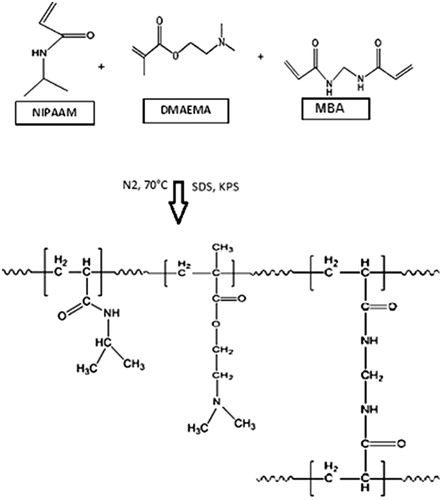
Abstract
In the present study, magnetic and thermo/pH-sensitive (multiresponsive) nanocomposites based on N-isopropylacrylamide (NIPAAM) were synthesized and characterized. Nanocomposites were synthesized by free radical emulsion polymerization of NIPAAM as thermosensitive monomer and N,N-dimethyl-aminoethyl methacrylate (DMAEMA) as pH-sensitive monomer in the presence of methylene-bis-acrylamide as cross-linking agent. Doxorubicin, an anti-cancer drug, was loaded into these nanocomposites via equilibrium swelling method. Thermo/pH-sensitive cross-linked poly (NIPAAM–DMAEMA)–Fe3O4 nanocomposites were characterized by Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), and vibrating sample magnetometer (VSM). The volume of the loaded drug and drug release amount was determined by UV measurements. The results showed that this thermo/pH-sensitive magnetic nanocomposite has a high drug-loading efficiency. Doxorubicin was released at 40 °C and pH 5.8 more than the 37 °C and pH 7.4.
Introduction
Doxorubicin (Adriamycin) is one of the most important cancer chemotherapeutic agents (Bristow et al. Citation1981). Doxorubicin has been used in many cancer treatments, including acute leukemia, lung, bladder, ovarian, and gastric cancers (Xu et al. Citation2013). It works via interfering with the growth of quickly growing cancer cells where it binds and intercalates into the DNA strand, therefore, inhibiting further DNA and RNA biosynthesis, finally causing cell death (DiMarco Citation1975, Goodman and Lee Citation1977, Mu and Feng Citation2001). However, it is considerably limited when compared to the strength of conventional intravenous chemotherapy demonstrations, not only in site non-specificity but also in additional serious side effects, especially cardiotoxicity (Tacar et al. Citation2013). Drug delivery systems such as polymeric nanocarriers (Minko et al. Citation2000, Zeighamian et al. Citation2015), liposomes (Ishida et al. Citation2001), and dendrimers (Lai et al. Citation2007) can intensify the antitumor efficiency and reduce toxicity of free doxorubicin (Hami et al. Citation2014). Among the several materials employed in controlled drug delivery systems, polymers have attracted more attention because of their variability compound and ease of design for the specific purpose (Rösler et al. Citation2012). The selected polymer must fulfill several requirements including biodegradability, biocompatibility, and ease of processing (Lin et al. Citation2005). Smart polymers are attractive candidates to the improvement of delivery systems (Davaran and Entezami Citation1997). These polymers can respond to many stimulants for example pH, temperature, magnetic field, chemical and biological stimulants and thus have an extensive range of applications such as sensors, drug delivery, gene delivery, and tissue engineering (Shimizu et al. Citation2010, Sima et al. Citation2014). The researches have been especially focused on the use of thermo and pH-sensitive polymers (Khodaverdi et al. Citation2010). Poly N-isopropylacrylamide (PNIPAAM) is one of the most widely used thermosensitive polymers (Jaiswal et al. Citation2014), PNIPAAM has a lower critical solution temperature (LCST) of 32 °C in water. Because of its reversible formation and splitting of hydrogen bonds between the amide groups and the encircling water molecules, this polymer tends to alternate from hydrophilic below the LCST to hydrophobic above the LCST (Ding et al. Citation1998). Below the LCST, PNIPAAM chains are solvable in water