Abstract
Mesenchymal stem cells (MSCs) are a population of multipotent progenitors which reside in bone marrow, fat, and some other tissues and can be isolated from various adult and fetal tissues. Self-renewal potential and multipotency are MSC’s hallmarks. They have the capacity of proliferation and differentiation into a variety of cell lineages like osteoblasts, condrocytes, adipocytes, fibroblasts, cardiomyocytes. MSCs can be identified by expression of some surface molecules like CD73, CD90, CD105, and lack of hematopoietic specific markers including CD34, CD45, and HLA-DR. They are hopeful tools for regenerative medicine for repairing injured tissues. Many studies have focused on two significant features of MSC therapy: (I) systemically administered MSCs home to sites of ischemia or injury, and (II) MSCs can modulate T-cell-mediated immunological responses. MSCs express chemokine receptors and ligands involved in cells migration and homing process. MSCs induce immunomedulatory effects on the innate (dendritic cells, monocyte, natural killer cells, and neutrophils) and the adaptive immune system cells (T helper-1, cytotoxic T lymphocyte, and B lymphocyte) by secreting soluble factors like TGF-β, IL-10, IDO, PGE-2, sHLA-G5, or by cell–cell interaction. In this review, we discuss the main applications of mesenchymal stem in Regenerative Medicine and known mechanisms of homing and Immunomodulation of MSCs.
Introduction
Mesenchymal stem cells (MSCs) are non-hematopoietic multipotent progenitor cells, which reside in bone marrow (Ben-Ami et al. Citation2011). The presence of non-hematopoietic stem cells in bone marrow were first recommended 130 years ago by Cohnhein, a German pathologist, (Chamberlain et al. Citation2007) who observed a class of the multipotent progenitors with fibroblast-like morphology which were spindle-shaped, plastic adherent, and non-phagocytic (Mohammadian and Shamsasenjan Citation2013). These cells which are also known as multipotent stromal or mesenchymal cells were first characterized by Friedenstein and his colleagues in 1970. In vitro, MSCs are able to divide up to 50 times in about 10 weeks (Lotfinegad Citation2014).
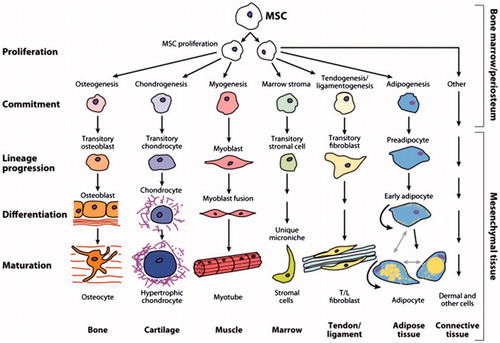
Self-renewal potential and multipotency are MSC’s hallmarks (Mohammadian and Shamsasenjan Citation2013, Moriscot et al. Citation2005, Shi et al. Citation2011). In vitro and in vivo, these cells are capable of differentiating to the variety of cell lineages like adipocyte, chondrocyte, osteocyte, tenocyte, fibroblast, cardiomyocyte, skeletal myocyte, Neuronshepatocyte, and stromal cell (Gebler et al. Citation2012, Krampera et al. Citation2006b, Wang et al. Citation2009). In addition, MSCs supply cytokines and growth factors which support expansion of hematopoietic and embryonic stem cells (Aggarwal and Pittenger Citation2005); therefore, they support hematopoietic stem cells homing and self-renewal in bone marrow (Maitra et al. Citation2004) ().
It is estimated that MSCs are about 0.001% of mononucleated cells in BM, despite the fact that the amount of them decline with age (Mohammadian and Shamsasenjan Citation2013, Mueller and Glowacki Citation2001, Kitoh et al. Citation2004). In addition to bone marrow, MSCs have been isolated from other supplies including liver, lung, brain, adipose tissue, peripheral blood, cornea, synovium, thymus, dental pulp, periosteum, tendon, spleen, fallopian tube, placenta, aminiotic fluid, Whartons jelly, and umbilical cord blood (Lotfinegad Citation2014).
Mesenchymal stem cells (MSCs) are well-known for the expression of surface markers such as: including CD105(SH2), CD73(SH3,4), stromal antigen-1, CD90, CD44, CD166(VCAM), CD54, CD102(ICAM-2), and CD49(VLA). (Aggarwal and Pittenger Citation2005) Conversely, MSCs are distinguished from hematopoietic cells by lacking hematopoietic specific markers including CD11b, CD11c, CD14, CD19, CD31, CD34, CD45, CD79a, HLA-DR, lymphocyte function-associated antigen 1 (LFA1), glycophorin A, as well as platelet, and endothelial cell markers (Ghannam et al. Citation2010a, Shi et al. Citation2011, Volarevic et al. Citation2011). It is believed that MSCs are remains of the embryonic stem cells which exist in adult human body and express the embryonic stem cell transcription factors including: HOX, SSEA-1 (stage-specific embryonic antigen 1), Nanong, Oct-4, Rex-1, and GATA-4 (Krampera et al. Citation2006b, Lotfinegad Citation2014).
In vitro and in vivo, MSCs release IL (Interleukin)-6, IL-7, IL-8, IL-10, IL-11, IL-12, IL-14, IL-15, sHLA (soluble human leukocyte antigen)-G5, PGE2 (prostaglandin E2), M-CSF (macrophage colony-stimulating factor), IDO (Indole 2,3-dioxygenase),TGF-β (transforming growth factor-β), HGF (hepatocyte growth factor), iNOS (inducible nitric oxide synthase), Galectin-3, and HO (hemooxygenase). Pevsner-Fischer et al. displayed that cultured MSCs express TLR (Toll like receptor) molecules 1–9. Activation of MSCs by TLR ligands stimulate IL-6 secretion and NFkB nuclear translocation (Pevsner-Fischer et al. Citation2007, Yagi et al. Citation2010). It is identified that TLRs mediate responses of bone marrow-derived progenitor cells. A recent study described the significance of TLRs in migration and immune regulation of MSCs (Nagai et al. Citation2006, Pevsner-Fischer et al. Citation2007, Ryan et al. Citation2007, Yagi et al. Citation2010).
