Abstract
Chrysin were well-documented as having significant biological roles particularly cancer chemo-preventive activity. However, the poor water solubility of chrysin limited their bioavailability and biomedical applications. In this study, we encapsulate the chrysin into PLGA-PEG nanoparticles for local treatment. In regard to the amount of the drug load, IC50 was significant decreased in nanocapsulated chrysin in comparison with free chrysin. This was confirmed through decrease of miR-18a, miR-21, and miR-221 genes expression by real-time PCR. The results demonstrated that PLGA-PEG-chrysin complexes can be more effective than free chrysin. Therefore, PLGA-PEG can be a better nanocarrier for this kind of hydrophobic flavonoid.
Keywords:
Introduction
Gastric cancer is one of the most prevalent cancers, which usually diagnosed in an advanced state, in which healing radical surgical resection is not successful. In the case of complex gastric cancer, several non-operative handling modalities, such as radiotherapy and chemotherapy, are applied, but recurrently survival is not delayed, and side effects are brutal (Eatemadi et al. Citation2014b).
As side effects of chemotherapeutic agents without survival advantage are not trivial, development of innovative carriers and new agents, make available anticancer activity through new mechanisms of action, and so is needed to reduce side effects and increase efficacy (Eatemadi et al. Citation2014a, Goldberg et al. Citation2014, Schroeder et al. Citation2012).
In recent times, substantial consideration has been focused on presenting naturally occurring chemopreventive agents to reverse, retard, or inhibit the multiple stages of carcinogenesis. A wide range of phenolic agents, particularly those present in dietary and medicinal plants, have been documented to have power over substantial antimutagenic and anticarcinogenic activities (Surh Citation1999).
Some simple and polyphenols found in natural foods such as honey, namely, chrysin (CR), quercetin (QU), acacetin (AC), caffeic acid phenylesters (CAPE), caffeic acid (CA), galangin (GA), kaempferol (KP), apigenin (AP), pinocembrin (PC), and pinobanksin (PB), have developed as talented pharmacological agents in prevention and treatment of cancer (Zarzecki et al. Citation2014). One of important agent that not well its anticancer properties studied is chrysin (5,7-dihydroxyflavone) (Jaganathan and Mandal Citation2009).
Flavones, such as chrysin, scutellare, apigenin, and baicaleinin were recently recognized as having important biological roles in nitrogen fixation, chemical defenses (Ursin et al. Citation1995, Vatten and Kvinnsland Citation1992), anti-inflammatory, anti-oxidant effects (Ursin et al. Citation1995), and in diverse range of human and rat cell types cancer its chemopreventive activity has revealed via induction of apoptosis. However, the effects of chrysin on human Gastric cancer need further studies. However, the poor water solubility of chrysin and other flavonoids limited their bioavailability, biocompatibility, and biomedical applications. In this study, we encapsulate the chrysin into poly (d, l-lactic-co-glycolic acid) poly (ethylene glycol) (PLGA-PEG) nanoparticles for local treatment (Eatemadi et al. Citation2015, Mellatyar et al. Citation2014). MicroRNAs (abbreviated miRNA or miR) are small non-coding RNA molecule (containing about 22 nucleotides), have roles in post-transcriptional regulation of gene expression and RNA silencing. In cancer patients, the function of some miRs may be deregulated, such as miR-18a, miR-21, and miR-221 (le Sage et al. Citation2007, Si et al. Citation2007, Tsang and Kwok Citation2009). In this study, we hypothesize that chrysin, especially in form of encapsulated, have strong effect onmiR-18a, miR-21, and miR-221 expression in AGS human gastric carcinoma cell line.
Materials and methods
Materials
Chrysin, PEG, streptomycin, penicillin G, 3(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), glycolide, dimethyl sulphoxide (DMSO), stannous octoate (Sn (Oct) 2), polyvinyl alcohol (PVA), d, l-lactide and dichloromethane (DCM) were purchased from Sigma-Aldrich (St. Louis, MO). Primers were purchased from Exiqon, Denmark. AGS human gastric carcinoma cell line was obtained from Pasteur Institute of Iran. Fetal bovine serum (FBS), Trypsin-EDTA, and RPMI-1640 were from Gibco, Invitrogen (Paisley, Scotland, UK) miRNA detection and analysis performed by MiRCURY LNA Universal RT microRNA PCR system (Exiqon, Vedbæk, Denmark). Nanodrop spectrophotometer was Bio Photometer. Real-time PCR was done on a Rotor-Gene 6000 (Corbett Research, Mortlake, Australia). Fourier transform infrared spectroscopy (FTIR) Perkin Elmer Series and KYKY model EM3200 was used for Infrared spectra and Scanning electron microscopy (SEM), respectively.