and the polymer is in a swollen phase, (Purushotham and Ramanujan Citation2010) when the outside temperature is raised it undergoes a sudden and abrupt shrinkage in volume above the LCST (Wang et al. Citation2008). However, one possible problem with using PNIPAAM is that its LCST, the temperature at which a phase transition occurs, is lower than body temperature (32 °C). To increase the LCST of PNIPAAM above body temperature, it has been co-polymerized with different monomers, for example acrylamide (AAm) (Hu et al. Citation2007) and poly(2-(N,N-dimethylamino)ethyl methacrylate) (PDMAEMA). PDMAEMA is a pH responsive cationic polymer containing a tertiary amine group (pKa 7.0) (Ebrahimi et al. Citation2016a, El-Masry et al. Citation2007, Jin-Hwan et al. Citation2016) that can be protonized at low pH (Zhang et al. Citation2004), and a thermosensitive polymer with an LCST of about 50 °C (Ward and Georgiou Citation2011). The LCST of P(DMAEMA) is powerfully reliant on pH and no LCST has been observed at low pH (Yuk et al. Citation1997). Therefore, P(DMAEMA) is frequently used only to formulate pH-sensitive materials (Brahim et al. Citation2003, Ebrahimi et al. Citation2016b). Combination of PNIPAM and PDMAEMA would propose thermal and pH double response, and excellent efficacy in the controlled drug delivery system (Zhang et al. Citation2010). Fe3O4 nanoparticles coated with multiresponsive polymers have been given great attention due to their several applications in the fields of biotechnology and medication (Gupta and Gupta Citation2005, Ito et al. Citation2005, Yong et al. Citation2008). These methods are capable of site-specific targeting and controlled and sustained drug release (Banerjee and Chen Citation2008, Yallapu et al. Citation2010) under magnetic fields (Deng et al. Citation2005, Satarkar and Hilt Citation2008a, Citation2008b, Wang et al. Citation2009). In this study, poly (NIPAAM–DMAEMA)–Fe3O4 nanocomposite was synthesized, and doxorubicin was encapsulated in this nanocomposite with the floating and swelling method (Davaran et al. Citation2014). The physicochemical properties of the nanocomposites and the release of drug at different temperatures and pHs were studied to disclose the relation between temperature/pH and drug release, which is very essential in planning a smart drug delivery system (Kurd et al. Citation2015).
Experimental
Materials
N-isopropyl acrylamide (NIPAAM) was purchased from Acros Organics (Morris, NJ), and recrystallized in n-hexane and dried under vacuum at 25 °C before use. N,N-dimethyl-aminoethyl methacrylate (DMAEMA) monomers, N-N-methylene bis acrylamide (MBA), and potassium per sulfate (KPS) were purchased from Merck (Darmstadt, Germany). Sodium dodecyl sulfate (SDS) was purchased from Sigma (St. Louis, MO). Fe3O4 nanoparticles were obtained from US Research Nanomaterials Inc. (Houston, TX). Doxorubicin hydrochloride was received from Sigma-Aldrich. Sodium monohydrogen phosphate and dihydrogen phosphate were obtained from Sigma-Aldrich Co. NaOH was obtained from Merck Chemical Co. (Germany). Nuclear magnetic resonance (NMR) spectroscope (Brucker DRX, Billerica, MA), scanning electron microscopy (SEM) measurements were conducted using LEO 1430VP, vibrating sample magnetometer (VSM) (Meghnatis Daghigh Kavir Co, Kashan, Iran). The drug loading capability and release behavior were determined using a UV-Vis 2550 spectrometer (Shimadzu, Kyoto, Japan). The infrared spectra of copolymers were recorded on a Perkin Elmer 983 IR spectrometer (Perkin Elmer, Boston, MA) at room temperature.
