Abstract
Since so many years ago, tissue damages that are caused owing to various reasons attract scientists’ attention to find a practical way to treat. In this regard, many studies were conducted. Nano scientists also suggested some ways and the newest one is called tissue engineering. They use biodegradable polymers in order to replace damaged structures in tissues to make it practical. Biodegradable polymers are dominant scaffolding materials in tissue engineering field. In this review, we explained about biodegradable polymers and their application as scaffolds.
Introduction
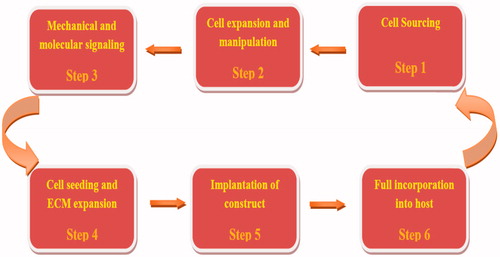
Tissue engineering is an interdisciplinary science that applies chemistry, material science, engineering, and medicine aiming to repair and replace tissues and organs (Cui et al. Citation2010, Walmsley et al. Citation2015). Tissue engineering strategies are classified into three main components containing scaffold, cells (differentiated or undifferentiated), and biological signaling molecules such as growth factors (GFs) (Pereira et al. Citation2011). The overall regeneration strategy based on the principles is shown in . Scaffolds are three-dimensional (3D) structure that can be produced by synthetic polymers, natural polymers and purely biological molecules, such as collagen, elastin, hyaluronic acid, and other extracellular matrix (ECM) molecules (Bačáková et al. Citation2014). The scaffold must be able to mime the structure and biological function of natural extracellular matrix (ECM) in terms of both chemical composition and physical structure (Zhou and Lee Citation2011). The extracellular matrix (ECM) is a various composition of proteoglycans, proteins, and signaling molecules. ECM is originally known for its role in providing structural support to cells and as a location for cell migration (Owen and Shoichet Citation2010). Appropriate scaffolds for tissue engineering applications should be biodegradable, biocompatible, nontoxic, nonmutagenic, and nonimmunogenic. Furthermore, they should be able to provide appropriate mechanical support and show favorable surface properties, such as helping adhesion, proliferation and differentiation of cells (Bačáková et al. Citation2014, Zhou and Lee Citation2011). Polymeric scaffolds play a main role in tissue engineering through cell seeding, proliferation, and new tissue formation in three dimensions. These scaffolds have shown great ability in the research of tissue engineering (Sakai et al. Citation2013). Biodegradable polymers due to reducing inflammatory reactions, nontoxic and degradation by enzymes in the body have many applications in medicine and pharmacy. Biodegradable polymers are generally classified into two types, namely synthetic polymers and natural polymers (Liu et al. Citation2012). Synthetic polymers are compatible with body, biodegradable, and absorbable. These polymers are easily changed into different 3D matrix structure. Natural polymers are metabolized into metabolites that could be used or is easily removed by kidney clearance (Tian et al. Citation2012). Natural polymers are originated from both tissues like collagen and plants like lactic acid (Abbasi et al. Citation2016).
Synthetic polymers
Synthetic polymers can be obtained by polymerization of monomers. Porous scaffolds fabricated from biocompatible and biodegradable polymers play vital roles in tissue engineering and regenerative medicine. The development of tissue engineering, tissue induction, and other types of regenerative medicine depend strongly upon the techniques of 3D porous scaffolds composed of organic and inorganic substrates, very frequently, biocompatible and biodegradable polymers. Synthetic polymers, such as polyglycolic acid, polylactic acid, polycaprolactone, poly (N-isopropylacrylamide), and their copolymers have been used in tissue engineering science. Synthetic polymers have acceptable processing flexibility and no immunological concerns compared with natural ECM proteins (Liu et al. Citation2012). Polylactic acid (PLA) and its copolymers, such as polylactide-co-glycolide (PLGA), PLGA–PEG. The biodegradable polyester family has been regarded as one of the few synthetic biodegradable polymers with controllable biodegradability, excellent biocompatibility, and high safety. Some properties of synthetic biodegradable polymers are shown in (Eatemadi et al. Citation2016).
Table 1. Properties of synthetic biodegradable polymers.