Methods
Synthesis of PLGA-PEG
PLGA-PEG was synthesized through a ring open polymerization of d, l-lactide and glycolide followed by adding PEG. PEG and PLGA were co-polymerized under vacuum using stannous octoate (Sn(Oct)2: stannous 2-ethylhexanoate) as catalyst. Under a nitrogen atmosphere, Glycolide (0.570 g), PEG 1.54 g (45% w/w) and d,l-lactide (2.882 g), were melted in bottleneck flask at 140 °C. The mixture of reaction comprise 0.05% (w/w) stannous octoate and a 3:1 proportion of d,l-lactide to glycolide was prepared and heated to 180 °C and maintained for 4 h.
Chrysin loading determination of its encapsulation efficiency
Chrysin were encapsulated in PLGA-PEG nanoparticle using double emulsion (w/o/w), probe type sonication, and solvent evaporation methods. Briefly, 200 mg PLGA-PEG and pure 20 mg chrysin was dissolved in dichloromethane (DCM) and sonicated for 1 min to yield the primary emulsion of s/o. Polyvinyl alcohol (PVA) 1% (1:1) and dimethyl sulphoxide (DMSO) was added to w/o emulsion then sonicated for 1 min to produce w/o/w emulsion. Using rotary evaporator, remaining solvents of this emulsion were evaporated. Then obtaining emulsion was centrifuged 30 min at 10,000 × g.
To determine chrysin loading and efficiency of chrysin encapsulation, the supernatant was separated and used for comparing with the total amount of chrysin. Using an ultraviolet 2550 spectrophotometer (Shimadzu), the amount of non-capsulated chrysin in supernatant was measured with an absorbance peak in 348 nm. The percentage of chrysin encapsulated on the nanoparticles [EquationEquation (1)](1) and drug loading [EquationEquation (2)]
(2) was measured using the following formula.
(1)
(2)
The FTIR spectrum was gained from a neat film cast of the chloroform copolymer solution between KBr tablets. The 1H NMR spectra were recorded in CDCI3. SEM was used as determination of shape and size of PLGA-PEG nanoparticles.
Cell viability and MTT-based cytotoxicity test
AGS human gastric carcinoma cell line were preserved in RPMI 1640 culture medium supplemented with 2 mM l-glutamine, 10% fetal bovine serum, 0.2 mg amphotericin B, 2 mg sodium bicarbonate, Penicillin G (80 mg/mL), and Strepto-mycin (50 mg/mL) at 37 °C in 5% humidified CO2 incubator (Davoudi et al. Citation2014).
3(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay was done to determine AGS cell viability. Under the exponential phase of growth, cells were exposed to chrysin loaded PCL-PEG and free chrysin. Cytotoxic effect of chrysin loaded PCL-PEG and free chrysin was assessed by MTT assay in 24, 48 and 72 h after treatment. About 10,000 cells/well were seeded on 96-well plate and allowed them to attach to plate for 24 h. Then each concentration triplicate was treated in the triplicate manner with different concentrations (0–160 μM) of chrysin loaded PCL-PEG and free chrysin for 24, 48, and 72 h and control received same amount of solvent (DMSO). After 24, 48, and 72 h, medium was removed. Then the cells were fed with fresh medium (200 μl) and were incubated for 24 h at 37 °C.
