6 g/dL Hb concentration and mole ratios of 8 between Vc and HBOCs) were prepared in 0.2 mol/L PBS solution. The pH values of these prepared HBOCs solutions were adjusted to be 6.5 ± 0.05, 6.8 ± 0.05, 7.1 ± 0.05, 7.4 ± 0.05, 7.7 ± 0.05 and 8.0 ± 0.05, respectively. After the Vc being added into the HBOCs solution, the antioxidant reactions were processed for about 8 h when the reactions were nearly completely. The MetHb contents after reactions in HBOCs solutions were detected and the results were shown in terms of the profiles of pH values versus MetHb contents.
Effects of temperatures on antioxidant protection in HBOCs solution
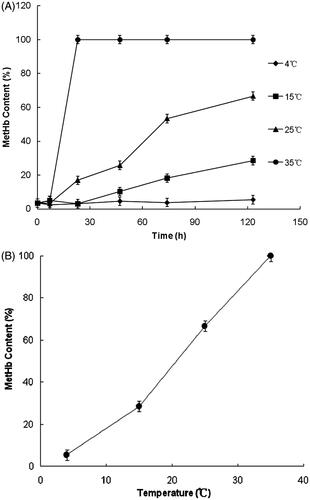
The effects of temperatures on Vc antioxidant reactions in HBOCs solutions were investigated at the temperatures of 4, 15, 25 and 35 °C, respectively. The HBOCs sample solutions were prepared according to the method mentioned in the section “Effects of mole ratio between Vc and hemoglobin on antioxidant protection”. The property factors of the sample HBOCs solutions were of pH 7.5 ± 0.05, Hb concentration 6.0 g/dL and the mole ratio of 8 between Vc and Hb. The antioxidant reactions were trigged by Vc addition. During the reaction process, the MetHb contents of HBOCs solution were termly monitored until 123 h after the reactions started, which could enhance the differences in results at different temperatures.
MetHb content measurement
In this study, the oxidation degrees of HBOCs sample solution were illustrated by the MetHb content, which was measured by the Evelyn–Malloy method (Evelyin and Malloy Citation1938).
UV spectrum of HBOCs solution before and after Vc addition
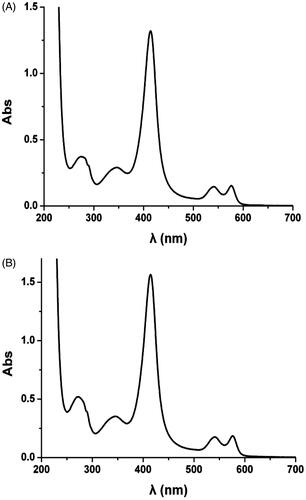
To investigate the effect of Vc reaction on the structure of Hb, the UV spectrums of HBOCs solutions were detected before and after Vc addition. The HBOCs sample solutions were prepared according to the methods mentioned in the section “Effects of temperatures on antioxidant protection in HBOCs solution”. Before Vc addition, 60 μL HBOCs sample solutions was added into 0.2 mol/L PBS solution with pH 7.5, and the prepared diluted HBOCs solution was UV scanned in the wavelength of 200–700 nm by UV/Visible Spectrophotometer (Ultraspec 6300™, Amersham Bioscience, Sweden). After the Vc addition, the antioxidant reactions were processed for 72 h to ensure the reaction completion and the reacted solution was UV scanned again with the same operation of solution before Vc addition.
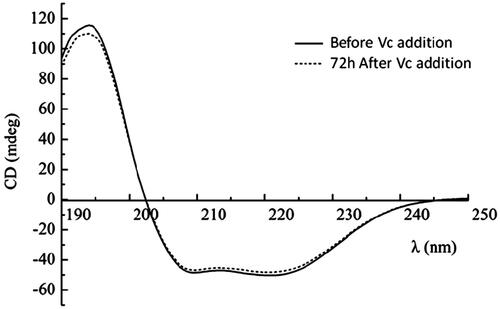
CD analysis of HBOCs before and after Vc addition
The changes of secondary structure of HBOCs were monitored through the CD analysis by Circular Dichroism Analyzer (J-815, JASCO, Japan). Thirty microliters of HBOCs sample solution was added into 3 mL 0.05 mol/L PBS solutions with pH 7.5 in the cuvette for the CD analysis and the CD spectra (190–250 nm) were then obtained. The HBOCs solution before and after Vc addition were both CD measured.