Polyglycolic acid (PGA)
Polyglycolic acid/or polyglycolide (PGA) is one of biodegradable and biocompatible aliphatic polyesters, that is, widely used in medical applications. PGA can be prepared starting from glycolic acid by ring-opening polymerization (Ikada and Tsuji Citation2000, Middleton and Tipton Citation2000). Common role of PGA as a biodegradable structure material has led to its assessment in other biomedical fields. Tissue engineering scaffolds, which are made of PGA, have been used in medical applications. Many studies in the field of tissue engineering are done using the PGA nanofibers (Boland et al. Citation2001). Kobayashi et al. prepared the nanocomposite combined of PGA and collagen. Their results of the animal models showed that the PGA-collagen nanocomposites were completely occupied and vascularized within 5 days after the implantation (Kobayashi et al. Citation2013). Patrascu et al. produced polyglycolic acid-hyaluronan (PGA-HA) scaffolds and analyze chondrogenic potential of freeze-dried (PGA-HA) implants preloaded with mesenchymal stem cells (MSCs) in vitro and in a rabbit articular cartilage defect model. Results demonstrated that MSC-loaded PGA-HA scaffolds have chondrogenic stimulation and are a good option for stem cell-mediated cartilage renewal (Patrascu et al. Citation2013). In studies based on tissue engineering, it can be concluded that using PGA polymers could be suitable scaffold for the regeneration of cartilage and blood vessels. Kobayashi et al. combined PGA and collagen to produce nanocomposite as scaffolds to be used in inducing vascularization, but another study is on cartilage regeneration. Patrascu et al. used polyglycolic acid and hyaluronan to prepare scaffolds-stimulating cartilage. Mesenchymal stem cells were cultured on the scaffold, and the results showed that the mesenchymal stem cell growth and differentiation are performed well enough so that mentioned scaffold is suitable for cartilage regeneration.
Polylactic acid
Polylactic acid or polylactide (PLA) is a biodegradable, bioadsorbable, thermoplastic aliphatic polyester, that is, derived from renewable resources. Lactic acid has two optical isomers, L- and D-lactic acid. PLA can be prepared from lactide by ring-opening polymerization (Middleton and Tipton Citation2000). PLA is used as medical implants in the form of screws, pins, rods, orthopedic device, and as a mesh (Lasprilla et al. Citation2012). One of the medical applications of polylactic acid in Chang’s study was conducted. In this study, polylactic acid has been used as a semipermeable microcapsule containing enzymes, hormones, vaccines, and other biological products. The reasons for using polylactic acid as semipermeable microcapsules are its biodegradability and also it produced nontoxic metabolite in the body after destroyed (Chang Citation1976). PLA is also used as a biodegradable and biocompatible material in tissue engineering science. Lin et al. produced hydroxyapatite (HA) mineralized on chitosan (CS)-coated poly (lactic acid) (PLA) nanofibers for bone regeneration. Their results show that this composite has similar structural, combination, and biological functions of natural bone and can help as a good choice for bone regeneration (Lin et al. Citation2014). In another study in the field of tissue engineering, Hao-Yang Mi et al. prepared composed thermoplastic polyurethane (TPU) and polylactic acid (PLA) tissue engineering scaffolds at different ratios. PLA was diffused as spheres into the TPU matrix. Their results show that the nanocomposite scaffolds PLA/TPU with surface roughness, biocompatibility, and mechanical properties that have the possible to be used as synthetic scaffolds in tissue engineering science (Mi et al. Citation2013). Based on these studies, it can be concluded that polylactic acid can be used as scaffolds for bone regeneration and other tissues. As noted above, Lin et al. used lactic acid to produce nanofibers containing hydroxyapatite, which is useful for bone mineralization. The nanofibers can be used as a scaffold for the growth and differentiation of osteoblast. These scaffolds can be a good choice for bone regeneration. But Hao-Yang Mi et al. combined polylactic acid and polyurethane to design scaffold that has good features such as surface roughness and being tunable. This scaffold with characteristics such as roughness and being tunable is a suitable candidate for soft and hard tissue regeneration (Ebrahimi et al. Citation2016).
Polycaprolactone
Polycaprolactone (PCL) is a biocompatible, bioadsorbable, and biodegradable polyester. PCL is synthesized by ring-opening polymerization of ɛ-caprolactone using a catalyst (SnO2) and heat (Middleton and Tipton Citation2000). PCL is used as medical implant, dental splints, and targeted drug delivery. PCL is also used in tissue engineering. Zheng R et al. produced electrospun membranes with different GT/PCL ratios. Results show that three kind of membranes with various GT/PCL ratios exhibited biocompatibility with chondrocytes. They also demonstrated that the high PCL content was unfavorable for 3D cartilage regeneration. It can be concluded from their studies that electrospun GT/PCL is a good candidate for cartilage and other tissue regenerations (Zheng et al. Citation2014). Uma Maheshwari et al. (Citation2014) produced a polymer–ceramic bilayer nanocomposite scaffold based on electrospun polycaprolactone (PCL)/polyvinyl alcohol (PVA) bilayer nanofibers mixed with hydroxyapatite nanoparticles (HAp) for bone regeneration. Results demonstrated that (PVA/HAp/PCL) nanofibers are biocompatible scaffolds for bone tissue engineering application (Uma Maheshwari et al. Citation2014). Nanofibers are prepared from polycaprolactone in combination with other polymers provide suitable scaffolds for tissue engineering. Recent studies show that polycaprolactone is a biocompatible scaffold to be used in regeneration of bone and cartilage.
Poly (lactic-co-glycolic acid)
Poly (lactic-co-glycolic acid) or PLGA is a biodegradable and biocompatible copolymer, which is used in medical application, therapeutic tools, and drug delivery systems. PLGA is synthesized by ring-opening copolymerization of two different monomers of glycolic acid and lactic acid (Middleton and Tipton Citation2000). PLGA is used in tissue engineering. Junmin Qian et al. prepared poly (dl-lactic-coglycolic acid)/nano-hydroxyapatite (PLGA/nHA) scaffolds. Results showed that the combination of nHA into PLGA reduced the degree of crystallinity of PLGA and significantly enhanced the compactive modulus of biomorphic scaffolds. It can be concluded from their studies that the biomorphic PLGA/nHA composite scaffolds are useful for bone tissue engineering (Qian et al. Citation2014). Scaffolds made of polylactic-containing hydroxyapatite can stimulate osteoblasts, which is important for bone regeneration.
