Abstract
A rapid and sensitive high-performance liquid chromatography (HPLC) method for vitamin C (Vc) determination was developed in hemoglobin-based oxygen carriers (HBOCs) solution. After polymerized hemoglobin precipitation, Vc could be tested out within 5 min. The standard curve was linear in Vc quantity of 0.00–3.00 μg. Vc recovery was 103.56–108.68%, and relative standard deviation values of the repeatability and precision were below 1.00%. The limits of detection and limits of quantification were 0.002 ppm and 0.01 ppm, respectively. Furthermore, the application of established HPLC methods in methemoglobin reduction by Vc under different pH indicated that higher pH values could accelerate the reduction. This study suggested the potential application of established HPLC method in HBOCs research and development.
Introduction
Hemoglobin-based oxygen carriers (HBOCs) have attracted more and more attentions during the past decades due to their great potential applications in many areas such as blood substitutes (Roche et al. Citation2015, Song et al. Citation2014), oxygen therapeutics (Buehler et al. Citation2008, Hai Citation2012), isolated organ protection (Wu et al. Citation2011) and so on. Usually, the hemoglobin was chemically or genetically modified to form complex molecules with designed functions to enhance their oxygen affinity or safety properties (Wang et al. Citation2014). After extracted from the red blood cells, the cell-free hemoglobin was subjected to be oxidized because of the absence of protective reductase system, including superoxide dismutase, catalase, methemoglobin (MetHb) reductase and so on. The oxidation of hemoglobin not only cause the accumulation of non-functional MetHb but also leads to the formation of superoxide iron, peroxide and other free radicals, which have become one of the main obstacles in the research and development of HBOCs (Winslow Citation2013).
To inhibit the uncontrolled oxidation process of HBOCs, administration of certain bioactive compounds with antioxidation functions were proved to be effective for the HBOCs protection (Chen et al. Citation2013, Dorman et al. Citation2002). Ascorbates are a group of antioxidants have been proved important for physiological status. Vitamin C of this class has displayed the distinct efficiency in oxidation prohibition and MetHb reduction in HBOCs solution under the physiological pH value conditions (Chen et al. Citation2013). Numerous studies have showed that the Vc is associated with many physiological effects such as antioxidant activities, free radical scavenging, nutritional roles and so on (Harrington et al. Citation2010). Therefore, the beneficial effects of Vc on human health would certainly enhance its safety to be antioxidant for HBOCs protection.
In the previous work, Vc has showed notable antioxidant effects on HBOCs solution and was considered to be potentially applicable in HBOCs research and development (Chen et al. Citation2013). For ensuring the safe dose and quality control, it is necessary to build a method for Vc content determination in HBOCs solution. Although an effective molybdenum blue method was proved to be effective for Vc detection in our previous work (Chen et al. Citation2015), a faster method with more sensitivity is still needed for the Vc content monitor in the further deeply research work.
The high-performance liquid chromatography (HPLC) method is now commonly adopted in chemical analysis and has a series of advantages regarding specificity, easy operation and high sensitivity, which are suitable for Vc detection in HBOCs solution. In this work, a rapid HPLC method with a C18 stationary phase was presented for reduced Vc testing in the acidic mobile phase, which is helpful for the Vc stability and hemoglobin isolation before Vc testing. In addition, the method was further applied in the chemical reaction dynamic process of MetHb reduction by Vc under different pH value to evaluate its applicability in the future research work especially about the pharmacokinetic studies.
Experimental
Equipments
A Waters e2695 HPLC with a Waters 2489 UV-detector (WATERS company, USA) was used. The software of WATERS-Empower (WATERS company, USA) was adopted for the data acquisition. Chromatographic separation was carried by using a Chromstar Aq-C18 column (250 × 4.6 mm, 5 μm) (Swell company, Chengdu, China). Samples and standard solutions were chromatographed at the temperature of 25.0 ± 2.0 °C. The mixture solution of 0.2% (w/v) metaphosphoric acid/methanol/acetonitrile (90:8:2, v/v/v) was used as mobile phase with the flow rate of 1.0 ml/min. The wavelength of UV-detection was 245 nm.