Medical applications of MSCs
Stem cells in common and MSC in particular, due to their significant potential in proliferation and differentiation, are considered as perfect candidates for regenerative medicine applications (McTaggart and Atkinson Citation2007). Major role of MSCs is correlated to their various therapeutic properties: sustained self-renewal and expansion, multipotentiality, anti-inflammatory and immunomodulatory effects, secretion of mediators that initiate or support tissue renovation, and tissue substitution (Caplan and Dennis Citation2006, Du et al. Citation2013, Waszak et al. Citation2012). One of the most significant properties that make MSCs a special tool for cell based therapeutic approaches is their ability to escape from immune rejection; therefore, HLA-matching is not that much important for their implantation and HLA-mismatched donors can be chosen too (Dazzi and Marelli Berg Citation2008, Siegel et al. Citation2009). Besides, the anti-inflammatory activity of MSCs resulted in the production of anti-inflammatory macrophages, which are important in increasing tissue repair (Kim and Hematti Citation2009).
The proof-of-principle essential to utilize of MSCs in vivo has been demonstrated by a series of trials: (I) MSCs might engraft into mouse tissues after transplantation and show a site-specific differentiation, which is due to their exclusive immunological properties that permit engraftment with no rejection; (II) in humans, autologous in vitro expanded MSCs could be transplanted intravenously with no toxicity; (III) co-transplantation of autologous MSCs with HSCs lead to improved HSC engraftment; and (IV) allogeneic transplantation of MSCs reduced the frequency and intensity of acute and chronic graft versus host disease (GVHD) (Gebler et al. Citation2012, Sato et al. Citation2010, Tolar et al. Citation2010).
Mesenchymal stem cells (MSCs) have been brought into the clinic for numerous reasons: for differentiation and repairing injured tissues, for increasing hematopoietic engraftment following transplant via the production of growth factors (116 117), as well as for immunosuppressant in GVHD (Koç et al. Citation2000, Lazarus et al. Citation1995). Some of the other considerable therapeutic area include ischemic cardiac disease (Beyth et al. Citation2005), chronic obstructive pulmonary disease Crohn’s and Behç et al.’s disease (Du et al. Citation2013, Mazaheri et al. Citation2012), autoimmune diseases (Corcione et al. Citation2006, Sotiropoulou et al. Citation2006), damaged liver and kidneys (McTaggart and Atkinson Citation2007), bone disorders (e.g., osteogenesis imperfecat) (Sillence et al. Citation1978, Horwitz et al. Citation1999, Citation2001, Citation2002), neurological defects (Nauta et al. Citation2006), metachromatic leukodystrophy, and Hurler’s disease (Groth and Ringdén Citation1984, Krivit et al. Citation1999). Moreover, MSCs also have therapeutic potential used in treating pulmonary fibrosis and acute renal nephropathy, and also in inhibiting the progress of diabetes. MSCs transplantation promotes the development and expansion of b cells and renal glomeruli as well as decreasing collagen declaration and inflammation in fibrosis (Lee et al. Citation2009, Vija et al. Citation2009) (Urban et al. Citation2008). Furthermore, MSCs infusion can be very useful in cord blood transplantation where the restricted amount of stem cells delays engraftment and favors graft rejection. Cell therapy approach has also been utilized as GVHD prophylaxis in HSC transplantation. The therapeutic efficiency was related to reduced antigen-specific Th1/Th17 cell expansion, increased production of IL-10 and generation of CD4+, CD25+, and FoxP3+ Treg cells with the ability to suppress self-reactive T effectors responses (Ghannam et al. Citation2010b, Angoulvant et al. Citation2004).
MSCs homing
Homing is the process by which cells migrate to, and engraft within. Although the homing of leukocytes to the inflammation sites is well studied, the process of progenitor cell homing to the sites of ischemia or damage is weakly identified (Imhof and Aurrand-Lions Citation2004, Luster et al. Citation2005). Homing involves a cascade of incidents begun to interactions between flowing cells and the vascular endothelium at the target tissue (Stage I). This procedure is mediated via “homing receptors” expressed on circulating cells that connect related endothelial co-receptors, causing in cell-tethering, and rolling contacts on the endothelial surface. This is typically followed by chemokine generated activation of integrin adhesiveness (Stage II), hard adhesion (Stage III), and extravasation (Stage IV) (Sackstein Citation2005, Yagi et al. Citation2010).
Integrins have been identified to play a significant role in cell adhesion, migration, and chemotaxis (Ridger et al. Citation2001, Werr et al. Citation1998). Integrin α4/β1-VCAM interaction has been known to regulate T cell and NK cell trafficking (Woodside et al. Citation2006). Integrin β1 is involved in cell adhesion, which is essential for the anchorage of the engrafted cells. As expected, blockade of integrin β1 reduces neutrophil migration into the lung through inflammation (Ridger et al. Citation2001, Yagi et al. Citation2010). Ruster et al. explained that MSCs interact with endothelial cells in an organized style, not only through integrin α4/β1-VCAM-1 interaction or integrin β1, but also via the endothelial phenotype, P-selectin, MMP-2 production, and cytokines (Rüster et al. Citation2006). Fibronectin attaches extracellular matrix components like collagen, fibrin, and heparan sulfate proteoglycans. It plays a significant role in cell adhesion, growth, and migration as well as differentiation; and it is important in injury healing process. Integrin α4/β1-fibronectin interaction plays a significant role in transmigration of MSCs into the extracellular matrix (Ruoslahti Citation1984, Valenick et al. Citation2005).
Mesenchymal stem cells (MSCs) express chemokine receptors and ligands involved in cell migration, including CCR1, CCR2, CCR3, CCR4, CCR7, CCR8, CCR10, CCL2, CCL3, CCL4, CCL5, CCL7, CCL20, CCL26, CX3CL1, CXCL1, CXCL2, CXCL3, CXCL5, CXCL8, CXCL10, CXCL11, and CXCL12. In contrast, they do not express CXCR4, which suggests that CXCR4 can simply be significant for trafficking of mature stem cell population (Hoogduijn et al. Citation2010, Lotfinegad Citation2014, McTaggart and Atkinson Citation2007, Yagi et al. Citation2010). Stromal cell-derived factors 1 (SDF-1) which is formally intended Chemokine (C-X-C motif) ligand 12 (CXCL12) is a small chemokine that activates leukocytes and is frequently stimulated by pro-inflammatory stimuli like TNF-α or IL-1 (Fedyk et al. Citation2001). CXCR4 is the receptor for this chemokine and the SDF-1-CXCR4 interaction which is the main mechanism of homing, considered to be exclusive (Ma et al. Citation1998). MSCs considerably migrated in response to SDF-1 and CX3CL, constant with their expression of chemokine receptors CXCR4 or CX3CR1, respectively (Yagi et al. Citation2010).