Preparation of (NIPAAM–DMAEMA) co-polymer
A copolymer of NIPAAM–DMAEMA was synthesized through free radical emulsion polymerization method. SDS was used as an emulsifying agent. NIPAAM (3.8 g), DMAEMA (0.37 g), cross-linking agent MBA (0.125 g, 3 wt%), and 0.125 g of SDS were dissolved in 230 ml of distilled water. The dissolved oxygen was removed by passing nitrogen gas for 30 min. After heating the solution to 70 °C, 0.16 g of KPS in 20 ml of water was added under vigorous stirring. The reaction became turbid and the reaction continued for 4 h at constant temperature in nitrogen atmosphere and was afterwards cooled to room temperature, and then freeze-dried.
Preparation of doxorubicin-loaded magnetic hydrogel nanocomposite
The doxorubicin-loaded magnetic hydrogel nanocomposite was prepared by dissolving the hydrogel in water in the presence of doxorubicin and Fe3O4. Ten milligrams of Fe3O4 nanoparticles and 50 mg of doxorubicin were dispersed in 5 ml of water and then were sonicated for 15 min. Then, 500 mg of freeze-dried poly (NIPAAM–DMAEMA), was dispersed in the solution and then sonicated again for 10 min. The solution was stirred at 25 °C (lower than the LCST) for 2 days, to allow the swelling of nanogel and the encapsulation of doxorubicin and Fe3O4 in the nanogel. After that, the solution was centrifuged, the aqueous phase was removed, DOX-loaded magnetic hydrogel nanocomposites were separated by centrifuging at 12,000 rpm for 30 min. The resulting DOX-loaded magnetic hydrogel nanocomposites were frozen and lyophilized to obtain the dried product.
Measurement of drug-loading
The content of doxorubicin in the separated supernatant was measured using UV spectrophotometry at 483 nm. This value was then compared with the total amount of doxorubicin to determine the doxorubicin-loading efficiency of the nanocomposites. Drug-loading efficiency was calculated according to the following equation:
In vitro drug release kinetics
To study the drug release profile of synthesized poly (NIPAAM–DMAEMA)–Fe3O4 nanocomposites, 10 mg doxorubicin-loaded nanocomposites were incubated with 15 ml phosphate buffer of pH 5.8 and pH 7.4, and samples were incubated at temperatures 37 °C and 40 °C. At determined time intervals, suspension was centrifuged at 15,000 rpm for 5 min and 2 ml of supernatant was withdrawn and replaced with fresh buffer. The amount of doxorubicin released in the medium was analyzed at 483 nm.
Characterization
Fourier transforms infrared (FTIR) spectroscopy
The chemical structures of the synthesized polymers and DOX-loaded nanocomposite were studied by FTIR spectroscopy. All samples were mixed with KBr and were pressed to disk. IR spectra of the samples were scanned in the range from 4000 to 400 cm−1.
1H-NMR spectroscopy
The chemical structures of the copolymers were determined by 1H-NMR (Bruker spectra spin 400 MHz, Leipzig, Germany).
Morphology of the DOX-loaded nanocomposite
The morphological properties of the synthesized copolymers and DOX-loaded nanocomposite were observed with the SEM (VEGA, TESCAN, Brno, Czech Republic). One drop of copolymers and DOX-loaded nanocomposite were placed on aluminum foil and left to dry. After coating, the sample was observed under 10 kV with SEM.
Magnetic properties
The VSM was used to appraise the magnetic properties of the nanocomposite and compare it to the magnetic properties of the Fe3O4 nanoparticles. The sample was placed in an applied field range of 0–10 kOe.
Measurement of swelling behavior
The classical gravimetric method was used to measure the swelling ratio of the nanocomposites. (NIPAAM–DMAEMA) copolymer sample was submerged in distilled water at different levels of pH (7.4, 5.8) and at different temperatures (37 °C, 40 °C), and left to equilibrate for 24 h. After removing excess water by filter paper it was then weighted. The percentage that the copolymer swelled was calculated using the formula:
where Wt is the weight of the copolymer at predetermined time and W0 is the dry mass of the copolymers.
