Abstract
A patient with complex regional pain syndrome type II was successfully treated using free anterolateral thigh flap transfer with digital nerve coaptation to the cutaneous nerve of the flap. Release of the scarred tissue and soft tissue coverage with targeted sensory nerve coaptation were useful in relieving severe pain.
Introduction
Complex regional pain syndrome (CRPS) is an intractable clinical condition, first reported in the 1800s. It is characterized by burning pain, hyperalgesia, edema, and sudomotor changes, without or with identifiable associated nerve injury (type I and type II CRPS, respectively) [Citation1]. The pathogenesis of CRPS is unclear but involves both peripheral and central neurologic components, which complicates understanding of the condition. No single treatment has proven completely effective; thus, there are multiple options and approaches for managing CRPS. Surgical intervention is not usually indicated for CRPS type I, except in cases of sympathectomy or stimulator implantation [Citation2]; however, surgical approaches may lead to satisfactory outcomes for CRPS type II [Citation3]. We present a case of CRPS type II that was successfully managed with free anterolateral thigh flap transfer.
Case report
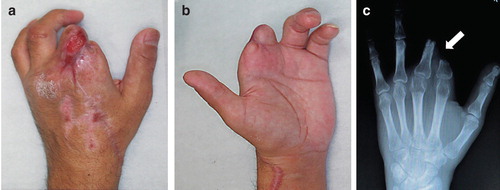
A 45-year-old man with failed replantation of the left index and middle fingers had undergone pedicled reverse forearm flap coverage of the stumps of the amputated fingers before being referred to our center. The pedicled flap failed to survive, probably because of inappropriate flap design and dissection procedures (). The wound was treated conservatively after removing necrotic flap tissue. The wounds later epithelialized, and malpositioning of the two stumps developed due to formation of cicatricial tissue between the stumps (). A burning sensation developed throughout the left upper arm, resulting in ipsilateral shoulder joint contracture. CRPS type II was diagnosed by anesthesiologists on the basis of the criteria of the International Association for the Study of Pain [Citation1]. The patient received treatment with oral gabapentin, sympathetic nerve block, and periodic epidural block; however, the symptoms remained poorly controlled. Eight months after the initial finger amputation injury, the patient was referred to our center for pain treatment and possible surgical intervention.
Figure 1. Photograph taken by the patient showing a pedicled reverse forearm flap that totally failed to cover the stumps.
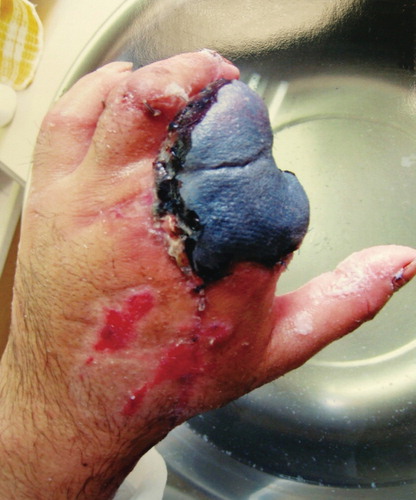
Figure 2. (a, b) Photographs showing the condition at presentation. The stumps of the fingers were almost dry with cicatricial tissue. The patient reported severe pain expanding over the region of the upper arm. (c) Preoperative radiograph showing malposition of the proximal phalanx of the index and middle fingers (arrow) caused by severe scar contracture around the two stumps.
Procedure
The patient’s pain symptoms were carefully evaluated preoperatively by a plastic surgeon and anesthesiologist, after which the indications for surgical intervention were discussed with the patient.
The principal complaint was burning pain at the space between the stumps of the index and middle fingers. This pain substantially limited the patient’s activities of daily living, and the disabilities of the arm, shoulder, and hand (DASH) global scale score was 53.3. Use of lidocaine for digital nerve block of the proximal common digital nerve in the second digital space resulted in effective pain relief, which was not possible with previous nonsurgical treatment. We, therefore, concluded that surgical intervention was indicated for treatment of the patient’s condition.
The surgical procedure called for the undesirable scar to be removed and replaced by generous soft tissue to cover the stumps and the space between the stumps created after contracture release. Ray amputation of the index finger allows for accommodation of soft tissue for coverage of stumps, without the need for a soft tissue flap. However, it reduces palm width and can result in loss of grip power. Our patient declined ray amputation and we therefore planned to transfer the free flap to the affected area.