As revealed in studies, on engraftment of hematopoietic stem/progenitor cells, interaction between CXCR4 and its ligand, SDF-1, co-operates a significant function in homing and mobilization (Chamberlain et al. Citation2007, Peled et al. Citation1999). Moreover, MSCs have an important role in co-transplantation of hematopoietic stem cells by producing SDF-1, Flt-3 ligand, and stem cell factor, together with expressing extra-cellular matrix proteins including fibronectin, laminin, and vimentin, which have a critical role in HSC homing in the bone marrow niche (Akbari et al. Citation2007, Delalat et al. Citation2009, Horwitz et al. Citation2011, Mohammadian and Shamsasenjan Citation2013).
Immunomodulatory effects of MSCs on immune system
It has been approved that the potency of MSCs to modulate immune responses is due to both cell–cell interactions and paracrine effects (Lotfinegad Citation2014). MSCs can reduce immune responses by influencing both natural and adaptive immunity (Ben-Ami et al. Citation2011). MSCs can inhibit innate immune system cells (DC, NK, monocyte, and neutrophil) and adaptive immune system cells (B, TH1, and T CTL) (Spaggiari et al. Citation2009).
Innate immune system cells
Natural killer cells
Natural killer cells (NK cells) are main effector cells of the innate immunity and are generally thought to play a basic role in antiviral responses (Yagi et al. Citation2010).
MSCs inhibit IL-2-induced NK cell proliferation, mostly via the soluble immunosuppressive factors, transforming growth factor-β (TGF-β), soluble human leukocyte antigen-G (sHLA-G), prostaglandinE2 (PGE2), and indoleamine 2,3-dioxygenase (IDO) in addition to cell–cell contact (Abdi et al. Citation2008, Ben-Ami et al. Citation2011, Gebler et al. Citation2012, Gonen-Gross et al. Citation2010, Lotfinegad Citation2014, Spaggiari et al. Citation2008, Yagi et al. Citation2010). Recently, Prigione et al. discovered that the inhibitory effect of MSCs on the proliferation of invariant NK T (iNKT, Vα24 + Vβ11+) and γδT(Vδ2+) cells in the peripheral blood is mediated by secreting PGE2, before IDO and TGF-β1. On the other hand, cytokine production and cytotoxic activity of the NK cells only moderately affected by MSCs (Prigione et al. Citation2009, Shi et al. Citation2011).
MSCs may have more influence on the innate immunity by their inhibitory effects on natural killer cell cytotoxicity via down-regulating of NKp30, NKp44, NKG2D, and DNAM-1, by enhancing receptor expression on NK cells and also by inhibiting proliferation and suppressing of IFN-γ production (Ben-Ami et al. Citation2011, Spaggiari et al. Citation2006, Citation2008). Although several studies revealed that MSCs suppress NK cell proliferation and IFN-γ production (Aggarwal and Pittenger Citation2005, Maccario et al. Citation2005, Rasmusson et al. Citation2003, Ryan et al. Citation2007, Shi et al. Citation2011, Sotiropoulou et al. Citation2006), Krampera et al. described that NK cells which co-cultured with MSCs in the presence of IL-2 for 4–5 days had shown reduced cytolytic activity against K562 cell line and this suppressive effect might be attributed to the IFN-γ production by NK cells (Shi et al. Citation2011). General aspects of immunomodulatory effects of MSCs on NK cells are illustrated in .
Dendritic cells
Dendritic cells (DCs), depending on their maturation and activation phase, have an important role in initiating of primary immune responses. Immature DCs have the ability of antigen up taking and processing and so they act as guard in peripheral tissues (Banchereau and Steinman Citation1998, Mellman and Steinman Citation2001, Yagi et al. Citation2010). MSCs may also regulate immune responses via interacting with DCs.
Myeloid DCs are the most potent antigen presenting cells which are essential in the induction of immunity and tolerance. During maturation, immature DCs acquired the expression of co-stimulatory molecules, and up-regulated the expression of MHC class I and class II molecules together with additional cell surface markers like CD11c, CD80, CD83, and CD86. MSC postpones the up-regulation of CD1A, CD40, CD80 (B7–1), CD86 (B7–2), and HLA-DR through DC maturation even as CD83 rose (Delalat et al. Citation2009, Ruoslahti Citation1984). In vitro, MSCs can inhibit the differentiation of monocytes and CD34+ hematopoietic progenitor cells into the DCs, via a declined cell surface expression of MHC class II, CD11c, CD83, and co-stimulatory molecules on mature DCs, in addition to a reduced production of IL-12 and TNFα; and subsequently give rise to immature DCs which could consequently provide anergic T-cells. Ramasamy et al. explained that the cell cycle in DCs was arrested in the G0/G1 phase due to their contact with MSCs (Ma et al. Citation1998). This outcome is partly mediated by the production of IL-6 or PGE-2 by activating MSCs, which is directly responsible for blocking DC maturation (Aggarwal and Pittenger Citation2005, Djouad et al. Citation2007, Ghannam et al. Citation2010a, Jiang et al. Citation2005, Spaggiari et al. Citation2009). Spaggiari et al. confirmed that MSCs powerfully inhibited the maturation and function of monocyte-derived DCs by means of inhibitory mediator of MSCs derived, PGE2 (Fedyk et al. Citation2001). IL-6 has been reported to be involved in the inhibition of monocyte differentiation to DCs as well as diminishing their stimulation capacity on T cells (Horwitz et al. Citation2011). These results suggest that MSCs might direct DC maturation to an anti-inflammatory or regulatory phenotype which is responsible for a desired T-cell response (Aggarwal and Pittenger Citation2005, Djouad et al. Citation2007, Ghannam et al. Citation2010a, Jiang et al. Citation2005, Spaggiari et al. Citation2009).