Results and discussion
Preparation of (NIPAAM–DMAEMA) co-polymer
Synthesis of cross-linked copolymer of NIPAAM–DMAEMA was prepared by free radical emulsion copolymerization of the monomers in the presence of KPS as the initiator and MBA as the cross-linking agent (). The reaction efficiency of these copolymers was around 89%.
Preparation of doxorubicin-loaded magnetic hydrogel nanocomposite
Fe3O4 nanoparticles were loaded in (NIPAAM–DMAEMA) co-polymer with the methods of swelling. The interaction between the copolymer and doxorubicin at neutral pH is related to hydrogen bonding and it is certified by FTIR spectra. The loading efficiency of doxorubicin-loaded nanocomposites was determined according to the formula that was illustrated in the Methods section. The results exhibited that doxorubicin was loaded into the nanocomposites with an encapsulation efficiency of 75%.
In vitro drug release
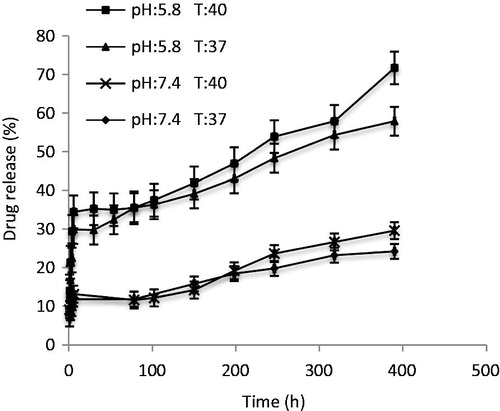
The release behavior of the nanocomposites was studied for 17 days (400 h) in PBS (0.1 M, pH 7.4, 5.8) at temperatures 37 °C and 40 °C. The ratio of cumulative release of DOX at pH = 5.8, t = 40 °C was significantly higher compared to pH = 7.4 and t = 37 °C (). After 400 h, 25% of the encapsulated DOX was released at pH = 7.4 and t = 37 °C and 70% of the encapsulated DOX was released at pH = 5.8 and t = 40 °C. The low pH, high temperature can lead to a higher released rate, because in the pH range of 0–6, the amine group in DOX is protonated and exists in the form of –NH3+, so that it cannot form hydrogen bond (Yang et al. Citation2010), also, protonated DOX has a higher solubility (Qi et al. Citation2010). At 37 °C, the nanocomposites are still in a swollen state. The increased drug release at 40 °C is attributed to the collapse of the nanocomposites.
FTIR spectroscopy
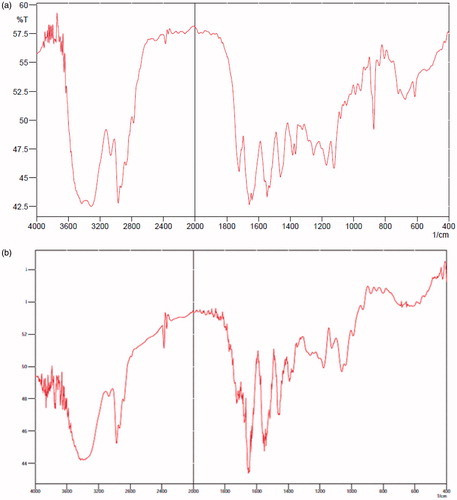
The FTIR spectra of the synthesized copolymers and DOX-loaded nanocomposites are given in . From the infrared spectra shown in , the absorption peaks at 1650–1660 cm−1 belonged to the amide peaks of NIPAAM units. The peaks at 1370–1390 and 2980 cm−1 were assigned to the stretching modes of the –CH(CH3)2 and –(CH3)2 groups in NIPAAM, respectively. DMAEMA with a band at 1715 cm−1 due to the C=O group and a signal at 1157 cm−1 attributed to the stretching vibration of C–C(=O)–O. peaks of doxorubicin at 1750 related to carbonyl groups of cyclohexanone ring of doxorubicin. The absorption peaks at 570 cm−1 belonged to the stretching vibration mode of Fe–O bonds in Fe3O4. After the copolymer was loaded with DOX, the absorption bonds of carbonyl and N–H groups of the NIPAAM shifted from 1660 and 1550 cm−1 to 1649 and 1545 cm−1 which showed that hydrogen bonds form between DOX and (NIPAAM–DMAEMA) co-polymer.