Then 50 μL of 2 mg/mL MTT dissolved in PBS was added to each well. Plates were covered with aluminum foil and then were incubated for 4 h at 37 °C. Then the content of all wells was removed and 200 μL pure DMSO and 25 μL Sorensen’s glycine buffer were added to it. Finally, ELISA plate reader (with a reference wavelength of 630 nm) was applied to calculate the absorbance measurement at 570 nm. The viability of the cells was calculated by this formula [EquationEquation (3)](3) :
(3)
RNA extraction, cDNA synthesis, and real-time PCR
AGS cells were treated with different concentrations of Nano and pure chrysin (0, 25, 35, 55, and 70 μM) for 24 h. Then, total RNA were extracted according Exiqon miRCURY RNA isolation kit (Exiqon, Denmark) The purity/quantity and integrity of total RNA was proved using Nanodrop 1000 (NanoDrop ND1000 spectrophotometer; Thermo Fisher Scientific, Waltham, MA). According to the instructions of the manufacturer, complementary DNA (cDNA) was synthesis by LNA universal RT miRNA PCR kit (Exiqon, Denmark) then, quantitative real-time PCR technique was used to determine miR-18a, miR-21, and miR-221 expression levels.
Relative miR-18a, miR-21, and miR-221 expression levels was normalized by miR-16 and relative expression of miR-18a, miR-21, and miR-221calculated by this formula [EquationEquation (4)](4) :
(4) where E (target) is referred to the real-time efficiency of target gene transcript, while E (reference) is referred to real-time efficiency of reference gene transcript. ΔCP reference is referred to CP deviation of (control-sample) of the reference gene transcript and ΔCP (target) is referred to CP deviation of (control-sample) of the target gene transcript.
Data analysis and statistics
GraphPad Prism 6.01 (GraphPad Software Inc., La Jolla, CA). was used for all plotting graphs and data analysis. Statistical analysis was performing by ANOVA test (by one-way analysis of variance). When P values was smaller than 0.05, the result was assumed of statistical importance. Results were expressed as the mean ± standard deviation (SD).
Results
FT-IR spectrum of PLGA-PEG
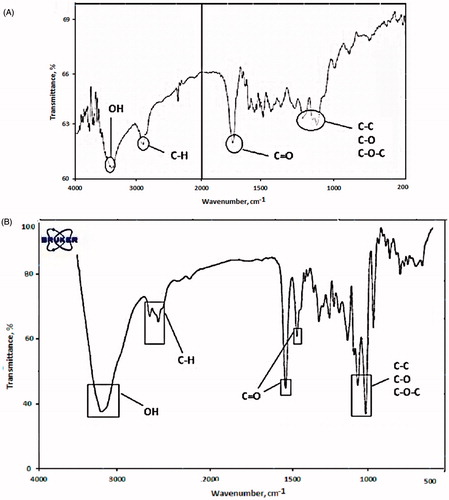
Successfully, PLGA-PEG co-polymer was synthesized by ring-opening copolymerization of PEG and PLGA. Functional groups were characterized using FTIR analysis. shows that the bands at 3000–2840 cm−1 are due to C-H stretch of CH. The bands at 1300–1090 cm−1 are due to interaction between C–C and C–O. Absorption at 1186–1089.6 cm−1 is due to C–O stretch. After encapsulation, some alteration accrued in FT-IR spectra. As shown in , the bands at 2840–3000 cm−1 are due to C–H stretch of CH. The bands at 1090–1300 cm−1 are due to interaction between C–C and C–O. The bands at 1085–1150 cm−1are assigned to ether of PEG.
1HNMR spectrum of synthesized copolymer
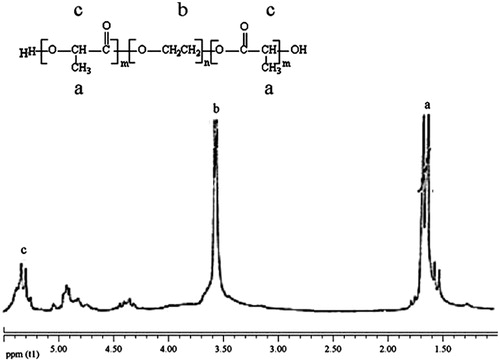
To confirm the structure of the copolymer, the 1H-NMR spectrum was traced. As shown in 1HNMR spectrum of synthesized copolymer (), the peak at 3.7, 1.6, 4.8, 4.3, and 5.3 ppm were assigned to the methylene protons of PEG, methyl of lactic, methylene of glycolic, methylene attach to aster oxygen, and hydrogen of methylene lactic. All the FT-IR and 1H-NMR results indicated that the PLGA-PEG copolymer formed successfully.