The effect of MSCs is controlled through primary phases of DC maturation, as verified by alterations in the expression of the DC surface markers CD80, CD86, CD83, and the secretion of the polarizing cytokine IL-12. MSCs have been shown to modify the cytokine secretion profile of DCs. DCs which are generated in the presence of MSCs, secrete low levels of inflammatory cytokines like IFN-γ, IL-12, and TNF-α, high levels of regulatory cytokines like IL-1β, IL-10 as well as low levels of MHC class II antigens; thus, induce further anti-inflammatory responses or generate tolerant DCs phenotype. Most recent studies suggest that antigen processing and presentation by MHC class II surface molecules is impaired in these DCs (Abdi et al. Citation2008, Nauta et al. Citation2006, Ryan et al. Citation2005).
CD14+ monocytes can activate MSCs to secrete soluble factors like IL-1β which inhibit alloreactive T-cells (Groh et al. Citation2005, Le Blanc and Ringden Citation2007). Significantly, DCs which co-cultured by MSCs showed a decreased potential to activate CD4+ cells in mixed lymphocyte culture (MLC) (Aggarwal and Pittenger Citation2005, Beyth et al. Citation2005, Jiang et al. Citation2005, Le Blanc and Ringden Citation2007, Maccario et al. Citation2005, Zhang et al. Citation2004). A new study reported that adipose derived MSCs are more potent immunomodulators in human DCs differentiation than bone marrow derived MSCs (Peled et al. Citation1999).
Neutrophils
Neutrophils are the first cells which arrive to the inflammatory tissues and secrete cytokines MSCs may also induce its immunomodulatory effects by interacting with neutrophils (Ben-Ami et al. Citation2011, Raffaghello et al. Citation2008).
MSCs inhibit the in vitro secretion of hydrogen peroxide in activated neutrophils, therefore these stem cells can potentially control the intensity of a respiratory burst in inflammatory stimulation (Ben-Ami et al. Citation2011, Raffaghello et al. Citation2008). MSCs can decrease the intensity of the respiratory burst and apoptosis which is a vital factor of the phagocytic role of neutrophils. This may be a serious process whereby MSCs can control the intensity of tissue damage following ischemic and ischemia/reperfusion damage (Hirata et al. Citation1993, Mazaheri et al. Citation2012). In addition, the production of IL-6 via MSCs has been reported toward stoppage apoptosis of lymphocytes (Prigione et al. Citation2009) and neutrophils (Djouad et al. Citation2007, Ghannam et al. Citation2010a, Jiang et al. Citation2005, Raffaghello et al. Citation2008, Xu et al. Citation2007).
Adaptive immune system cells
B cells
B cells have a crucial role in adaptive immune system by differentiating to plasma cells and antibody secretion (IgA, IgG, and IgM). They also play a major role in autoimmune diseases (Le Blanc and Ringden Citation2007).
MSCs are capable of modulating the immune response of B cells. It has been demonstrated that in a co-culture of activated B-cells with mesenchymal stem cells, the B-cells proliferation as well as antibody secretion (IgA, IgG, and IgM) in plasma cells were inhibited (Corcione et al. Citation2006, Lotfinegad Citation2014). MSCs arrest B cells in G0/G1 phase of the cell cycle and avoid apoptosis (Campagnoli et al. Citation2001, Mohammadian and Shamsasenjan Citation2013).
Allogeneic MSCs have been shown to restrain the proliferation, activation, and IgG secretion of B cells in BXSB mice that are utilized as an investigation model for human systemic lupus erythematous (Augello et al. Citation2005, Deng et al. Citation2005, Nauta and Fibbe Citation2007). Also, MSCs can cause an increase in the CD40 expression and CD40 ligand ectopic hyper expression in the B cells of BXSB mice (Deng et al. Citation2005, Shi et al. Citation2011). Krampera et al. demonstrated that MSCs decreased the proliferation of B cells in the presence of IFN-γ. The suppressive effect of IFN-γ is probably related to its ability to stimulate the secretion of IDO via MSCs, which in turn suppresses the proliferative response of effectors cells through the tryptophan pathway (Krampera et al. Citation2006a, Nauta and Fibbe Citation2007). MSCs down-regulated the expression of chemokine receptors CXCR4, CXCR5, and CCR7B; and decrease the chemotaxis of CXCL12 (CXCR4 ligand), CXCL13, and CXCR5 ligand, suggesting that elevated numbers of MSCs influence the chemotactic properties of B cells (Abdi et al. Citation2008, Chamberlain et al. Citation2007, Corcione et al. Citation2006, Deng et al. Citation2005, Le Blanc and Ringden Citation2007, Shi et al. Citation2011, Volarevic et al. Citation2011).
As B cell activation is mostly T cell-dependent, the influence of MSCs on T cells activity might also suppress B cells functions indirectly. In addition, MSC has a direct influence on B cell activity by cell contact and secretion of paracrine molecules (Augello et al. Citation2005, Corcione et al. Citation2006, Gerdoni et al. Citation2007, Weil et al. Citation2011). These findings cannot support the potential of therapeutic application of MSCs in autoimmune diseases, where the B cells play a major role (Le Blanc and Ringden Citation2007).
T cells
Human MSC limits the development of CD4+ and CD8+ T cells by soluble factors (Corcione et al. Citation2006, Di Nicola et al. Citation2002, Le Blanc and Ringden Citation2007, Potian et al. Citation2003, Tse et al. Citation2003). The suppressive factor is not constitutively produces by MSCs; since cell culture supernatants do not suppress T-cell proliferation (Augello et al. Citation2005, Le Blanc and Ringden Citation2007, Le Blanc et al. Citation2004, Maitra et al. Citation2004, Potian et al. Citation2003). Tse et al. displayed that nearness to MSCs significantly suppress T cell responsiveness which recommended that direct interaction between lymphocytes and MSCs was more significant than soluble mediators (Tse et al. Citation2003, Yagi et al. Citation2010). Krampera et al. also confirmed that immunosuppressive responses need MSC–T-cell interaction in culture (Krampera et al. Citation2003, Yagi et al. Citation2010).
Mesenchymal stem cells show immunosuppressive properties by inhibiting the response of naive and memory T cells in mixed lymphocyte culture (MLC) induced by mitogens (Koç et al. Citation2000). Suppression is MHC free (Nauta and Fibbe Citation2007) and mainly evident if MSCs is added on the earliest day of the 6-day culture. The suppression is appeared to be dosage dependent. Reserve manifests are detected when more numbers of MSCs are present (MSC:lymphocyte ratio >1:10). In contrast, adding of MSCs at a low ratio (1:100–1:10 000) increases proliferation (Le Blanc and Ringden Citation2007, Le Blanc et al. Citation2003, Liu et al. Citation2004, Potian et al. Citation2003).