Chemicals
The raw cord blood was kindly obtained from Stem Cell Bank of Sichuan Province. HBOCs solution used in this study was prepared through the crosslinking reactions between ultra-purified hemoglobin and glutaraldehyde, which was established method before (Li et al. Citation2009).
Methanol and acetonitrile were chromatographic grades were purchased from Merck (Kenilworth, NJ) and the prepared mobile phase for chromatographic separation was filtered by cellulose acetate membrane (0.45 μm) before used. Vc standard were purchased from National Standard Reference Material Centre in China. Other reagents were analytical grade and used without further purification.
Pretreatment of samples
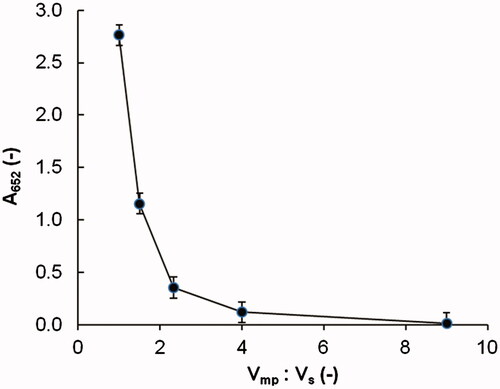
Before chromatographic analyzed for reduced Vc contents, the polymerized hemoglobin should be isolated from the sample solution in advance for its interference effects on the Vc testing and the acidic mobile phase solution was used as the precipitation solution. Therefore, the addition amounts of precipitation solution should be investigated to ensure the full precipitation of polymerized hemoglobin in the sample solution. In this study, X ml HBOCs sample solutions (with concentration of 3.0%, w/v) and Y ml acidic mobile phased solutions were mixed in the Eppendorf tubes (with labeled volume of 2 ml) and the series of mixture solutions were listed in the . The pretreated mixture solution was then centrifuged at 4500 g for 5 min at 4.0 ± 1.0 °C in the centrifuge of Biofuge Stratos and the supernatant was collected and filtered through the hydrophilic filter membrane with 0.45 μm. Then, with the mobile phase solution as blank, the absorption values of filtered solutions at the wavelength of 652 nm (A652) were tested on a UV detector of Professional 6300. Lower A652 values mean the more effective precipitation of the polymerized hemoglobin.
Table I. The series of pretreated mixture solutions for the hemoglobin precipitation.
Reduced Vc assay validation
The method validation was carried out through experiments of linearity, precision, repeatability, limits of quantification (LOQ), limits of detection (LOD) and stability, through which the suitability and reliability of this method could be evaluated.
Linearity of standard curve
For the preparation of 500 ppm stock solution, 0.0250 ± 0.0010 g Vc standard was weighed precisely and dissolved in 50 ml mobile phase solution. Then, the aliquots of prepared stock solution were diluted to a series of working standard solutions with required concentration range from 0.0 to 100.0 ppm, which were prepared in replicates of two.
After filtered by cellulose acetate membrane (0.45 μm), 30 μl working standard solutions were injected into the HPLC unit under the chromatography condition described in the “Equipments” section. The standard curve was constructed by plotting the peak area against the Vc concentrations. The least-square linear regression of the results data was employed to analyze the linearity of a standard curve by the coefficient of linear correlation (R value).
Repeatability, precision, stability and recovery
Vc working solutions with a certain concentration of 0.5, 5 and 50 ppm were employed for the validation experiments, including repeatability, precision, stability and recovery. Repeatability was calculated from chromatography to analyze results of six freshly prepared Vc standard solution diluted from the stock solution, respectively. Precision was determined by analyzing each calibration sample for six times. Repeatability and precision both were evaluated by the relative standard deviation (RSD).