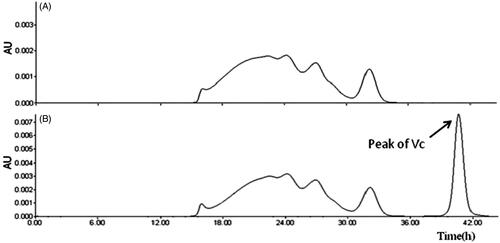
Molecular weight of HBOCs detected by size exclusion chromatography
The molecular weight of HBOCs was measured through the size exclusion chromatography (SEC) analysis by waters HPLC (e2695) with 2489 UV-detector. The SEC analysis was carried out on a column of Sephadex 200. The column was equilibrated and eluted with 0.15 mol/L PB solution with pH value of 7.5 at a flow rate of 1 mL/min at the temperature of 25 °C. The results of the SEC measurement were analyzed by the software of Empower.
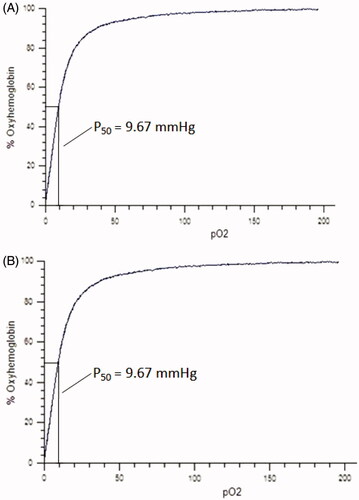
Testing of oxygen binding affinity
The oxygen binding affinity of the HBOCs sample solutions was tested through oxygen equilibrium curves measurement using the HEMOX™-ANALYZER (TCS Scientific Corp.) in PBS buffer with pH 7.4 at the temperature of 37 °C. The oxygen equilibrium curves were used for obtaining the value of P50, which was defined as the partial pressure of oxygen being related to the half ratio of oxygen saturated hemoglobin and was considered the sign of the oxygen affinity of HBOCs samples.
Results
Effects of mole ratio between Vc and hemoglobin on antioxidant protection
At the temperature of 4 °C, the effects of mole ratios between Vc and hemoglobin were investigated in HBOCs solutions with mole ratios of 0, 1, 3, 8 and 12, respectively. As shown in , under the investigated conditions, the MetHb contents in HBOCs solution increased from the initial value 6.25% to more than 9.00% in the duration of 8 h without the Vc condition, indicating the uncontrolled self-oxidant effect of HBOCs. However, under the condition of Vc addition, the MetHb contents gradually decreased with the Vc additions increasing. When the Vc addition amounts increased to the mole ratios of more than 3, the MetHb content decreasing extents were nearly kept constantly, suggesting the same antioxidant reaction rates between Vc and MetHb. Therefore, it could be suggested that the favorable Vc addition amounts could be set in the mole ratios of more than 3.
Effects of pH values on antioxidant protection in HBOCs solution
The effects of pH values on antioxidant by Vc were studied in the pH range from 6.5 to 8.0 at the temperature of 4 °C and the results are shown in . It could be seen that during the range of pH 6.5–8.0, the MetHb contents increased only at the pH value of 6.5, indicating the ineffective of Vc addition. At the other pH values, the MetHb contents all decreased remarkably, suggesting the effective antioxidant reactions at the higher pH value especially in the alkaline conditions.
Effects of temperatures on antioxidant protection in HBOCs solution
The effects of temperatures on Vc antioxidant protection in HBOCs solutions were researched under the conditions of pH 7.5 and mole ratio at 8 between Vc and HBOCs. The results are shown in . It could be seen that the MetHb contents kept nearly the same with the initial value only at the temperature of 4 °C and increased to different extents at the higher temperature (15, 25, 35 °C). Furthermore, the MetHb contents increased more rapidly at the higher temperature, indicating the higher self-oxidant rates at the higher temperatures. These results suggested that the lower temperatures especially at 4 °C would be suitable for the antioxidant protected by Vc in HBOCs solutions.
UV spectrum of HBOCs solution before and after Vc addition
The effects of Vc addition on the functional groups of HBOCs were investigated by the UV spectrum analysis and the results are shown in . As shown by UV spectrum analysis, the normal HBOCs samples before Vc addition showed the main three peaks at the wavelength of 414, 540 and 576 nm, which were the characteristic absorption peaks of the heme group, deoxyhemoglobin and oxyhemoglobin, respectively. However, the main three peaks presented no shifts in and the UV spectrum curves were almost the same with that in . These results indicated that the antioxidant reaction by Vc did not alter the functional group of the HBOCs.
CD analysis of HBOCs before and after Vc addition
The effects of antioxidant reaction by Vc on the secondary structures of HBOCs were investigated by the CD analysis and the results are shown in . As reported previously, there was only α-helix in the secondary structures of hemoglobin, which was presented by the two negative peaks and one positive peak at the wavelength of 208, 220 and 192 nm in . As for the HBOCs solution 72 h after Vc addition, the CD analysis curve presented almost no differences with that of HBOCs solution before Vc addition. Thus, it could be concluded that there was no effects of Vc antioxidant reactions on the secondary structure of the HBCOs molecules.