MSCs may inhibit the cell division through the gathering of cells in the G0/G1 phase of the cell cycle. At the molecular level, cyclin D2 expression is down-regulated, whereas p27 expression is up-regulated; this might clarify why T cell proliferation, before activation, and IFN-γ secretion are affected by MSC (Glennie et al. Citation2005, Shi et al. Citation2011). A recent study demonstrated that B7-H4, a negative co-stimulatory molecule, is involved in the immunosuppressive effect of MSCs on T cell activation and proliferation by arresting the cell cycle and inhibition of the nuclear translocation of nuclear factor (NF)-kappa B (Sensebe et al. Citation2010).
MSCs may inhibit T-cell proliferation through the secretion of indoleamine 2,3-dioxygenase (IDO). IDO, which is induced by IFN-γ, can catalyze the alteration of tryptophan to kynurenine and also inhibit T-cell responses through tryptophan reduction (Le Blanc and Ringden Citation2007, Munn et al. Citation1998). Meisel et al. revealed that human MSCs do not express IDO constitutively, and the expression is stimulated by IFN-γ. IFN-γ stimulates IDO enzyme activity in a dose-dependent manner. The importance of IDO activity was identified in T cells stimulated by mitomycin C-treated PBMC in the presence of MSCs (Le Blanc and Ringden Citation2007, Meisel et al. Citation2004).
MSCs display their immunosuppressive potentials through the other mechanisms too. Matrix metalloproteinase (MMPs), particularly MMP-2 and MMP-9, are produced by MSCs and mediate the suppressive activity of MSCs through the reduction of CD25 expression on responding T cells (Ding et al. Citation2009). MSCs can inhibit T-cell proliferation by interacting of the inhibitory programmed death 1 (PD-1) molecule to its ligands PD-L1 and PD-L2, thus producing soluble factors (such as TGF-β or IL-10) that suppress T-cell proliferation (Nauta and Fibbe Citation2007, Volarevic et al. Citation2009, Citation2011). Recently, it has been revealed that the production of HLA-G5 by MSCs may suppress T-cell proliferation, as well as natural killer and T-cell cytotoxicity, and also may increase the generation of regulatory T (Treg) cells. Cell contact between MSCs and activated T cells stimulate IL-10 production, which is necessary to induce the release of soluble HLA-G5 (Ghannam et al. Citation2010a, Nasef et al. Citation2009, Selmani et al. Citation2008).
A recent study reported that MSCs might stimulate apoptosis in activated T cells [CD3+ and bromodeoxyuridine (BrdU)+], but not in the resting T cells [CD3+ and BrdU−]. In vivo, this may lead to the reduction of delayed-type hypersensitivity (DTH) response by inducing NO production (Lim et al. Citation2010). NO (nitric oxide) prevents the T cells proliferation by suppressing the phosphorylation of signal transducer and activator of transcription-5 (STAT5), an essential transcription factor for T cell activation and proliferation (Bingisser et al. Citation1998, Shi et al. Citation2011). Altogether, MSC increases TH2 generation, IL-4 production, regulatory T cell response, and also decrease activation by foreign antigen, cytotoxic T cell, and IFN-γ production (Weil et al. Citation2011).
T helper cells
The T helper cells (Th cells) are subpopulations of CD4+ T-cells that play an important role in the adaptive immune system and provide help to other cells of the immune system releasing cytokines or causing cell activation (Akbari et al. Citation2007).
Signals that support T-helper cell 1 (TH1) development such as CD3, CD28, IL-4, IL-2, and IL-12, can also cause naive T cells maturation into IFN-γ secreting cells. IFN-γ secretion decreases in the presence of MSCs in culture. Hence, MSCs stimulates a bias towards TH2 differentiation (Aggarwal and Pittenger Citation2005, Le Blanc and Ringden Citation2007). Furthermore, MSCs has also been reported to influence the cytokine secretion profile of the different T-cell subsets, since in vitro, their addition to an activated T-cell culture reduces production of the pro-inflammatory cytokines like IFN-γ, TNF-α, IL-6, IL-17, and increases anti-inflammatory cytokines like IL-4 and IL-10. Altogether, these findings could show a possible MSC-mediated alternation in Th1/Th2 balance (Kong et al. Citation2009, Zappia et al. Citation2005).
MSC inhibits naive T cells to differentiate into the TH17 (Meisel et al. Citation2004). MSC reduces antigen-specific Th1/Th17 cell expansion as well as cytokine (IFN-γ and IL-17) production by Th1/Th17 cells. MSCs also induce the Th2 cells to increase the production of IL-4 and IL-10 in lymph node joints (Aggarwal and Pittenger Citation2005, Krampera et al. Citation2003, Shi et al. Citation2011, Zappia et al. Citation2005). MSCs can also decrease the expression of major histocompatibility complex class E (MHC class E) on T-helper cells (Ghannam et al. Citation2010b, Mazaheri et al. Citation2012).
Cytotoxic T cells
Cytotoxic T cells (CTL) are subpopulations of CD8+ T-cells that play an important role in the cellular immune system to kill cancer cells, infected cells or damaged cells (Akbari et al. Citation2007).
MSCs which co-cultured in mixed lymphocyte culture (MLC) suppress CD8+ T-cell activity. MSCs, if added by the start of the MLC, can suppress CD8+ T-cell mediated lysis (Le Blanc and Ringden Citation2007, Rasmusson et al. Citation2003). However, cytotoxicity is not affected if MSCs were added in the cytotoxic stage (Angoulvant et al. Citation2004, Le Blanc and Ringden Citation2007, Maccario et al. Citation2005, Potian et al. Citation2003, Rasmusson et al. Citation2003). Lysis was partly stopped by adding IL-2. MSCs might inhibit the afferent phase of alloreactivity and prevent the development of cytotoxic T cells (Angoulvant et al. Citation2004, Le Blanc and Ringden Citation2007). CD25 and CTLA-4 (cytotoxic T lymphocyte-associated antigen-4) surface expression, and Foxp3 mRNA levels, were not dependent forward when CD4+ T cells were cultured in the company with MSCs (Krampera et al. Citation2006a).