Poly (N-isopropylacrylamide)
Poly(N-isopropylacrylamide) (PNIPAAm) is a thermosensitive polymer. It is synthesized by free-radical polymerization from N-isopropylacrylamide monomers in the presence of the initiator (Schild Citation1992). Due to unique physical and chemical properties, it has many applications, such as biosensors, tissue engineering, and drug delivery. In studies, PNIPAAm is used to regenerate damaged tissue. For example, Sá-Lima H and et al. prepared scaffolds based on PNIPAAm polymers and studied capacity of poly (N-isopropylacrylamide)-g-methylcellulose (PNIPAAmg-MC) thermoreversible hydrogel as a 3D support for the regeneration of articular cartilage. They proved the feasibility of using PNIPAAm-g-MC thermoresponsive hydrogel as a 3D scaffold for cartilage regeneration by minimal-invasive approaches and also demonstrated an increase in synthesis of glycosoaminoglycans during culture time (Sá-Lima et al. Citation2011). It can be obtained that use of (PNIPAAm-g-MC) as thermosensitive scaffolds can be effective in regenerating cartilage and combination of PNIPAAm with other biological materials can be a suitable scaffold for tissue engineering applications.
Poly ((DL-lactic acid-co-glycolic acid)-g-ethylene glycol) (PLGA-g-PEG))
Poly((DL-lactic acid-co-glycolic acid)-g-ethylene glycol) (PLGA-g-PEG) is a copolymer of Poly (DL-lactic acid-co-glycolic acid) and poly (ethylene glycol). PLGA-g-PEG that is another biodegradable and bioadsorbable polymer used in tissue engineering and drug delivery systems. Sidney et al. (Citation2014) prepared porous scaffolds composed of poly (DL-lactic acid-co-glycolic acid) (PLGA) and polyethylene glycol (PEG) with and without the anti-inflammatory drug named diclofenac sodium for bone regeneration with the ability to release anti-inflammatory drugs. Produced scaffold was loaded with the various concentration of diclofenac sodium. The results of these studies suggest the possible use of PLGA/PEG scaffolds for the localized delivery of anti-inflammatory drugs in bone regeneration (Sidney et al. Citation2014).
Poly(caprolacton/ethylene glycol) copolymer
PCL–PEG copolymer is another biodegradable and biocompatible polymer, which is synthesized by ring-opening polymerization in the presence of catalyzer. PCL–PEG is used in tissue engineering to regenerate damaged tissue. In a recent study conducted by Niu et al. (Citation2014) scaffolds were prepared from copolymers of poly (ɛ-caprolactone) (PCL) and polyethylene glycol (PEG) for peripheral nerve regeneration. In this study, Niu et al. (Citation2014) prepared scaffolds of block polymers based on poly (ɛ-caprolactone) and polyethylene glycol (PEG) with high surface area porosity for cell adhesion and cell differentiation that would be a suitable environment for the regeneration of damaged tissue. The results of these studies on the peripheral nerves in animal models suggest that nerve guide scaffolds of poly (ɛ caprolactone) and poly(ethylene glycol) (PEG) have better peripheral nerve regeneration (Niu et al. Citation2014).
Poly(caprolacton/lactide) copolymer
PCL–PLA copolymer is another biodegradable, biocompatible, and bioadsorbable polymer that has many applications in tissue engineering. PCL–PLA copolymer is synthesized by ring-opening polymerization. In recent years, PCL–PLA copolymer nanofiber has many applications in the regeneration of damaged tissue and drug delivery systems based on nanofiber. A study by Karami et al (Citation2013) has been performed that they used PCL/PLA hybrid nanofibers containing herbal drug for the treatment of wounds. In this study, Karami et al. prepared PCL/PLA hybrid nanofibers containing the herbal drug Thymol (Dorman and Deans Citation2000). Thymol has antimicrobial (Dorman and Deans Citation2000), antibacterial effects (Zarrini et al. Citation2010), and antifungal activity (Numpaque et al. Citation2011). Because of these properties, it can be used as a wound-healing agent. The results of these studies indicate that the electrospun PCL/PLA hybrid nanofibers containing Thymol had led to notable wound healing (Karami et al. Citation2013).
Natural polymers
Natural polymers are polymers produced by biological systems, such as microorganisms, plants, and animals. Natural polymers have many uses, such as an adhesive bandage, absorbent, prepared cosmetics, drug delivery, and medical scaffolds (Malafaya et al. Citation2007, Shanmugam et al. Citation2005). Natural polymers have a number of advantages and disadvantages. Similar to the host tissue, ability to communicate with the biological systems, metabolic compatibility, being nontoxic and low inflammatory reactions, ability to degrade by enzyme, use of heir degradation products in cellular metabolism are some of the advantages. On the other hand, due to temperature sensitivity, natural polymers are destroyed before they reach the melting point, and its complex structure makes it difficult to process. The possibility of disease transmission to human from other species due to preparation of a variety of natural polymers by plant and animal resources are known disadvantages of these polymers (Sonia and Sharma Citation2012). The following two types of natural polymers are available: (1) polysaccharides such as chitin, chitosan, and alginate; (2) proteins such as collagen and gelatin (Leach et al. Citation2003).