1H-NMR spectroscopy
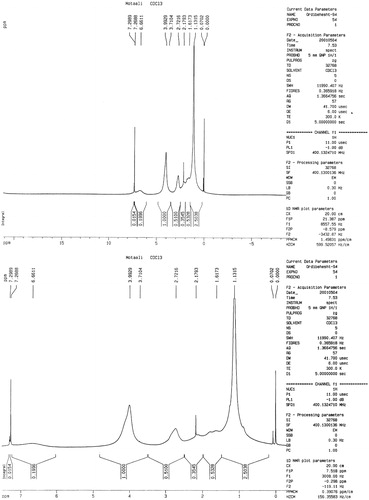
The chemical composition of the copolymers was determined with 1H-NMR by integrating the signals pertaining to each monomer. As an example, shows 1H-NMR spectra of poly (NIPAAM–DMAEMA). The signals belonging to NIPAAM are found in 1.61 ppm related to (CH2–CH), 1.13 ppm related to ((CH3)2CH), 3.99 ppm related to N–CH–(CH3)2, 2.17 ppm related to (CH–C=O). The signals belonging to DMAEMA are found in 3.98 ppm related to –C(O)–O–CH2–, 2.7 ppm related to the methylene (N–CH2) protons of the DMAEMA units.
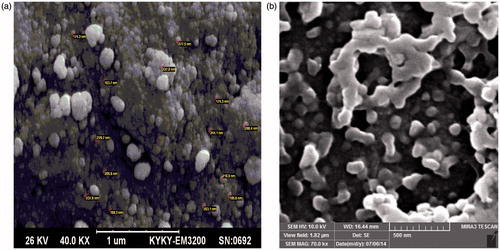
Morphology of the nanoparticle
shows the SEM images of copolymers and DOX-loaded nanocomposite. As shown, the size of the DOX-loaded nanocomposite is greater than that of the copolymer, which can be explained by this fact that some of the Fe3O4 are absorbed on the surface of the copolymers and the interaction between their charged surfaces are acted as a cue for growth in size of DOX-loaded nanocomposites.
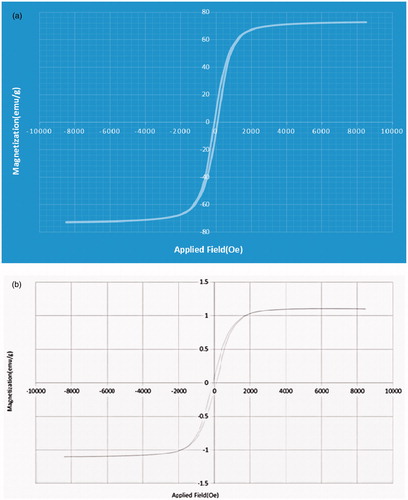
Magnetic properties
shows the magnetic curve of the Fe3O4 nanoparticles and the DOX-loaded nanocomposite. The magnetic saturation for DOX-loaded nanocomposite is about 1.3 emu/g, less than the pure Fe3O4 nanoparticles (70 emu/g). This difference suggests that a large amount of copolymer incorporated the Fe3O4 nanoparticles and doxorubicin.
Measurement of swelling behavior
shows the swelling behavior of the nanocomposites at corresponding pH and temperature levels. As seen, the swelling behavior of nanocomposites is directly related to pH and inversely related to temperature. Usually, the main reason for this unique characteristic of the nanocomposites can be ascribed to the unique and quick interchange of the hydrophilic and hydrophobic states (Feil et al. Citation1993, Inomata et al. Citation1990, Tokuhiro et al. Citation1991, Zhang et al. Citation2001). At high temperatures, the interaction between the hydrophobic parts of NIPAAM in the nanocomposites increases, resulting in the decrease of hydrogen bonding between copolymers and water, and hence it collapses (Tomar et al. Citation2014).