Size and size distribution of nanoparticles
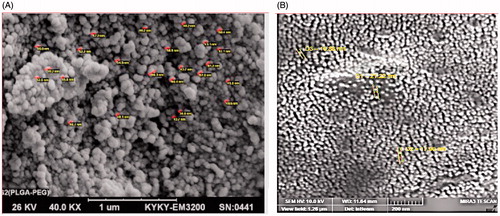
In order to investigate the physicochemical characterization of PLGA-PEG, the nanoparticles were monitored using SEM images. The micrographs of PLGA-PEG copolymers and PLGA-PEG-loaded Chrysin were shown in , respectively. The size of PLGA-PEG was 40–65 nm. After encapsulation of Chrysin on PLGA-PEG nanoparticles, the size of particles alter to 70–300 nm, which means dispersion of the particles was considerably improved.
Entrapment efficiency and drug loading
Based on EquationEquations (1)(1) and Equation(2)
(2) , encapsulation efficiency (EE) and drug loading (DL) was 98.6 and 16.19%, respectively.
Cell viability assay
The cytotoxicity of different concentration of pure chrysin and chrysin loaded in PLGA-PEG (0–160 μM) on AGS human gastric carcinoma cell line were analyzed by MTT-assay technique. These serial dilution of pure and encapsulated chrysin were treated on AGS for 24, 48, and 72 h and results suggested that pure chrysin and chrysin loaded in PLGA-PEG could inhibit cell proliferation by time and dose dependent manner. The pure chrysin had cytotoxic effect on AGS cell line with inhibitory concentration at 50% (IC50), 68.24 μM for 24 h, 56.16 μM for 48 h, and 42.32 μM for 72 h. On the other hand, the chrysin loaded PLGA-PEG, had cytotoxic effect of 58.24 μM for 24 h, 44.21 μM for 48 h, and 36.8 μM for 72 h at IC50 (). These results indicated that PLGA-PEG nanoparticle could improve the efficiency of chrysin in cell growth inhibition.
Table 1. IC50 value of pure and nano chrysin.
Quantitative real-time PCR
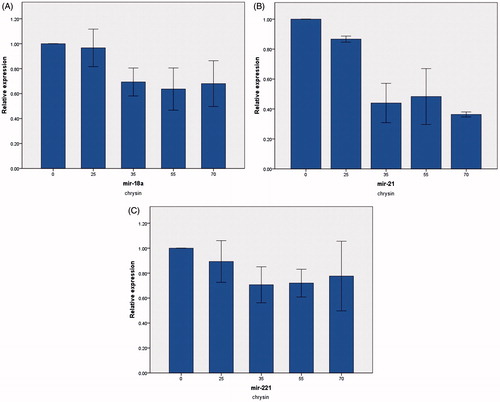
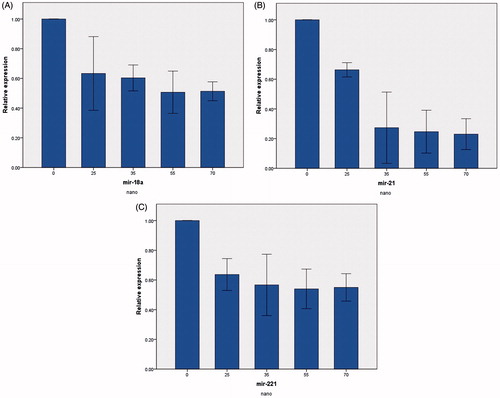
The levels of miR-18a, miR-21, and miR-221 expression were measured by real-time PCR. miR-18a, miR-21, and miR-221 mRNA levels were normalized by miR-16 (housekeeping gene). Different concentration of pure chrysin (0, 25, 35, 55, and 70 μM) for 24 h treatment decreased relative miR-18a, miR-21, and miR-221 expression (). Whereas chrysin loaded in PLGA-PEG nanoparticle in same concentration reduced further relative mRNA expression of miR-18a, miR-21, and miR-221 (). The results suggested that chrysin loaded in PLGA-PEG had better effect in decline miR-18, miR-21, and miR-221 expression rather than pure chrysin.