Molecular weight of HBOCs detected by SEC
The molecular weight of HBOCs before and after Vc addition was tested through SEC analysis and the spectrums are shown in . It could be seen that, compared to the curve in , the spectrum curve in was almost the same with that in expect the extra Vc peak, indicating no effects of Vc on the molecular weight and its distribution of HBOCs samples.
Testing of oxygen binding affinity
The oxygen equilibrium curves of HBOCs solution before and 72 h after Vc addition are shown in . It could be seen that the curves before and after the Vc addition were almost the same, indicating the same oxygen binding behavior of HBOCs sample solution before and after Vc addition. In addition, the P50 values presented in the two curves both were about 9.67 mmHg, which suggested that the Vc addition did not alter the oxygen binding affinity of HBOCs. It could be concluded from these results that the antioxidant reaction by Vc in HBOCs solution did not have the effects on the oxygen-carrying functions of HBOCs molecules.
Discussion
The oxygen binding affinity is one of the most physiological functions of HBOCs. The self-oxidation process was considered to be an important side-reaction of the oxygen binding process (Bian et al. Citation2012), which leading to the formation of MetHb. In the inner environment of red blood cell, the MetHb content usually could be controlled below 3% due to the protection of reductases. After being extracted from the red blood cell, the self-oxidation process was out of control because of absence of reductases and thus results the increasing of MetHb contents, leading to the invalidation of oxygen-binding function of polyHb and the formation of a series of harmful products such as free Fe3+and superoxide radicals, which were considered the reasons to the side effects of HBOCs in clinical trials (Alayash Citation2014). Therefore, it is very important to prohibit the oxidation of polymerized hemoglobin in the research and development of HBOCs.
As a large-scaled natural products and nutritional compounds in vivo, Vc has always been proved to be effective in a variety of antioxidant biochemical reactions in physiological conditions. As for the antioxidant protection of polymerized hemoglobin, several kinds of reduction agents were reported to be valid in the research and development of HBOCs include N-acetyl-l-cysteine, glutathione, SOD and so on (Bian and Chang Citation2015, Fronticelli and Koehler Citation2009, Kleinhans et al. Citation2006). Compared to the peptides and reductases, the Vc seems to be more applicable in the industrial development due to its advantages such as massive production, easy-get and low-cost. Usually, Vc could reduce the MetHb to be the ferrous hemoglobin and scavenging the free radicals through redox reactions (Atyabi et al. Citation2012, Su et al. Citation2014).
The results from this study showed that the pH value is an important factor to effect the antioxidant reactions by Vc. It was reported that MetHb formation would be accelerated under the presence of H+, indicating that the condition of lower pH values is unfavorable for the hemoglobin preservation. However, Vc was more capable for reacting with the oxidizing compounds in HBOCs solution due to its more powerful reduction capability in the alkaline conditions. Thus, it could be concluded that the Vc is more effect to be used as the antioxidant reagent for HBOCs in the range of pH 7.5–8.0. In addition, the MetHb formation was prohibited by the lower temperature, suggesting that the lower temperatures would be suitable for the HBOCs preservation with Vc as the antioxidant agents. In this study, Vc presented distinct antioxidant effects in the experimental conditions of pH 7.5–8.0 and temperature of 4 °C. Furthermore, the results of HBOCs characterization before and after Vc addition enhanced the protection effects of Vc on HBOCs, which ensured the feasibility of Vc being the antioxidant reagents for HBOCs.
Besides MetHb formation, free radicals were another group of harmful products from oxidation reaction of HBOCs. Free radicals were proved to be related to the side effects of HBOCs in clinical trials such as ischemia reperfusion injuries and myocardial infarction (Jain et al. Citation2015). So, it is necessary to monitor the free radicals scavenging in the research of HBOCs antioxidant. Vc has been proved to be an effective free radicals scavenger and its reaction with free radicals should certainly be inclusion in the study of antioxidant process. Therefore, the mechanisms of free radicals cleaning out in HBOCs solution by Vc would be the future work, which will deepen the understanding about the antioxidant of HBOCs by Vc and enhancing the antioxidant protection effects of Vc on HBOCs.
Declaration of interest
This study was financially supported by the Sichuan International Cooperation and Exchange Research Program (No. 2014HH0065), Sichuan Science and Technology Support Program (No. 2014SZ0123), Scientific Research Program of Sichuan Provincial Health and Family Planning Commission (No. 150256).
References
- Alayash AI. 2014. Blood Substitutes: why haven’t we been more successful? Trends Biotechnol. 32:177–185.