Polysaccharide-originated polymers
Chitin
Chitin exists in animal skeletal system, the lens of the eye, tendons, the outer layer of arthropods and insects and arachnids and crustaceans body (crab, shrimp, and lobster) and the internal parts of body in some animals, such as mollusks and plants, as well as in the cell wall of fungus (Malafaya et al. Citation2007, Ravi Kumar Citation2000). Chitin and chitosan (derivative of chitin) have many chemical and physical properties, such as high strength, biodegradability, and nontoxic. Chitin has a white appearance. Chitin has many applications in tissue engineering. Chitin can be used for wound healing because of biodegradability (Arun Kumar et al. Citation2015, Krajewska Citation2004).
Chitosan
Chitosan is a derivative of Glocan which is produced by repeated monomers of chitin. Boiling chitin in potassium hydroxide results in chitosan. Indeed, it is the deacetylated chitin and it is the most natural aminopolysaccharide (Malafaya et al. Citation2007). Chitosan has features, such as biocompatibility, being nontoxic and biodegradability. Chitosan has high blood compatibility and macrophages are less active (Khor and Lim Citation2003). It has many applications in the field of medical and pharmaceutical sciences (Jing et al. Citation2015, Şenel and McClure Citation2004). It is also widely used in tissue engineering. Chitosan without the use of toxic solution can be prepared in injectable form. This material is made as porous scaffold, hydrogel, and microspheres (Drury and Mooney Citation2003).
Alginate
Alginate is polysaccharide linear, homogeneous, and natural.Alginate mainly is prepared by dark and brown algae (George and Abraham Citation2006, Shanmugam et al. Citation2005). Because of its natural structure, it has many applications in the medical field, such as detoxifying in blood to absorb toxic metals (De Carvalho et al. Citation2004), assisting in the processing of many drugs (Patel et al. Citation2006), as molding materials in dental prosthetics (Rubel Citation2007), stabilizing cells and cell encapsulation (Smidsrød and Skja˚k-Br˦k Citation1990), healing of burns and reducing pain (Kneafsey et al. Citation1996), and tissue Engineering (Lee and Mooney Citation2001). Alginate scaffolds containing stem cells have been used to treat many diseases (Zhao et al. Citation2010).
Protein-originated polymers
Collagen
Collagen is a protein found in the extracellular matrix of animals. Collagen molecule is composed of three polypeptide chains. Three separate polypeptide chains called alpha, which is called tropocollagen involved in the collagen formation (Myllyharju Citation2003). Collagen of the skin, tendons, cartilage, and bone of animals are extracted. Collagen has features such as being biodegradable and easily destroyed by enzymes in the body, biocompatibility, low stimulation of the immune system and high cell binding (Lynn et al. Citation2004, Malafaya et al. Citation2007). Collagen has many applications in medical science including delivery systems, burn and wound healing (Doillon and Silver Citation1986), and tissue engineering (Glowacki and Mizuno Citation2008). Collagen is widely applied in the preparation of tissue engineering scaffolds.
Gelatin
Gelatin is solid substance, that is, translucent and colorless obtained from the hydrolysis of collagen inside animals' skin and bones (Malafaya et al. Citation2007, Shanmugam et al. Citation2005). Gelatin forms colloid and gel in water. Many chemical properties of gelatin are like collagen and mechanical properties of collagen are temperature dependent (Ross-Murphy Citation1992). Gelatin also has many applications in the field of medical sciences such as drug delivery systems (Olsen et al. Citation2003), shells of pharmaceutical capsules (Digenis et al. Citation1994), cell culture (Paguirigan and Beebe Citation2006), and preparation of tissue engineering scaffolds. Gelatin is widely applied in the field of tissue engineering.
Nanofiber scaffold preparation methods
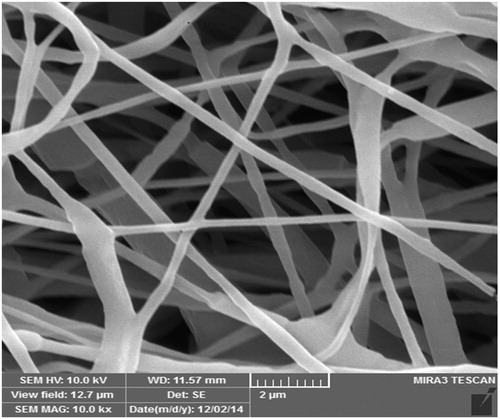
To prepare a suitable scaffold for tissue engineering, a variety of methods can be used. Scaffolds used in tissue engineering have a high porosity, biodegradable, and nontoxic, and it can also be able to increase the growth and cell differentiation. Methods that are used for preparing scaffolds include electrospinning method, solvent casting, freeze drying and gas foaming. Electrospinning technique is most commonly used techniques, which are widely used in the preparing scaffold in nanotechnology. Electrospinning as a simple and inexpensive method to produce very thin fibers from polymer solution. shows the PCL/PLA nanofiber produced by electrospinning device.