Regulatory T cells
The regulatory T cells (Tregs), are a subpopulation of T cells which modulate the immune system, maintain tolerance to self-antigens, and abrogate autoimmune disease. These cells generally suppress or downregulate induction and proliferation of effector T cells (Akbari et al. Citation2007).
While MSCs effectively inhibit T cells proliferation, they can protect the role of CD4+ CD25+ CD127–FoxP3+ regulatory T cells (Treg). MSCs increase the amount of CD4+ CD25+, CD4+ CTLA4+ cells, and CD4+ CD25+ CTLA4+ cells in IL-2-stimulated MLC (Aggarwal and Pittenger Citation2005, Le Blanc and Ringden Citation2007, Maccario et al. Citation2005). In contrast, the amount of CD25+ and CD38+ cells decrease in the presence of MSCs in mitogen-stimulated lymphocyte cultures (Groh et al. Citation2005, Le Blanc and Ringden Citation2007). MSC also produces bone morphogenic protein-2 (BMP-2) which mediates immunosuppressive response through the production of CD8+ regulatory T cells (Djouad et al. Citation2003, Le Blanc and Ringden Citation2007).
Prostaglandin E2 (PGE2), which is produced by cyclooxygenase (COX) enzymes, induces the regulatory T cells. MSCs constitutively express COX-1 and COX-2 together. When purified T cells were co-cultured with MSCs, both COX-2 and PGE2 production were increased PGE2 synthesis inhibitors restored the majority of the proliferation of lymphocytes co-cultured by MSCs (Aggarwal and Pittenger Citation2005, Arikawa et al. Citation2004, Le Blanc and Ringden Citation2007).
Conclusion
Rational understanding of MSCs mechanisms of action allows us to translate our basic knowledge of MSC biology into the design of new clinical therapies. The anti-proliferative potential and immunodulatory function of MSCs has been studied by different groups, with the hope that MSCs may be developed as a new therapeutic strategy for autoimmune disease, Hematopoietic Stem Cell Transplantation (HSCT), Bone marrow Transplantation (BMT), and cell-based regenerative therapy. MSCs have the capacity of homing and integration into the damaged tissues. MSCs provide immunomodulatory effects on the immune system via paracrine and/or cell–cell interaction, which may inhibit innate and adaptive immune system cells. Therefore, employing MSCs could lead to various therapeutic possibilities such as supporting tissue regeneration and correcting inherited disorders. Autologous MSC transplantation may have a high capability to develop the desired outcome in clinical therapies.
Acknowledgements
The authors thank Hematology and Oncology Research Center, Tabriz University of Medical Sciences for all supports provided.
Declaration of interest
The authors declare that they have no competing interests.
References
- Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. 2008. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 57:1759–1767.
- Aggarwal S, Pittenger MF. 2005. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 105:1815–1822.
- Akbari A, Mozdarani H, Akhlaghpoor S, Pourfatollah A, Soleimani M. 2007. Evaluation of the homing of human CD34+ cells in mouse bone marrow using clinical MR imaging. Pak J Biol Sci. 10:833–842.
- Angoulvant D, Clerc A, Benchalal S, Galambrun C, Farre A, Bertrand Y, et al. 2004. Human mesenchymal stem cells suppress induction of cytotoxic response to alloantigens. Biorheology. 41:469–476.
- Arikawa T, Omura K, Morita I. 2004. Regulation of bone morphogenetic protein 2 expression by endogenous prostaglandin E2 in human mesenchymal stem cells. J Cell Physiol. 200:400–406.
- Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, et al. 2005. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 35:1482–1490.
- Banchereau J, Steinman RM. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252.
- Ben-Ami E, Berrih-Aknin S, Miller A. 2011. Mesenchymal stem cells as an immunomodulatory therapeutic strategy for autoimmune diseases. Autoimmun Rev. 10:410–415.
- Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, et al. 2005. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 105:2214–2219.
- Bingisser RM, Tilbrook PA, Holt PG, Kees UR. 1998. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol. 160:5729–5734.
- Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. 2001. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 98:2396–2402.
- Caplan AI, Dennis JE. 2006. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 98:1076–1084.
- Chamberlain G, Fox J, Ashton B, Middleton J. 2007. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 25:2739–2749.
- Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al. 2006. Human mesenchymal stem cells modulate B-cell functions. Blood. 107:367–372.
- Dazzi F, Marelli Berg FM. 2008. Mesenchymal stem cells for graft versus host disease: close encounters with T cells. Eur J Immunol. 38:1479–1482.
- Delalat B, Pourfathollah AA, Soleimani M, Mozdarani H, Ghaemi SR, Movassaghpour AA, et al. 2009. Isolation and ex vivo expansion of human umbilical cord blood-derived CD34+ stem cells and their cotransplantation with or without mesenchymal stem cells. Hematology. 14:125–132.
- Deng W, Han Q, Liao L, You S, Deng H, Zhao RC. 2005. Effects of allogeneic bone marrow-derived mesenchymal stem cells on T and B lymphocytes from BXSB mice. DNA Cell Biol. 24:458–463.
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. 2002. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 99:3838–3843.
- Ding Y, Xu D, Feng G, Bushell A, Muschel RJ, Wood KJ. 2009. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and-9. Diabetes. 58:1797–1806.
- Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, et al. 2003. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 102:3837–3844.
- Djouad F, Charbonnier LM, Bouffi C, Louis Plence P, Bony C, Apparailly F, et al. 2007. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin 6 dependent mechanism. Stem Cells. 25:2025–2032.
- Du Z, Wei C, Cheng K, Han B, Yan J, Zhang M, et al. 2013. Mesenchymal stem cell-conditioned medium reduces liver injury and enhances regeneration in reduced-size rat liver transplantation. J Surg Res. 183:907–915.
- Fedyk ER, Jones D, Critchley HO, Phipps RP, Blieden TM, Springer TA. 2001. Expression of stromal-derived factor-1 is decreased by IL-1 and TNF and in dermal wound healing. J Immunol. 166:5749–5754.
- Gebler A, Zabel O, Seliger B. 2012. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. 18:128–134.
- Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, et al. 2007. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 61:219–227.