The experiment of stability was carried out by investigating the chromatography analysis results in change from the same calibration sample stored at 4.0 ± 1.0 °C with duration of 10 h. Recovery was determined by a comparison between the designed concentration of standard Vc added to samples and those measured from the chromatographic analysis. It should be pointed out that the standard Vc can only be added into the supernatant after centrifugation due to the reaction between the reduced Vc and HBOCs solutions. Recovery was calculated as the measured value/resigned value ×100.
Limits of detection and quantification
The sensitivity of the developed method was evaluated by the LOD and LOQ, which were determined by gradient dilution of standard Vc solutions for obtaining signal/noise ratios of about 3:1 and 10:1, respectively.
Preparation of HBOCs and methemoglobin reduction by Vc under different pH values
The HBOCs solution was prepared through purified cord hemoglobin polymerized by glutaraldehyde as described previously (Zhou et al. Citation2013). The prepared HBOCs solution was exposed in the aerobic condition at 25 °C to form MetHb until the MetHb content rises up to 40% above. Then, the pH of MetHb solution was adjusted to the expected value and Vc was added to start the reduction reaction for the duration of 10 h in the anaerobic condition at 15 °C. The conversion process of MetHb to ferrous hemoglobin was monitored by multi-wave method (Wang et al. Citation2015) on a UV detector of Professional 6300. The Vc concentration in HBOCs solution was also termly determined by the validated HPLC method in this study.
For the reaction process of Vc contents decreasing, the rate constant k is certainly a negative number. Thus, k was adapted to illustrate the rate of reaction. The kinetic parameters of Vc in the HBOCs solutions with different pH values were calculated by the Mathematica software (Wolfram Research, Britain). The larger the k value, the more rapid the reaction. In addition, T1/2 was defined as the time of the half concentration of Vc in HBOCs solution during the reaction process. The kinetic parameters of k and T1/2 were used to illustrate the reaction rate in this study.
Results
Pretreatment of HBOCs samples
The HBOCs sample solutions were pretreated by a series volume of acidic mobile phase solutions to precipitate the polymerized hemoglobin. After centrifuged at 4500 g for 5 min at 4.0 ± 1.0 °C, the supernatants were collected and the results of absorbance at a wavelength of 652 nm (A652) were shown in . It can be seen that the A652 values were dramatically decreased as the volume ratio (Vmp:Vs) increased, indicating more effective precipitation by more addition amount of acidic mobile phase solution. When the volume ratio (Vmp:Vs) increased to 9, the A652 value of the collected supernatant was about 0.008, which could be considered almost the same with the blank solution. Thus, it could be concluded that the polymerized hemoglobin could be completely precipitated by the acidic mobile phase solution with the volume ratio (Vmp:Vs) of 9.
Linearity of standard curve
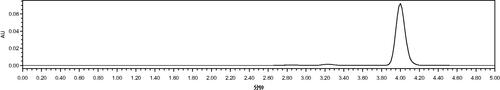
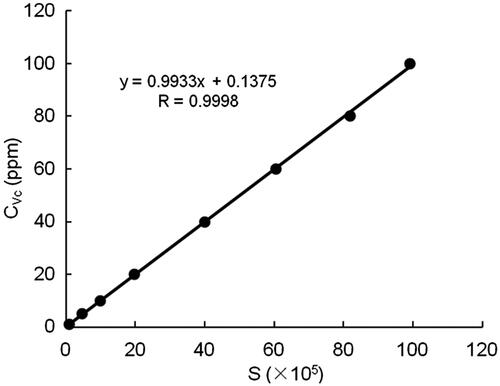
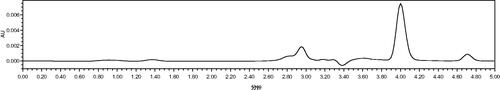
After preparation of a series of standard Vc solution with a different concentration (0–100 ppm) solution, 30 μl sample solution were administered (equal to 0.00–3.00 μg Vc quantity). The retention time of Vc was about 4.0 min and the peak could be remarkably presented (seen in ). The standard curve of Vc could be obtained by running analysis standard samples and the results were illustrated in . The coefficient of correlation was 0.9998, indicating the good linearity between the A652 and the amounts of Vc in sample solutions.