Synthesis method of nanofibers by electrospinning
Nanofibers are fibers with a diameter of less than 100 nm, which can be used as scaffolds in tissue engineering (Li and Xia Citation2004). Polymeric nanofibers have dramatically been considered due to their properties in nanoscale dimensions. Electrospinning is one of the most common methods for producing nanofibers. Electrospinning is a method for producing polymer fibers in nm diameters. In this way, the liquid solution and the polymer solution can be used (Huang et al. Citation2003). Electrospinning method for producing nanofibers consists of three components: a high voltage supply, small diameter capillary needle, and collector. In this process, the high voltage causes the jet of polymer solution. Before reaching the collector, jet solution is vapor or solid and the small fibers are collected. The electric field is applied through the electrode into the solution and collector. By increasing field intensity, fluid is drawn in hemispherical shape from tip of syringe and tends to form a cone which is known as the Taylor cone. With further increase in the electric field due to reaching the critical value, a jet is formed at the tip of the Taylor cone. In the low electrical potential, electrostatic repulsive force is balanced with the surface tension force. But, in the high electrical potential, electrostatic force overcomes the surface tension of the liquid and causing the formation of the jet. The jet is moving a certain distance in a straight way and then it is curved and moves spiral path through it. Jets instability and tension in the spiral path lead to thinning and evaporation of the solvent to produce nanofibers that finally reach collector (Agarwal et al. Citation2008, Pham et al. Citation2006). shows a schematic of the electrospinning device (Fallahzadeh et al. Citation2010).
Solvent casting
Solvent casting property for the scaffolds preparation is extremely simple and easy. It is totally based on leading the evaporation of some solvent in order to form scaffolds by one of the two routes. One method is to the dip the mold into polymeric solution and to permit enough time to draw off the solution. As a result, a layer of polymeric membrane is shaped. Other method is to add the polymeric solution in to a mold and provided the enough time to evaporate the solvent that make a layer of polymeric membrane, which adhere to the mold (Mikos and Temenoff Citation2000). One of the major disadvantages of this technique is the toxic solvent denatures the protein and may affect other solvent. To overcome these problems, scaffolds are completely dried by vacuum procedure to eliminate toxic solvent. In solvent casting method, a polymer is dissolved in an organic solvent, particles, mainly salts, with specific dimensions are then added to the solution, The mixture is shaped into its final geometry. For example, it can be cast onto a glass plate to produce a membrane or in a 3D mold to produce a scaffold. When the solvent evaporates, it creates a structure of composite material consisting of the particles together with the polymer and the composite material is then placed in a bath which dissolves the particles, leaving behind a porous structure.
Freeze drying
Freeze-drying technique is used for the production of porous scaffold. This technique is based on leading the principle of sublimation. Polymer is first dissolved in a solvent to form a solution of preferred concentration. The solution is frozen and solvent is removed by lyophilization under the high vacuum that fabricates the scaffold with high porosity and inter connectivity (Haugh et al. Citation2009). The pore size can be controlled by the freezing rate and pH. A fast freezing rate produces smaller pores. Controlled solidification in a single direction has been used to create a homogeneous 3D pore structure. Main advantage of this technique is that it does not require high temperature (von Heimburg et al. Citation2001).
Gas foaming
This technique uses high-pressure carbon dioxide gas for the fabrication of highly porous scaffolds. The porosity and porous structure of the scaffold depend upon the amount of gas dissolvent in the polymer. This process involves exposing highly porous polymer with carbon dioxide at high pressure to saturate the polymer with gas (Sachlos and Czernuszka Citation2003). Under this condition, dissolved carbon dioxide becomes unstable and will phase separates from the polymer. The carbon dioxide molecule becomes cluster to minimize the free energy, as a result pore nucleation is created. These pores cause the significant expansion of polymeric volume and decrease in polymeric density. A 3D scaffold is formed after completion of foaming process. The gas-foaming scaffold fabrication techniques do not require the utilization of organic solvent and high temperature.
In vivo and in vitro application of nanofibers
Nanofibers are widely used in in vitro and in vivo application. In a study that was conducted by Zong et al. (Citation2015), nanofibers have been used as drug delivery systems to cancer cells. In this study (Zong et al. Citation2015), cisplatin loaded on nanofibers poly(ethylene oxide)/polylactide and its effect on cervical cancer cells examined. The experimental results showed that the antitumor effect and safety are in balance and this nanofibers are a good candidate for in vivo drug release in cancer cells (Zong et al. Citation2015). Another study on the use of nanofibers in bone conductivity investigated. The study was conducted by Ardeshirylajimi et al. (Citation2015) prepared nanofibers and coated with bioactive glass.They in vitro studies were performed on MG-63 cells and results showed that nanofibers polyethersulfone coated with bioactive glass increases bone growth markers and the scaffolds is biocompatible with the media (Ardeshirylajimi et al. Citation2015). Damaged nerve regeneration is important for nerve function. In a study conducted by Su and Shih (Citation2015) prepared polycaprolactone nanofiber with or without carbon nanotubes by electrospinning method. Studies for the growth and differentiation of nerve were performed on PC12 cell line by electric stimulation. The results showed that when carbon nanotubes are used in the scaffold structure differentiation of nerve cells in the scaffold increases. It can be concluded that carbon nanotubes because of their high electrical conductivity led to the rapid growth of nerve cells in the scaffold (Su and Shih Citation2015). These are just a sample of in vivo and in vitro studies was nanofibers. These studies can be understood that nanofibers due to mimic the extracellular matrix can be a good candidate for the reconstruction of damaged tissue in medical applications.