- Ghannam S, Bouffi C, Djouad F, Jorgensen C, Noël D. 2010a. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res Ther. 1:2–10.
- Ghannam S, Pène J, Torcy-Moquet G, Jorgensen C, Yssel H. 2010b. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 185:302–312.
- Glennie S, Soeiro I, Dyson PJ, Lam EW-F, Dazzi F. 2005. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 105:2821–2827.
- Gonen-Gross T, Goldman-Wohl D, Huppertz B, Lankry D, Greenfield C, Natanson-Yaron S, et al. 2010. Inhibitory NK receptor recognition of HLA-G: regulation by contact residues and by cell specific expression at the fetal-maternal interface. PLoS One. 5:e8941.
- Groh ME, Maitra B, Szekely E, Koç ON. 2005. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol. 33:928–934.
- Groth CG, Ringdén O. 1984. Transplantation in relation to the treatment of inherited disease. Transplantation. 38:319–326.
- Hirata Y, Sugita T, Gyo K, Yanagihara N. 1993. Experimental vestibular neuritis induced by herpes simplex virus. Acta Oto-Laryngol. 113:79–81.
- Hoogduijn MJ, Popp F, Verbeek R, Masoodi M, Nicolaou A, Baan C, et al. 2010. The immunomodulatory properties of mesenchymal stem cells and their use for immunotherapy. Int Immunopharmacol. 10:1496–1500.
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. 1999. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 5:309–313.
- Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD, et al. 2001. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 97:1227–1231.
- Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, et al. 2002. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA. 99:8932–8937.
- Horwitz EM, Maziarz RT, Kebriaei P. 2011. MSCs in hematopoietic cell transplantation. Biol Blood Marrow Transplant. 17:S21–S29.
- Imhof BA, Aurrand-Lions M. 2004. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 4:432–444.
- Jiang X-X, Zhang Y, Liu B, Zhang S-X, Wu Y, Yu X-D, et al. 2005. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 105:4120–4126.
- Kim J, Hematti P. 2009. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 37:1445–1453.
- Kitoh H, Kitakoji T, Tsuchiya H, Mitsuyama H, Nakamura H, Katoh M, et al. 2004. Transplantation of marrow-derived mesenchymal stem cells and platelet-rich plasma during distraction osteogenesis – a preliminary result of three cases. Bone. 35:892–898.
- Koç ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, et al. 2000. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 18:307–313.
- Kong Q-f, Sun B, Bai S-s, Zhai D-x, Wang G-y, Liu Y-m, et al. 2009. Administration of bone marrow stromal cells ameliorates experimental autoimmune myasthenia gravis by altering the balance of Th1/Th2/Th17/Treg cell subsets through the secretion of TGF. J Neuroimmunol. 207:83–91.
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, et al. 2003. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 101:3722–3729.
- Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, et al. 2006a. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 24:386–398.
- Krampera M, Pasini A, Pizzolo G, Cosmi L, Romagnani S, Annunziato F. 2006b. Regenerative and immunomodulatory potential of mesenchymal stem cells. Curr Opin Pharmacol. 6:435–441.
- Krivit W, Peters C, Shapiro EG. 1999. Bone marrow transplantation as effective treatment of central nervous system disease in globoid cell leukodystrophy, metachromatic leukodystrophy, adrenoleukodystrophy, mannosidosis, fucosidosis, aspartylglucosaminuria, Hurler, Maroteaux-Lamy, and Sly syndromes, and Gaucher disease type III. Curr Opin Neurol. 12:167–176.
- Lazarus H, Haynesworth S, Gerson S, Rosenthal N, Caplan A. 1995. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transpl. 16:557–564.
- Le Blanc K, Ringden O. 2007. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 262:509–525.
- Le Blanc K, Tammik L, Sundberg B, Haynesworth S, Ringden O. 2003. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 57:11–20.
- Le Blanc K, Rasmusson I, Götherström C, Seidel C, Sundberg B, Sundin M, et al. 2004. Mesenchymal stem cells inhibit the expression of CD25 (interleukin 2 receptor) and CD38 on phytohaemagglutinin activated lymphocytes. Scand J Immunol. 60:307–315.
- Lee JW, Gupta N, Serikov V, Matthay MA. 2009. Potential application of mesenchymal stem cells in acute lung injury. Expert Opin Biol Ther. 9:1259–1270.
- Lim J-H, Kim J-S, Yoon I-H, Shin J-S, Nam H-Y, Yang S-H, et al. 2010. Immunomodulation of delayed-type hypersensitivity responses by mesenchymal stem cells is associated with bystander T cell apoptosis in the draining lymph node. J Immunol. 185:4022–4029.
- Liu J, Lu X, Wan L, Li Y, Li S, Zeng L, et al. 2004. Suppression of human peripheral blood lymphocyte proliferation by immortalized mesenchymal stem cells derived from bone marrow of Banna Minipig inbred-line. Transplant Proc. 36:3272–3275.
- Lotfinegad P. 2014. Immunomodulatory nature and site specific affinity of mesenchymal stem cells: a hope in cell therapy. Adv Pharm Bull. 4:5–10.
- Luster AD, Alon R, von Andrian UH. 2005. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 6:1182–1190.
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, et al. 1998. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4-and SDF-1-deficient mice. Proc Natl Acad Sci. 95:9448–9453.
- Maccario R, Podestà M, Moretta A, Cometa A, Comoli P, Montagna D, et al. 2005. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 90:516–525.
- Maitra B, Szekely E, Gjini K, Laughlin M, Dennis J, Haynesworth S, et al. 2004. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 33:597–604.
- Mazaheri T, Esmaeilzadeh A, Mirzaei MH. 2012. Introducing the immunomodulatory effects of mesenchymal stem cells in an experimental model of Behçet’s disease. J Med Hypoth Ideas. 6:23–27.
- McTaggart SJ, Atkinson K. 2007. Mesenchymal stem cells: immunobiology and therapeutic potential in kidney disease (Review Article). Nephrology (Carlton). 12:44–52.
- Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. 2004. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2, 3-dioxygenase-mediated tryptophan degradation. Blood. 103:4619–4621.
- Mellman I, Steinman RM. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell. 106:255–258.
- Mohammadian M, Shamsasenjan K. 2013. Mesenchymal stem cells: new aspect in cell-based regenerative rherapy. Adv Pharm Bull. 3:433–438.