Repeatability, precision, stability and recovery
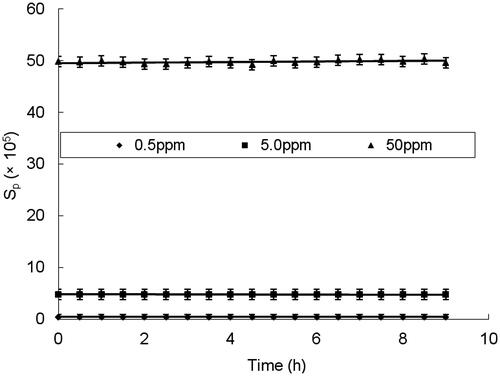
The validation of this HPLC method for Vc determination include repeatability, precision, stability and recovery were carried out through analysis of prepared Vc standard solution by plotting the peak area (Sp) against the Vc concentrations (CVc). Three samples with a concentration of 0.5, 5 and 50 ppm were prepared and then analyzed by the established methods. The results of repeatability, precision, stability and recovery were listed in , , and , respectively. It could be seen that the RSD values were below 2% in both results of the repeatability and precision experiments. The results of the stability experiments showed that the peak area almost kept equal in 9 h after the sample solution pretreated, indicating the stability of the reduced Vc in the acidic mobile phase solution. In addition, the recoveries of samples with different concentrations were 108.68%, 105.86% and 103.56%, respectively. All the results strongly proved that the established HPLC method is accurate and reliable for reduced Vc detection in HBOCs sample solution.
Table II. Results of the repeatability experiment.
Table III. Results of the precision experiment.
Table IV. Results of the recovery experiment.
Limits of detection and quantification
The LOD and LOQ were defined as the minimum amounts capable of giving chromatographic signals intensity higher than the background noise by three times and 10 times, respectively. In this study, the results of sensitive experiments showed that the LOD was 0.002 ppm (equal to the Vc amounts of 60 pg) and the LOQ was 0.01 ppm (equal to the Vc amounts of 300 pg), which were much lower than the Vc addition in HBOCs solutions. Thus, this established HPLC method could be considered to be sensible enough for quantification of Vc in HBOCs research and development.
Application in study of methemoglobin reduction by Vc under different pH values
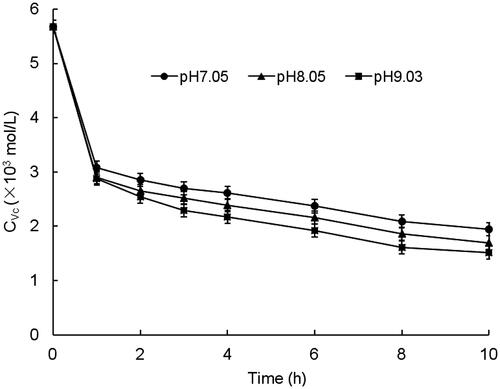
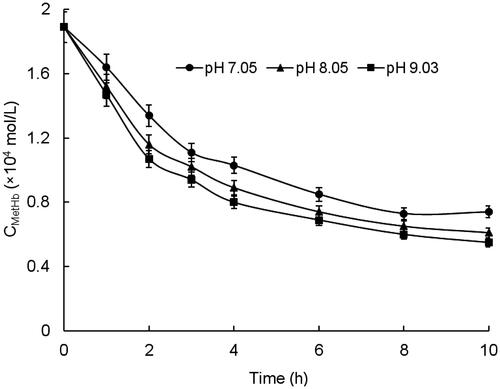
The experiments of MetHb reduction by Vc were carried out under different pH conditions and the results were illustrated in . In the HPLC chromatograms of HBOCs samples, the retention time of reduced Vc was about 4.00 min, which was the same with that of the Vc standard in , indicating no interferences on the reduced Vc testing. The kinetic parameters of Vc in the HBOCs solutions with different pH values were given in . It was found that the Vc concentrations in HBOCs solution were rapidly decreased in the first hour during the reaction process and then decreased more and more slowly as the reaction time increasing. During the reaction process, the MetHb contents were decreased simultaneously with the reduced Vc concentration decreasing, indicating the reaction process of MetHb reduction by Vc. On the other hand, it could also be seen that the reactions of MetHb reduction by Vc were remarkably effected by the pH values of the HBOCs solution. The higher the pH values, the rapid the reaction process, which was presented by the values of k and T1/2. All these results suggest that higher pH values may have beneficial effects on the antioxidant protection effects of Vc on HBOCs solution.