Conclusion
In brief, the main goal of tissue engineering is to repair damaged tissues. In this regard, we need some physical structures with possibility cell attachment, cell migration, cell culture, cell differentiation and finally to be replaced in tissues. These structures are produced by biodegradable polymers that play external matrix role in bioengineering. Different methods are used to make scaffolds, such as solvent casting, gas foaming, freeze drying, and electrospinning. Electrospinning is the most common method due to resulting in nanofibers in different sizes.
Funding information
This work is funded by a 2015 grant Drug Applied Research Center, Tabriz University of Medical Sciences.
Disclosure statement
The authors report no declaration of interest. The authors alone are responsible for the content and writing of the article.
References
- Abbasi E, Akbarzadeh A, Kouhi M, Milani M. 2016. Graphene: Synthesis, bio-applications, and properties. Artif Cells Nanomed Biotechnol. 44:150–156.
- Agarwal S, Wendorff JH, Greiner A. 2008. Use of electrospinning technique for biomedical applications. Polymer. 49:5603–5621.
- Ardeshirylajimi A, Farhadian S, Adegani JF, Mirzael S, Zomorrod SM, Langroudi L, et al. 2015. Enhanced osteoconductivity of polyethersulphone nanofibres loaded with bioactive glass nanoparticles in in vitro and in vivo models. Cell Prolif. 48:455–464.
- Arun Kumar R, Sivashanmugam A, Deepthi S, Iseki S, Chennazhi KP, Nair SV, Jayakumar R. 2015. Injectable chitin-poly (ɛ-caprolactone)/nano hydroxyapatite composite microgels prepared by simple regeneration technique for bone tissue engineering. ACS Appl Mater Interfaces. 7:9399–9409.
- Bačáková L, Novotná K, Pařízek M. 2014. Polysaccharides as cell carriers for tissue engineering: the use of cellulose in vascular wall reconstruction. Physiol Res. 63:29–47.
- Boland ED, Wnek GE, Simpson DG, Pawlowski KJ, Bowlin GL. 2001. Tailoring tissue engineering scaffolds using electrostatic processing techniques: a study of poly (glycolic acid) electrospinning. J Macromol Sci Part A. 38:1231–1243.
- Chang T. 1976. Biodegradable semipermeable microcapsules containing enzymes, hormones, vaccines, and other biologicals. J Bioeng. 1:25–32.
- Cui W, Zhou Y, Chang J. 2010. Electrospun nanofibrous materials for tissue engineering and drug delivery. Sci Technol Adv Mater. 11:014108.
- De Carvalho W, Canilha L, Mussatto SI, Dragone G, Morales MLV, Solenzal AIN. 2004. Detoxification of sugarcane bagasse hemicellulosic hydrolysate with ion-exchange resins for xylitol production by calcium alginate-entrapped cells. J Chem Technol Biotechnol. 79:863–868.
- Digenis GA, Gold TB, Shah VP. 1994. Cross-linking of gelatin capsules and its relevance to their in vitro-in vivo performance. J Pharm Sci. 83:915–921.
- Doillon CJ, Silver FH. 1986. Collagen-based wound dressing: effects of hyaluronic acid and fibronectin on wound healing. Biomaterials. 7:3–8.
- Dorman H, Deans S. 2000. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 88:308–316.
- Drury JL, Mooney DJ. 2003. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 24:4337–4351.
- Eatemadi A, Daraee H, Zarghami N, Melat Yar H, Akbarzadeh A. 2016. Nanofiber: Synthesis and biomedical applications. Artif Cells Nanomed Biotechnol 44:111–121.
- Ebrahimi E, Akbarzadeh A, Abbasi E, Khandaghi AA, Abasalizadeh F, Davaran S. 2016. Novel drug delivery system based on doxorubicin-encapsulated magnetic nanoparticles modified with PLGA-PEG1000 copolymer. Artif Cells Nanomed Biotechnol 44:290–297.
- Fallahzadeh S, Bahrami H, Akbarzadeh A, Tayarani M. 2010. High-Isolation Dual-Frequency Operation Patch Antenna Using Spiral Defected Microstrip Structure. IEEE Antenn Wireless Propag Lett 9:122–124.
- George M, Abraham TE. 2006. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan – a review. J Control Release. 114:1–14.
- Glowacki J, Mizuno S. 2008. Collagen scaffolds for tissue engineering. Biopolymers. 89:338–344.