- Moriscot C, de Fraipont F, Richard MJ, Marchand M, Savatier P, Bosco D, et al. 2005. Human bone marrow mesenchymal stem cells can express insulin and key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or microenvironmental manipulation in vitro. Stem Cells. 23:594–603.
- Mueller SM, Glowacki J. 2001. Age related decline in the osteogenic potential of human bone marrow cells cultured in three dimensional collagen sponges. J Cell Biochem. 82:583–590.
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. 1998. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 281:1191–1193.
- Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, et al. 2006. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 24:801–812.
- Nasef A, Zhang Y, Mazurier C, Bouchet S, Bensidhoum M, Francois S, et al. 2009. Selected Stro 1 enriched bone marrow stromal cells display a major suppressive effect on lymphocyte proliferation. Int J Lab Hematol. 31:9–19.
- Nauta AJ, Fibbe WE. 2007. Immunomodulatory properties of mesenchymal stromal cells. Blood. 110:3499–3506.
- Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. 2006. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 177:2080–2087.
- Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. 1999. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 283:845–848.
- Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, et al. 2007. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 109:1422–1432.
- Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. 2003. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 171:3426–3434.
- Prigione I, Benvenuto F, Bocca P, Battistini L, Uccelli A, Pistoia V. 2009. Reciprocal interactions between human mesenchymal stem cells and gammadelta T cells or invariant natural killer T cells. Stem Cells. 27:693–702.
- Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, et al. 2008. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. 26:151–162.
- Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. 2003. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 76:1208–1213.
- Ridger VC, Wagner BE, Wallace WA, Hellewell PG. 2001. Differential effects of CD18, CD29, and CD49 integrin subunit inhibition on neutrophil migration in pulmonary inflammation. J Immunol. 166:3484–3490.
- Ruoslahti E. 1984. Fibronectin in cell adhesion and invasion. Cancer Metastasis Rev. 3:43–51.
- Rüster B, Göttig S, Ludwig RJ, Bistrian R, Müller S, Seifried E, et al. 2006. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 108:3938–3944.
- Ryan JM, Barry FP, Murphy JM, Mahon BP. 2005. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond). 2:8–14.
- Ryan J, Barry F, Murphy J, Mahon B. 2007. Interferon does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 149:353–363.
- Sackstein R. 2005. The lymphocyte homing receptors: gatekeepers of the multistep paradigm. Curr Opin Hematol. 12:444–450.
- Sato K, Ozaki K, Mori M, Muroi K, Ozawa K. 2010. Mesenchymal stromal cells for graft-versus-host disease: basic aspects and clinical outcomes. J Clin Exp Hematop. 50:79–89.
- Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, et al. 2008. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 26:212–222.
- Sensebe L, Krampera M, Schrezenmeier H, Bourin P, Giordano R. 2010. Mesenchymal stem cells for clinical application. Vox Sang. 98:93–107.
- Shi M, Liu ZW, Wang FS. 2011. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 164:1–8.
- Siegel G, Schäfer R, Dazzi F. 2009. The immunosuppressive properties of mesenchymal stem cells. Transplantation. 87:S45–SS49.
- Sillence DO, Rimoin DL, Danks DM. 1978. Clinical variability in osteogenesis imperfecta-variable expressivity or genetic heterogeneity. Birth Defects Orig Artic Ser. 15:113–129.
- Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. 2006. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 24:74–85.
- Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. 2006. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 107:1484–1490.
- Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. 2008. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 111:1327–1333.
- Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. 2009. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 113:6576–6583.
- Tolar J, Le Blanc K, Keating A, Blazar BR. 2010. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells. 28:1446–1455.
- Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. 2003. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation (Baltimore). 75:389–397.
- Urban VS, Kiss J, Kovacs J, Gocza E, Vas V, Monostori v, et al. 2008. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 26:244–253.
- Valenick LV, Hsia HC, Schwarzbauer JE. 2005. Fibronectin fragmentation promotes 4 1 integrin-mediated contraction of a fibrin–fibronectin provisional matrix. Exp Cell Res. 309:48–55.
- Vija L, Farge D, Gautier J-F, Vexiau P, Dumitrache C, Bourgarit A, et al. 2009. Mesenchymal stem cells: stem cell therapy perspectives for type 1 diabetes. Diabetes Metab. 35:85–93.
- Volarevic V, Al-Qahtani A, Arsenijevic N, Pajovic S, Lukic ML. 2009. Interleukin-1 receptor antagonist (IL-1Ra) and IL-1Ra producing mesenchymal stem cells as modulators of diabetogenesis. Autoimmunity. 43:255–263.
- Volarevic V, Arsenijevic N, Lukic ML, Stojkovic M. 2011. Concise review: mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells. 29:5–10.
- Wang M, Yang Y, Yang D, Luo F, Liang W, Guo S, et al. 2009. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 126:220–232.
- Waszak P, Alphonse R, Vadivel A, Ionescu L, Eaton F, Thébaud B. 2012. Preconditioning enhances the paracrine effect of mesenchymal stem cells in preventing oxygen-induced neonatal lung injury in rats. Stem Cells Dev. 21:2789–2797.
- Weil BR, Manukyan MC, Herrmann JL, Abarbanell AM, Poynter JA, Wang Y, et al. 2011. The immunomodulatory properties of mesenchymal stem cells: implications for surgical disease. J Surg Res. 167:78–86.
- Werr J, Xie X, Hedqvist P, Ruoslahti E, Lindbom L. 1998. beta1 Integrins are critically involved in neutrophil locomotion in extravascular tissue in vivo. J Exp Med. 187:2091–2096.
- Woodside DG, Kram RM, Mitchell JS, Belsom T, Billard MJ, McIntyre BW, et al. 2006. Contrasting roles for domain 4 of VCAM-1 in the regulation of cell adhesion and soluble VCAM-1 binding to integrin alpha4beta1. J Immunol. 176:5041–5049.
- Xu G, Zhang Y, Zhang L, Ren G, Shi Y. 2007. The role of IL-6 in inhibition of lymphocyte apoptosis by mesenchymal stem cells. Biochem Biophys Res Commun. 361:745–750.
- Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, et al. 2010. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transpl. 19:667–679.
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. 2005. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 106:1755–1761.
- Zhang W, Ge W, Li C, You S, Liao L, Han Q, et al. 2004. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 13:263–271.