Table 1. Swelling behavior of the nanocomposites at pH and temperature
Conclusions
We have synthesized thermo/pH-sensitive (multiresponsive) nanocomposites containing NIPAAM–DMAEMA and Fe3O4 nanoparticles. For this purpose, a copolymer of NIPAAM–DMAEMA was synthesized through free radical emulsion polymerization method. Then Fe3O4 nanoparticles were incorporated with doxorubicin into NIPAAM–DMAEMA co-polymer. Our results showed that the poly (NIPAAM–DMAEMA) exhibited a noticeable thermo/pH-sensitive behavior. Thus this nanocomposite can be used for controlled drug delivery due to their thermo/pH-sensitive properties.
Funding information
This work is funded by 2015 grant Drug Applied Research Center, Tabriz University of Medical Sciences.
Disclosure statement
The authors report no declaration of interest. The authors alone are responsible for the content and writing of the paper.
References
- Banerjee SS, Chen D-H. 2008. Cyclodextrin conjugated magnetic colloidal nanoparticles as a nanocarrier for targeted anticancer drug delivery. Nanotechnology. 19:265602.
- Brahim S, Narinesingh D, Guiseppi-Elie A. 2003. Synthesis and hydration properties of pH-sensitive p (HEMA)-based hydrogels containing 3-(trimethoxysilyl) propyl methacrylate. Biomacromolecules. 4:497–503.
- Bristow MR, Mason JW, Billingham ME, Daniels JR. 1981. Dose-effect and structure–function relationships in doxorubicin cardiomyopathy. Am Heart J. 102:709–718.
- Davaran S, Entezami AA. 1997. Acrylic type polymers containing ibuprofen and indomethacin with difunctional spacer group: synthesis and hydrolysis. J Control Release. 47:41–49.
- Davaran S, Alimirzalu S, Nejati-Koshki K, Nasrabadi HT, Akbarzadeh A, Khandaghi AA, et al. 2014. Physicochemical characteristics of Fe3O4 magnetic nanocomposites based on poly(N-isopropylacrylamide) for anti-cancer drug delivery. Asian Pac J Cancer Prev. 15:49–54.
- Deng Y, Wang C, Shen X, Yang W, Jin L, Gao H, et al. 2005. Preparation, characterization, and application of multistimuli-responsive microspheres with fluorescence-labeled magnetic cores and thermoresponsive shells. Chem-A Eur J. 11:6006–6013.
- DiMarco A. 1975. Adriamycin (NSC-123127); mode and mechanism of action. Cancer Chemother Rep. 6:91–106.
- Ding XB, Sun ZH, Wan GX, Jiang YY. 1998. Preparation of thermosensitive magnetic particles by dispersion polymerization. React Funct Polym. 38:11–15.
- Ebrahimi E, Khandaghi AA, Valipour F, Babaie S, Asghari F, Motaali S, et al. 2016a. In vitro study and characterization of doxorubicin-loaded magnetic nanoparticles modified with biodegradable copolymers. Artif Cells Nanomed Biotechnol. 44:550–558.
- Ebrahimi E, Abbasi E, Akbarzadeh A, Khandaghi AA, Davaran S. 2016b. Novel drug delivery system based on doxorubicin-encapsulated magnetic nanoparticles modified with PLGA-PEG1000 copolymer. Artif Cells Nanomed Biotechnol. 44:290–297.
- El-Masry MM, Elnashar MM, El-Sherif HM. 2007. Amphoteric hydrogels for immobilization of enzymes using template polymerization technique. J Appl Polym Sci. 106:3571–3580.
- Feil H, Bae YH, Feijen J, Kim SW. 1993. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules. 26:2496–2500.