- Haugh MG, Murphy CM, O'Brien FJ. 2009. Novel freeze-drying methods to produce a range of collagen–glycosaminoglycan scaffolds with tailored mean pore sizes. Tissue Eng Part C Methods. 16:887–894.
- Huang ZM, Zhang YZ, Kotaki M, Ramakrishna S. 2003. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol. 63:2223–2253.
- Ikada Y, Tsuji H. 2000. Biodegradable polyesters for medical and ecological applications. Macromol. Rapid Commun. 21:117–132.
- Jing X, Mi HY, Peng J, Peng XF, Turng LS. 2015. Electrospun aligned poly(propylene carbonate) microfibers with chitosan nanofibers as tissue engineering scaffolds. Carbohydr Polym. 117:941–949.
- Karami Z, Rezaeian I, Zahedi P, Abdollahi M. 2013. Preparation and performance evaluations of electrospun poly (ɛ-caprolactone), poly (lactic acid), and their hybrid (50/50) nanofibrous mats containing thymol as an herbal drug for effective wound healing. J Appl Polymer Sci. 129:756–766.
- Khor E, Lim LY. 2003. Implantable applications of chitin and chitosan. Biomaterials. 24:2339–2349.
- Kneafsey B, O'Shaughnessy M, Condon KC. 1996. The use of calcium alginate dressings in deep hand burns. Burns. 22:40–43.
- Kobayashi H, Terada D, Yokoyama Y, Moon DW, Yasuda Y, Koyama H, Takato T. 2013. Vascular-inducing poly (glycolic acid)-collagen nanocomposite-fiber scaffold. J Biomed Nanotechnol. 9:1318–1326.
- Krajewska B. 2004. Application of chitin- and chitosan-based materials for enzyme immobilizations: a review. Enzyme Microb Technol. 35:126–139.
- Lasprilla AJR, Martinez GA, Lunelli BH, Jardini AL, Filho RM. 2012. Poly-lactic acid synthesis for application in biomedical devices – a review. Biotechnol Adv. 30:321–328.
- Leach JB, Bivens KA, Patrick CW Jr, Schmidt CE. 2003. Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds. Biotechnol Bioeng. 82:578–589.
- Lee KY, Mooney DJ. 2001. Hydrogels for tissue engineering. Chem Rev. 101:1869–1880.
- Li D, Xia Y. 2004. Electrospinning of nanofibers: reinventing the wheel? Adv Mater. 16:1151–1170.
- Lin CC, Fu SJ, Lin YC, Yang IK, Gu Y. 2014. Chitosan-coated electrospun PLA fibers for rapid mineralization of calcium phosphate. Int J Biol Macromol. 68:39–47.
- Liu X, Holzwarth JM, Ma PX. 2012. Functionalized synthetic biodegradable polymer scaffolds for tissue engineering. Macromol Biosci. 12:911–919.
- Lynn A, Yannas I, Bonfield W. 2004. Antigenicity and immunogenicity of collagen. J Biomed Mater Res Part B Appl Biomater. 71:343–354.
- Malafaya PB, Silva GA, Reis RL. 2007. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. origin as and for and delivery tissue applications. Adv Drug Deliv Rev. 59:207–233.
- Mi HY, Salick MR, Jing X, Jacques BR, Crone WC, Peng XF, Turng LS. 2013. Characterization of thermoplastic polyurethane/polylactic acid (TPU/PLA) tissue engineering scaffolds fabricated by microcellular injection molding. Mater Sci Eng C Mater Biol Appl. 33:4767–4776.
- Middleton JC, Tipton AJ. 2000. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 21:2335–2346.
- Mikos AG, Temenoff JS. 2000. Formation of highly porous biodegradable scaffolds for tissue engineering. Electron J Biotechnol. 3:23–24.
- Myllyharju J. 2003. Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol. 22:15–24.
- Niu Y, Chen KC, He T, Yu W, Huang S, Xu K. 2014. Scaffolds from block polyurethanes based on poly(ɛ-caprolactone) (PCL) and poly(ethylene glycol) (PEG) for peripheral nerve regeneration. Biomaterials. 35:4266–4277.
- Numpaque MA, Oviedo LA, Gil JH, Garcia CM, Durango DL. 2011. Thymol and carvacrol: biotransformation and antifungal activity against the plant pathogenic fungi Colletotrichum acutatum and Botryodiplodia theobromae. Trop Plant Pathol. 36:3–13.
- Olsen D, Yang C, Bodo M, Chang R, Leigh S, Baez J, et al. 2003. Recombinant collagen and gelatin for drug delivery. Adv Drug Deliv Rev. 55:1547–1567.
- Owen SC, Shoichet MS. 2010. Design of three-dimensional biomimetic scaffolds – biomimetic. J Biomed Mater Res A Part A. 94:1321–1331.
- Paguirigan A, Beebe D. 2006. Gelatin based microfluidic devices for cell culture. Lab Chip. 6:407–413.
- Patel YL, Sher P, Pawar AP. 2006. The effect of drug concentration and curing time on processing and properties of calcium alginate beads containing metronidazole by response surface methodology. AAPS PharmSciTech. 7:E24–E30.