There were two basic principles to be considered in the present case. One is coaptation of the nerve ends, with or without an interposing nerve graft, to prevent development of a painful neuroma and restore function. Although some neuroma formation may occur at the coaptation site, if nerve repair is adequate, a substantial number of nerve fibers will regenerate beyond the repair site to their distal targets.
The other fundamental principle is coverage of the painful neuroma with a well-vascularized soft tissue flap, which was reported to be effective in relieving pain in patients with intractable conditions [Citation4].
We selected a free sensate anterolateral thigh flap to cover the stumps. After removing the scar around the stumps, the index and middle proximal phalanx were freed from contracture (). Then, the digital nerves to these fingers were explored and identified. Because the pain center was localized in the space between the index and middle fingers, the digital nerve stumps in the second interdigital space were cut and coapted to the cutaneous nerve of the flap in an end-to-side or end-to-end manner. The other end of the cutaneous nerve in the flap was buried deeply in the soft tissue of the flap. The other two proper digital nerves (at the radial side of the index finger and ulnar side of the middle finger) were shortened by about 2 cm, and the new stumps were buried deeply in the intrinsic muscle [Citation5] (). The vascular pedicle of the flap was anastomosed to the distal end of the radial artery and cutaneous vein. A skin paddle was placed to partially divide and cover the two stumps and dorsum of the hand.
Figure 3. (a) A free anterolateral thigh flap (7 × 23 cm) was designed and harvested with its cutaneous nerve (arrows). (b) Schematic diagram of the digital nerves. (c) Intraoperative photo showing end-to-end (black arrow) and end-to-side (red arrow) neurorrhaphy between the digital nerves and cutaneous nerve of the flap. (d) Schematic diagram of the procedure. After releasing the contracture between the two stumps, the digital nerves between them are coapted to the cutaneous nerve of the flap using end-to-end (black arrow) and end-to-side (red arrow) neurorrhaphy. The other end of the cutaneous nerve of the flap is buried deeply in the soft tissue (green arrow). The other digital nerves are refreshed for about 3 cm and buried deeply in the muscle (blue arrows).
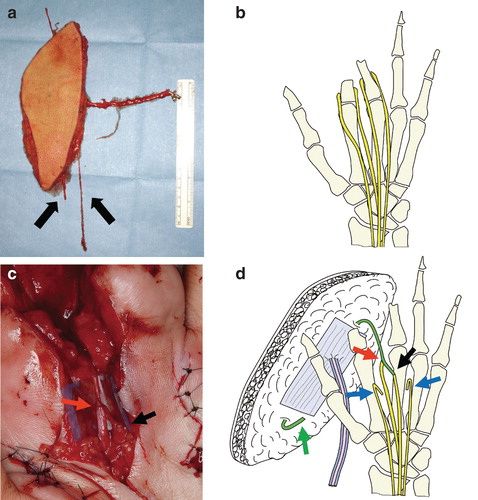
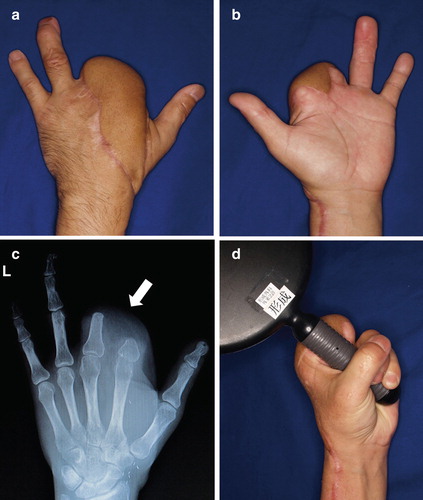
Figure 4. (a, b) Photographs and radiograph at 18 months postoperatively. The skin paddle of the flap was set to divide and cover the two stumps and dorsum of the hand. Almost all pain had disappeared at 18 months postoperatively. (c) Postoperative radiograph shows good alignment of the two stumps (arrow). (d) Range of motion, including that of the other fingers, substantially recovered. The patient can now grasp things easily without pain and enjoys motorbike riding.