Discussion and conclusion
Despite the fact that chemotherapeutic agents has toxic side effects in healthy tissues in treatment of human cancers, nanotechnology try to overcome with these problems by loading or encapsulating chemotherapeutic agents to nanomaterials (Fustier and Chang Citation2012, Tong and Langer Citation2015). Traditional medicine using Medicinal plants have been applied worldwide and have been revealed to be a source of effective anticancer agents (Richard et al. Citation2015).
In this study, we assayed anti-proliferation effects of chrysin-loaded PLGA-PEG and chrysin free on AGS human gastric carcinoma cell line. MTT assay showed that chrysin-loaded PLGA-PEG has more cell death effect than free chrysin on AGS human gastric carcinoma cell line in same condition.
Chrysin is a flavone were recognized as having important biological roles in nitrogen fixation, chemical defenses (Ursin et al. Citation1995, Vatten and Kvinnsland Citation1992) anti-inflammatory and anti-oxidant effects (Ursin et al. Citation1995). However, the poor water solubility of chrysin and other flavonoids limited their bioavailability, biocompatibility, and biomedical applications. PLGA-PEG is one of the most promising nanoparticle for drug delivery and safety of this nanoparticle approved by FDA (Zheng et al. Citation2015). Studies have revealed that encapsulating and loading drugs to PLGA-PEG nanoparticles reduces dosage of drug and causes low adverse side effects of the drugs (Le et al., Citation2015, Pillai et al. Citation2015).
Gastric cancer is the second most frequent cause of cancer-related death, and the fourth most frequently diagnosed cancer worldwide (Siegel et al. Citation2015). Recent advances in therapy and diagnosis have improved the clinical outcomes of early stage gastric cancer patients. However, many patients have an overall poor prognosis and present with advanced stage disease (Catalano et al. Citation2009). Most advanced stage gastric cancer patients require chemotherapy, but a gold standard treatment for this tumor type has not been established.
In this study, we used PLGA-PEG–chrysin complex nanoparticles and free chrysin to inhibit AGS human gastric carcinoma cell line. Our study indicated that when we treat cell lines with the same amounts of PLGA-PEG–chrysin complex and chrysin-free, under the same conditions, PLGA-PEG–chrysin complex is more effective and kill some more human gastric carcinoma cells. Our experiments showed that PLGA-PEG–chrysin complex nanoparticles significantly decrease miR-18a, miR-21, and miR-221 mRNA gene expression. In conclusion, our data show that PLGA-PEG–chrysin complex had inhibitory effect on AGS human gastric carcinoma cell line and this inhibition was time and dose-dependent. Cytotoxic effect of PLGA-PEG–chrysin complex in the cells was increased with increasing concentration of PLGA-PEG–chrysin complex. Data analysis showed that with increasing concentration of PLGA-PEG–chrysin complex, decreasing trends of miR-18a, miR-21, and miR-221 expression was observed. Briefly, our data showed that low dosage of PLGA-PEG-chrysin complex has more inhibitory mRNA on expression of miR-18a, miR-21, and miR-221 mRNA than chrysin free. Besides, PLGA-PEG–chrysin complex has fewer side effects on AGS human gastric carcinoma cell lines than chrysin free.
Declaration of interest
The authors declare that they have no conflict of interest. Authors would like to thank Hematology and Oncology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran for supporting this project (grant No 92/34), which was a part of thesis No 92/1–1/1.
References
- Catalano V, Labianca R, Beretta GD, Gatta G, De Braud F, Van Cutsem E. 2009. Gastric cancer. Crit Rev Oncol Hematol. 71:127–164.
- Davoudi Z, Akbarzadeh A, Rahmatiyamchi M, Movassaghpour AA, Alipour M, Nejati-Koshki K, et al. 2014. Molecular target therapy of AKT and NF-kB signaling pathways and multidrug resistance by specific cell penetrating inhibitor peptides in HL-60 cells. Asian Pac J Cancer Prev. 15:4353.
- Eatemadi A, Darabi M, Afraidooni L, Zarghami N, Daraee H, Eskandari L, Mellatyar H, Akbarzadeh A. 2015. Comparison, synthesis and evaluation of anticancer drug-loaded polymeric nanoparticles on breast cancer cell lines. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. DOI: 10.3109/21691401.2015.1008510.