- Patrascu JM, Krüger JP, Böss HG, Ketzmar AK, Freymann U, Sittinger M, et al. 2013. Polyglycolic acid-hyaluronan scaffolds loaded with bone marrow-derived mesenchymal stem cells show chondrogenic differentiation in vitro and cartilage repair in the rabbit model. J Biomed Mater Res B Appl Biomater. 101:1310–1320.
- Pereira H, Frias AM, Oliveira JM, Esprequeira-Mendes J, Reis RL. 2011. Tissue engineering and regenerative medicine strategies in meniscus lesions. Arthroscopy. 27:1706–1719.
- Pham QP, Sharma U, Mikos AG. 2006. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 12:1197–1211.
- Qian J, Xu W, Yong X, Zhang W. 2014. Fabrication and in vitro biocompatibility of biomorphic PLGA/nHA composite scaffolds for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 36:95–101.
- Ravi Kumar MNV. 2000. A review of chitin and chitosan applications. React Funct Polym. 46:1–27.
- Ross-Murphy SB. 1992. Structure and rheology of gelatin gels: recent progress. Polymer. 33:2622–2627.
- Rubel BS. 2007. Impression materials: a comparative review of impression materials most commonly used in restorative dentistry. Dent Clin North Am. 51:629–642.
- Sachlos E, Czernuszka J. 2003. Making tissue engineering scaffolds work. Review: the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur Cell Mater. 5:39–40.
- Sakai R, John B, Okamoto M, Seppala JV, Vaithilingam J, Hussein H, Goodridge R. 2013. Fabrication of polylactide-based biodegradable thermoset scaffolds for tissue engineering applications. Macromol Mater Eng. 298:45–52.
- Sá-Lima H, Tuzlakoglu K, Mano JF, Reis RL. 2011. Thermoresponsive poly (N-isopropylacrylamide)-g-methylcellulose hydrogel as a three-dimensional extracellular matrix for cartilage-engineered applications. J Biomed Mater Res A. 98:596–603.
- Schild HG. 1992. Poly(N-isopropylacrylamide): experiment, theory and application. Prog Polym Sci. 17:163–249.
- Şenel S, McClure SJ. 2004. Potential applications of chitosan in veterinary medicine. Adv Drug Deliv Rev. 56:1467–1480.
- Shanmugam S, Manavalan R, Venkappayya D, Sundramoorthy K, Mounnissamy VMS, Ayyappan T. 2005. Natural polymers and their applications. Natl Prod Radiance. 4:478–481.
- Sidney LE, Heathman TRJ, Britchford ER, Abed A, Rahman CV, Buttery LDK. 2014. Investigation of localized delivery of diclofenac sodium from poly(D, L-lactic acid-co-glycolic acid)/poly (ethylene glycol) scaffolds using an in vitro osteoblast inflammation model. Tissue Eng Part A. 21:362–373.
- Smidsrød O, Skja˚k-Br˦k G. 1990. Alginate as immobilization matrix for cells. Trends Biotechnol. 8:71–78.
- Sonia TA, Sharma CP. 2012. An overview of natural polymers for oral insulin delivery. Drug Discov Today. 17:784–792.
- Su WT, Shih YA. 2015. Nanofiber containing carbon nanotubes enhanced PC12 cell proliferation and neuritogenesis by electrical stimulation. Bio-Med Mater Eng. 26:189–195.
- Tian H, Tang Z, Zhuang X, Chen X, Jing X. 2012. Biodegradable synthetic polymers: preparation, functionalization and biomedical application. Prog Polym Sci. 37:237–280.
- Uma Maheshwari S, Samuel VK, Nagiah N. 2014. Fabrication and evaluation of (PVA/HAp/PCL) bilayer composites as potential scaffolds for bone tissue regeneration application. Ceram Int. 40:8469–8477.
- von Heimburg D, Zachariah S, Heschel I, Kuhling H, Schoof H, Hafemann B, Pallua N. 2001. Human preadipocytes seeded on freeze-dried collagen scaffolds investigated in vitro and in vivo. Biomaterials. 22:429–438.
- Walmsley GG, McArdle A, Tevlin R, Momeni A, Atashroo D, Hu MS, et al. 2015. Nanotechnology in bone tissue engineering. Nanomedicine. 11:1253–1263.
- Zarrini G, Delgosha ZB, Moghaddam KM, Shahverdi AR. 2010. Post-antibacterial effect of thymol. Pharm Biol. 48:633–636.
- Zhao L, Weir MD, Xu HHK. 2010. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials. 31:6502–6510.
- Zheng R, Duan H, Xue J, Liu Y, Feng B, Zhao S, et al. 2014. The influence of gelatin/PCL ratio and 3-D construct shape of electrospun membranes on cartilage regeneration. Biomaterials. 35:152–164.
- Zhou H, Lee J. 2011. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 7:2769–2781.
- Zong S, Wang X, Yang Y, Wu W, Li H, Ma Y, et al. 2015. The use of cisplatin-loaded mucoadhesive nanofibers for local chemotherapy of cervical cancers in mice. Eur J Pharm Biopharm. 93:127–135.