- Allen CJ, Tashiro J, Valle EJ, Thorson CM, Shariatmadar S, Schulman CI, et al. 2014. Initial hematocrit predicts the use of blood transfusion in the pediatric trauma patient. J Pediatr Surg. 49:1678–1682.
- Atyabi N, Yasini SP, Jalali SM, Shaygan H. 2012. Antioxidant effect of different vitamins on methemoglobin production: an in vitro study. Vet Res Forum. 3:97–101.
- Bian Y, Chang TM. 2015. A novel nanobiotherapeutic poly-[hemoglobin-superoxide dismutase-catalase-carbonic anhydrase] with no cardiac toxicity for the resuscitation of a rat model with 90 minutes of sustained severe hemorrhagic shock with loss of 2/3 blood volume. Artif Cells Nanomed Biotechnol. 43:1–9.
- Bian Y, Rong Z, Chang TM. 2012. Polyhemoglobin-superoxide dismutase-catalase-carbonic anhydrase: a novel biotechnology-based blood substitute that transports both oxygen and carbon dioxide and also acts as an antioxidant. Artif Cells Nanomed Biotechnol. 39:127–136.
- Buehler PW, D’Agnillo F, Schaer DJ. 2010. Hemoglobin-based oxygen carrier: from mechanisms of toxicity and clearance to rational drug design. Trends Mol Med. 16:447–457.
- Chen JY, Scerbo M, Kramer G. 2009. A review of blood substitutes: examining the history, clinical trial results, and ethics of hemoglobin-based oxygen carriers. Clinics. 64:803–813.
- Evelyn KA, Malloy HT. 1938. Microdetermination of oxyhemoglobin, methemoglobin, and sulfhemoglobin in single of blood. Biol Chem. 126:655–662.
- Greenburg AG. 2009. The ideal blood substitute. Crit Care Clin. 25:415–424.
- Fontes P, Lopez R, Plaats A, Vodovotz Y, Minervini M, Scott V. 2014. Liver preservation with machine perfusion and a newly developed cell-free oxygen carrier solution under subnormothermic conditions. Am J Transplant. 15:381–394.
- Fronticelli C, Koehler RC. 2009. Design of recombinant hemoglobin for use in transfusion fluids. Crit Care Clin. 25:357–371.
- Jahr JS, Walker V, Manoochehri K. 2007. Blood substitutes as pharmacotherapies in clinical practice. Curr Opin Anaesthesiol. 20:325–330.
- Jain AK, Mehra NK, Swarnakar NK. 2015. Role of antioxidant for the treatment of cardiovascular diseases: challenges and opportunities. Curr Pharm Des. 21:1–15.
- Karamian R, Komaki A, Salehi I, Tahmasebi L, Komaki H, Shahidi S, Sarihi A. 2015. Vitamin C reversed lead-induced deficits in hippocampal synaptic plasticity in rats. Brain Res Bull. 116:7–15.
- Kleinhans H, Mann O, Schurr PG, Kaifi JT, Hansen B, Izbicki JR, Strate T. 2006. Oxygen radical formation does not have an impact in the treatment of severe acute experimental pancreatitis using free cellular hemoglobin. World J Gastroenterol. 12:2914–2918.
- Li T, Zhang Z, Liao D, Chen Y, Yang C, Xu X, Liu J. 2012. The effect of polymerized placenta hemoglobin on renal ischemia/reperfusion injury. Artif Cells Nanomed Biotechnol. 40:396–399.
- Ortiz D, Barros M, Yan S, Cabrales P. 2014. Resuscitation from hemorrhagic shock using polymerized hemoglobin compared to blood. Am J Emerg Med. 32:248–255.
- Rempf C, Standl T, Schenke K, Chammas K, Gottschalk A, Burmeister MA, Gottschalk A. 2014. Administration of bovine polymerized haemoglobin before and during coronary occlusion reduces infarct size in rabbits. Br J Anaesth. 103:496–504.
- Silverman TA, Weiskopf RB. 2009. Hemoglobin-based oxygen carriers: current status and future directions. Transfusion. 49:2495–2515.
- Su X, Guo S, Huang X, Wang X, Qi D, Yang C. 2014. Control of oxidative reactions of hemoglobin in the design of blood substitutes: role of the Vc, NAC, TEMPO and their reductant system. Artif Cells Nanomed Biotechnol. 42:222–228.
- Vermeijden WJ, Klarenbosch JV, Gu YJ, Mariani MA, Buhre WF, Scheeren TL, et al. 2015. Effects of cell-saving devices and filters on transfusion in cardiac surgery: a multicenter randomized study. Ann Thorac Surg. 99:26–32.