- Eatemadi A, Daraee H, Karimkhanloo H, Kouhi M, Zarghami N, Akbarzadeh A, et al. 2014a. Carbon nanotubes: properties, synthesis, purification, and medical applications. Nanoscale Res Lett. 9:1–13.
- Eatemadi A, Daraee H, Zarghami N, Melat Yar H, Akbarzadeh A. 2014b. Nanofiber: synthesis and biomedical applications. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. DOI: 10.3109/21691401.2014.922568.
- Fustier C, Chang TM. 2012. PEG-PLA nanocapsules containing a nanobiotechnological complex of polyhemoglobin-tyrosinase for the depletion of tyrosine in melanoma: preparation and in vitro characterisation. J Nanomed Biother Discov. 2:1–9.
- Goldberg M, Manzi A, Aydin E, Singh G, Khoshkenar P, Birdi A, et al. 2014. Development of a nanoparticle-embedded chitosan sponge for topical and local administration of chemotherapeutic agents. J Nanotechnol Eng Med. 5:040905.
- Jaganathan SK, Mandal M. 2009. Antiproliferative effects of honey and of its polyphenols: a review. BioMed Res Int. 2009:830616.
- Le DTT, Dang LTM, Hoang NTM, La HT, Nguyen HTM, Le HQ. 2015. Anti-tumor activity of docetaxel PLGA-PEG nanoparticles with a novel anti-HER2 scFv. J Nanomed Nanotechnol. 6:1.
- le Sage C, Nagel R, Egan DA, Schrier M, Mesman E, Mangiola A, et al. 2007. Regulation of the p27(Kip1) tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation. EMBO J. 26:3699–3708.
- Mellatyar H, Akbarzadeh A, Rahmati M, Ghalhar MG, Etemadi A, Nejati-Koshki K, Zarghami N, Barkhordari A. 2014. Comparison of inhibitory effect of 17-DMAG nanoparticles and free 17-DMAG in HSP90 gene expression in lung cancer. Asian Pac J Cancer Prev. 15:8693–8698.
- Pillai JJ, Thulasidasan AKT, Anto RJ, Devika NC, Ashwanikumar N, Kumar GV. 2015. Curcumin entrapped folic acid conjugated PLGA–PEG nanoparticles exhibit enhanced anticancer activity by site specific delivery. RSC Adv. 5:25518–25524.
- Richard TS, Kamdje AHN, Mukhtar F. 2015. Medicinal plants in breast cancer therapy. J Dis Med Plants. 1:19–23.
- Schroeder A, Heller DA, Winslow MM, Dahlman JE, Pratt GW, Langer R, Jacks T, Anderson DG. 2012. Treating metastatic cancer with nanotechnology. Nat Rev Cancer. 12:39–50.
- Si M, Zhu S, Wu H, Lu Z, Wu F, Mo Y. 2007. miR-21-mediated tumor growth. Oncogene. 26:2799–2803.
- Siegel RL, Miller KD, Jemal A. 2015. Cancer statistics 2015.Cancer J Clin. 65:5–29.
- Surh YJ. 1999. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res Fundam Mol Mech Mutagen. 428:305–327.
- Tong R, Langer R. 2015. Nanomedicines targeting the tumor microenvironment. Cancer J. 21:314–321.
- Tsang WP, Kwok TT. 2009. The miR-18a* microRNA functions as a potential tumor suppressor by targeting on K-Ras. Carcinogenesis. 30:953–959.
- Ursin G, Longnecker MP, Haile RW, Greenland S. 1995. A meta-analysis of body mass index and risk of premenopausal breast cancer. Epidemiology. 6:137–141.
- Vatten LJ, Kvinnsland S. 1992. Prospective study of height, body mass index and risk of breast cancer. Acta Oncol. 31:195–200.
- Zarzecki MS, Araujo SM, Bortolotto VC, Paula MTd, Jesse CR, Prigol M. 2014. Hypolipidemic action of chrysin on Triton WR-1339-induced hyperlipidemia in female C57BL/6 mice. Toxicol Rep. 1:200–208
- Zheng L, Wang L, Qin J, Sun X, Yang T, Ni Y, Zhou Y. 2015. New biodegradable implant material containing hydrogel with growth factors of lyophilized PRF in combination with an nHA/PLGA scaffold. J Hard Tissue Biol. 24:54–60.