- Goodman MF, Lee GM. 1977. Adriamycin interactions with T4 DNA polymerase. Two modes of template-mediated inhibition. J Biol Chem. 252:2670–2674.
- Gupta AK, Gupta M. 2005. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 26:3995–4021.
- Hami Z, Amini M, Ghazi-Khansari M, Rezayat SM, Gilani K. 2014. Doxorubicin-conjugated PLA-PEG-folate based polymeric micelle for tumor-targeted delivery: synthesis and in vitro evaluation. DARU J Pharm Sci. 22:30.
- Hu S-H, Liu T-Y, Liu D-M, Chen S-Y. 2007. Nano-ferrosponges for controlled drug release. J Control Release. 121:181–189.
- Inomata H, Goto S, Saito S. 1990. Phase transition of N-substituted acrylamide gels. Macromolecules. 23:4887–4888.
- Ishida T, Kirchmeier M, Moase E, Zalipsky S, Allen T. 2001. Targeted delivery and triggered release of liposomal doxorubicin enhances cytotoxicity against human B lymphoma cells. Biochim Biophys Acta (BBA)-Biomembr. 1515:144–158.
- Ito A, Shinkai M, Honda H, Kobayashi T. 2005. Medical application of functionalized magnetic nanoparticles. J Biosci Bioeng. 100:1–11.
- Jaiswal MK, De M, Chou SS, Vasavada S, Bleher R, Prasad PV, et al. 2014. Thermoresponsive magnetic hydrogels as theranostic nanoconstructs. ACS Appl Mater Interfaces. 6:6237–6247.
- Jin-Hwan C, Young Kyung K, Kyo-Han K, Tae-Yub K, Vaezmomeni SZ, Samiei M, et al. 2016. Synthesis, characterization, biocompatibility of hydroxyapatite-natural polymers nanocomposites for dentistry applications. Artif Cells Nanomed Biotechnol. 44:277–284.
- Khodaverdi E, Rajabi O, Farhadi F, Jalali A, Tekie FMS. 2010. Preparation and investigation of poly (N-isopropylacrylamide-acrylamide) membranes in temperature responsive drug delivery. Iran J Basic Med Sci 13:102–110
- Kurd K, Khandagi AA, Davaran S, Akbarzadeh A. 2015. Cisplatin release from dual-responsive magnetic nanocomposites. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. doi: 10.3109/21691401.2015.1008513.
- Lai P-S, Lou P-J, Peng C-L, Pai C-L, Yen W-N, Huang M-Y, et al. 2007. Doxorubicin delivery by polyamidoamine dendrimer conjugation and photochemical internalization for cancer therapy. J Control Release. 122:39–46.
- Lin R, Ng LS, Wang C-H. 2005. In vitro study of anticancer drug doxorubicin in PLGA-based microparticles. Biomaterials. 26:4476–4485.
- Minko T, Kopečková P, Kopeček J. 2000. Efficacy of the chemotherapeutic action of HPMA copolymer-bound doxorubicin in a solid tumor model of ovarian carcinoma. Int J Cancer. 86:108–117.
- Mu L, Feng S. 2001. Fabrication, characterization and in vitro release of paclitaxel (Taxol®) loaded poly (lactic-co-glycolic acid) microspheres prepared by spray drying technique with lipid/cholesterol emulsifiers. J Control Release. 76:239–254.
- Purushotham S, Ramanujan R. 2010. Thermoresponsive magnetic composite nanomaterials for multimodal cancer therapy. Acta Biomater. 6:502–510.
- Qi J, Yao P, He F, Yu C, Huang C. 2010. Nanoparticles with dextran/chitosan shell and BSA/chitosan core—doxorubicin loading and delivery. Int J Pharm. 393:177–185.
- Rösler A, Vandermeulen GW, Klok H-A. 2012. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv Drug Deliv Rev. 64:270–279.
- Satarkar NS, Hilt JZ. 2008a. Hydrogel nanocomposites as remote-controlled biomaterials. Acta Biomater. 4:11–16.