Table V. Kinetic parameters for the reaction of Vc in HBOCs solutions at different pH values.
Discussion
Vc is a common nutritional compound in a variety of dietary and natural products and is very useful for the biochemical reaction especially for the antioxidant and free radicals scavenging in vivo (Putchala et al. Citation2013). For the blood compounds, it had been proved that MetHb could be reduced to ferrous hemoglobin by Vc in the physiological conditions (Krukoski et al. Citation2009). Thus, Vc is certainly effective for the HBOCs antioxidant protection. However, an over dose of Vc could remarkably decrease the pH value of the HBOCs solution, leading to the unstability of the polymerized hemoglobin. During the development of the HBOCs, it is necessary to monitor the Vc content in HBOCs solution products. Thus, a fast and sensitive method for Vc detection in HBOCs solution is needed for the research and development of HBOCs, especially for the pharmacokinetic studies. HPLC methods were commonly used in the biochemical analysis of many natural compounds due to the high sensitivity, fast operations and fine repeatability.
Before the Vc detection, the polymerized hemoglobin need to be isolated previously to avoid its interference on testing. So, the acidic mobile phase solution was used as the precipitation solution for hemoglobin and its effectiveness was validated by the results of the precipitation experiments. On the other hand, Vc is a powerful antioxidant and could react with several compounds in HBOCs solutions, leading to the instability in solution. So, during the recovery experiments, the standard Vc was added into the supernatant after precipitation and centrifugation to enhance the precision of the testing.
In this study, the established method for Vc determination was proved to be effectiveness by the results of kinetics study of MetHb reduction under different pH values. Although both the Vc and MetHb contents decreased in the process of the reaction, the concentration-time profiles of Vc and MetHb were not always the same during the reaction process, indicating the different reaction rates of Vc and MetHb in solution. In fact, MetHb is not the only active compounds reacted with Vc. Under the pH condition investigated in this study, Vc could rapidly react with several oxidizing compounds in HBOCs solutions such as free Fe3+ ions, free oxygen, combined oxygen and so on. So, during the reaction process, Vc concentration decreased more rapidly than MetHb, indicating other oxidizing compounds scavenging reaction. On the other hand, free radicals were a series of indexes need to be monitored during the reaction because of their important roles in the side effects of HBOCs. Due to the instantaneity property, free radicals were difficult to be captured (Chikvaidze et al. Citation2014). Therefore, the process and mechanisms of free radicals scavenging by Vc were still kept unclear up to now. Based on the establishment HPLC methods for Vc determination, the interaction of Vc and oxidizing compounds would be systematically investigated in the future work to ensure the safety and feasibility of Vc as antioxidant in HBOCs solution.
Declaration of interest
This research was financially supported by the Sichuan International Cooperation and Exchange Research Program (No. 2014HH0065), Sichuan Science and Technology Support Program (No. 2014SZ0123), Scientific Research Program of Sichuan Provincial Health and Family Planning Commission (No. 150256).
References
- Buehler PW, Vallelien F, Mikolajczyk MG, Schoedon G, Schweizer T, Alayash AI, Schaer DJ. 2008. Structural stabilization in tetrameric or polymeric hemoglobin determines its interaction with endogenous antioxidant scavenger pathways. Antioxid Redox Signal. 10:1449–1462.
- Chen G, Duan Y, Li S, Wang H, Liu J, Yang C. 2013. Antioxidant effects of Vc on human placenta hemoglobin-based oxygen carriers. The14th International Symposium of Blood Substitutes and Oxygen Therapeutics. Chengdu, China.
- Chen G, Mo L, Li S, Zhou W, Wang H, Liu J, Yang C. 2015. Separation and determination of reduced vitamin C in polymerized hemoglobin-based oxygen carriers of the human placenta. Artif Cells Nanomed Biotechnol. 43:152–156.
- Chikvaidze EN, Partskhaladze TM, Gogoladze TV. 2014. Electron spin resonance (ESR/EPR) of free radicals observed in human red hair: a new, simple empirical method of determination of pheomelanin/eumulanin ratio in hair. Magn Reson Chem. 52:377–382.
- Dorman SC, Kenny CF, Miller L, Hirsch RE, Harrington JP. 2002. Role of redox potential of hemoglobin-based oxygen carriers on methemoglobin reduction by plasma components. Artif Cells Blood Substit Immobil Biotechnol. 30:39–51.
- Hai CM. 2012. Systems biology of HBOC-induced vasoconstriction. Curr Drug Discov Technol. 9:204–211.
- Harrington JP, Orlig K, Zito SL, Wollocko J, Wollocko H. 2010. Structural and redox behavior of OxyVita, a zero-linked polymeric hemoglobin: comparison with natural acellular polymeric hemoglobins. Artif Cells Blood Substit Immobil Biotechnol. 38:64–68.
- Krukoski DW, Comar SR, Claro LM, Leonart MS, do Nascimento AJ. 2009. Effect of vitamin C, deferoxamine, quercetin and rutin against tert-butyl hydroperoxide oxidative damage in human erythrocytes. Hematology. 14:168–172.
- Li F, Zhang H, Wang J, Yang C. 2009. Purification and viral inactivation of hemoglobin from human placenta blood. Artif Cells Blood Substit Immobil Biotechnol. 37:57–60.
- Putchala MC, Ramani P, Sherlin HJ, Premkumar P, Natesan A. 2013. Ascorbic acid and its pro-oxidant activity as a therapy for tumours of oral cavity – a systematic review. Arch Oral Biol. 58:563–574.
- Roche CJ, Talwar A, Palmer AF, Cabrales P, Gerfen G, Friedman JM. 2015. Evaluating the capacity to generate and preserve nitric oxide bioactivity in highly purified earthworm erythrocruorin: a giant polymeric hemoglobin with potential blood substitute properties. J Biol Chem. 290:99–117.
- Song BK, Nugent WH, Moon-Massat PF, Pittman RN. 2014. Effects of a hemoglobin-based oxygen carrier (HBOC-201) and derivatives with altered oxygen affinity and viscosity on systemic and microcirculatory variables in a top-load rat model. Microvasc Res. 95:124–130.
- Wang Q, Hu T, Sun L, Ji S, Zhao D, Liu J, Ma G, Su Z. 2015. CO binding improves the structural, functional, physical and anti-oxidation properties of the PEGylated hemoglobin. Artif Cells Nanomed Biotechnol. 43:18–25.
- Wang Q, Sun L, Ji S, Zhao D, Liu J, Su Z, Hu T. 2014. Reversible protection of Cys-93(β) by PEG alters the structural and functional properties of the PEGylated hemoglobin. Biochim Biophys Acta. 1844:1201–1207.
- Winslow RM. 2013. Oxygen: the poison is in the dose. Transfusion. 53:424–437.
- Wu W, Li T, Liu J, Yang C. 2011. Pretreatment before ischemia induction with polymerized human placenta hemoglobin (PolyPHb) attenuates ischemia/reperfusion injury-induced myocardial apoptosis. Artif Cells Blood Substit Immobil Biotechnol. 39:3–6.
- Zhou W, Zhao L, Wang J, Li S, Chen G, Liu J, Yang C. 2013. An optimal polymerization conditions for poly-human placenta hemoglobin with lower mean molecular weight. Artif Cells Nanomed Biotechnol. 41:289–292.