- Satarkar NS, Hilt JZ. 2008b. Magnetic hydrogel nanocomposites for remote controlled pulsatile drug release. J Control Release. 130:246–251.
- Shimizu K, Fujita H, Nagamori E. 2010. Oxygen plasma-treated thermoresponsive polymer surfaces for cell sheet engineering. Biotechnol Bioeng. 106:303–310.
- Sima M, Fatemeh Zeinali S, Samad Mussa F, Mehdi Soleymani G, Abolfazl A. 2014. Current methods for synthesis of magnetic nanoparticles. Artif Cells Nanomed Biotechnol. 44:722–734.
- Tacar O, Sriamornsak P, Dass CR. 2013. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 65:157–170.
- Tokuhiro T, Amiya T, Mamada A, Tanaka T. 1991. NMR study of poly (N-isopropylacrylamide) gels near phase transition. Macromolecules. 24:2936–2943.
- Tomar LK, Tyagi C, Choonara YE, Kumar P, Pillay V. 2014. Rheological and swelling behavior of pH sensitive hydrogel particles. APCBEE Procedia. 9:192–196.
- Wang B, Xu X-D, Wang Z-C, Cheng S-X, Zhang X-Z, Zhuo R-X. 2008. Synthesis and properties of pH and temperature sensitive P(NIPAAm-co-DMAEMA) hydrogels. Colloids Surf B Biointerfaces. 64:34–41.
- Wang S, Zhou Y, Sun W. 2009. Preparation and characterization of antifouling thermosensitive magnetic nanoparticles for applications in biomedicine. Mater Sci Eng C. 29:1196–1200.
- Ward MA, Georgiou TK. 2011. Thermoresponsive polymers for biomedical applications. Polymers. 3:1215–1242.
- Xu Z, Guo M, Yan H, Liu K. 2013. Enhanced loading of doxorubicin into polymeric micelles by a combination of ionic bonding and hydrophobic effect, and the pH-sensitive and ligand-mediated delivery of loaded drug. React Funct Polym. 73:564–572.
- Yallapu MM, Foy SP, Jain TK, Labhasetwar V. 2010. PEG-functionalized magnetic nanoparticles for drug delivery and magnetic resonance imaging applications. Pharm Res. 27:2283–2295.
- Yang X, Chen L, Han B, Yang X, Duan H. 2010. Preparation of magnetite and tumor dual-targeting hollow polymer microspheres with pH-sensitivity for anticancer drug-carriers. Polymer. 51:2533–2539.
- Yong Y, Bai Y, Li Y, Lin L, Cui Y, Xia C. 2008. Preparation and application of polymer-grafted magnetic nanoparticles for lipase immobilization. J Magnet Magnet Mater. 320:2350–2355.
- Yuk SH, Cho SH, Lee SH. 1997. pH/Temperature-responsive polymer composed of poly ((N, N-dimethylamino) ethyl methacrylate-co-ethylacrylamide). Macromolecules. 30:6856–6859.
- Zeighamian V, Darabi M, Akbarzadeh A, Rahmati-Yamchi M, Zarghami N, Badrzadeh F, et al. 2015. PNIPAAm-MAA nanoparticles as delivery vehicles for curcumin against MCF-7 breast cancer cells. Artif Cells Nanomed Biotechnol. 44:735–742.
- Zhang Y, Zha L, Fu S. 2004. Kinetic analysis of poly (N-isopropylacrylamide-co-dimethylaminoethyl methacrylate) microgel latex formation. J Appl Polym Sci. 92:839–846.
- Zhang B-Y, He W-D, Li W-T, Li L-Y, Zhang K-R, Zhang H. 2010. Preparation of block-brush PEG-b-P (NIPAM-g-DMAEMA) and its dual stimulus-response. Polymer. 51:3039–3046.
- Zhang X-Z, Yang Y-Y, Chung T-S, Ma K-X. 2001. Preparation and characterization of fast response macroporous poly (N-isopropylacrylamide) hydrogels. Langmuir. 17:6094–6099.