Results
The postoperative course was uneventful, and pain symptoms gradually resolved. The oral medications were tapered and finally stopped at 4 months after the operation. The patient returned to work at 7 months postoperatively. At 18 months postoperatively, almost all pain had disappeared; however, the Semmes–Weinstein monofilament examination [Citation6] revealed that recovery in the flap was limited to protective sensation. Postoperatively, he was able to use the treated hand effectively and renewed his motorbike license. Pain relief was dramatic, and the patient’s activities of daily living greatly improved. The global score on the DASH improved to 7.5 at 18 months postoperatively.
Discussion
Although surgical intervention for peripheral nerves is not recommended for CRPS type I [Citation2], such procedures may be considered if the origin of pain is identified in patients with CRPS type II [Citation7]. In addition, patients who originally received a diagnosis of CRPS type I might have associated lesions, such as nerve entrapment or compression [Citation7], that lead to a later diagnosis of CRPS type II, which is more likely to require surgical intervention. Because the location of pain was identifiable and the effect of digital nerve block for pain relief was confirmed in our patient, we selected surgical intervention. We believed that decompression of the digital nerve by releasing the contracture between the two stumps and filling of the space around the nerves with ample, well-vascularized soft tissue [Citation4] would avoid recompression and aberrant axonal sprouting. The free anterolateral thigh flap is well suited for these requirements.
Management of the digital nerves in the freed stump is a concern. Numerous types of surgical treatments for painful neuroma have been reported. Although most are designed to inhibit aberrant axonal growth or avoid noxious stimuli in nerve stumps, Zhang et al. reported a different concept for avoiding neuroma formation, that is, use of microsurgical toe transfer to provide a pathway and target for regenerating axons [Citation8]. Our treatment is similar in that it uses a cutaneous nerve of the anterolateral thigh flap to provide a pathway for regenerating axons. Another option is end-to-side nerve coaptation of nerve stumps to the adjacent intact nerve [Citation9], such as the digital nerves of the thumb or ring finger, or the superficial branch of the radial nerve in our patient. However, we felt that surgical invasion of the adjacent intact area could expand the area of pain. We, therefore, selected treatments that were limited to the affected area. Two digital nerves (at the radial side of the index finger and ulnar side of the middle finger) were cut and the proximal ends were placed in the dorsal web space within intrinsic muscle [Citation5] and covered with healthy scarless tissue. Two refreshed digital nerve stumps, which were thought to be the main source of the burning sensation, were connected to the sensory nerve of the flap in an end-to-end or end-to-side manner. Although sensory recovery is relatively poor, an anterolateral thigh flap, with its cutaneous nerve, can be used as a sensate flap [Citation10]. In our patient, sensory recovery of the flap is not easy to assess; however, the length of the created pathway (about 15 cm) appears to have eliminated the possibility of redeveloping painful neuroma.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- IASP. Merskey MB, Bogduk N. editor. Classification of chronic pain: descriptions of chronic pain syndromes and definition of pain terms. 2nd ed. Seattle, WA: IASP Press; 1994
- Ackerman WE3rd, Ahmad M. Recurrent postoperative CRPS I in patients with abnormal preoperative sympathetic function. J Hand Surg Am 2008;33:217–22
- Placzek JD, Boyer MI, Gelberman RH, Sopp B, Goldfarb CA. Nerve decompression for complex regional pain syndrome type II following upper extremity surgery. J Hand Surg Am 2005;30:69–74
- Adani R, Tos P, Tarallo L, Corain M. Treatment of painful median nerve neuromas with radial and ulnar artery perforator adipofascial flaps. J Hand Surg Am 2014;39:721–7
- Whipple RR, Unsell RS. Treatment of painful neuromas. Orthop Clin North Am 1988;19:175–85
- Weinstein S. Fifty years of somatosensory research: from the Semmes-Weinstein monofilaments to the Weinstein Enhanced Sensory Test. J Hand Ther 1993;6:11–22; discussion 50
- Dellon AL, Andonian E, Rosson GD. CRPS of the upper or lower extremity: surgical treatment outcomes. J Brachial Plex Peripher Nerve Inj 2009;4:1
- Zhang F, Hu EC, Chen W, Lineaweaver WC. Treatment of painful neuroma of amputated phalanx with distal toe transfer: a case report. South Med J 2006;99:85–9
- Aszmann OC, Moser V, Frey M. [Treatment of painful neuromas via end-to-side neurorraphy]. Handchir Mikrochir Plast Chir 2010;42:225–32
- Kimata Y, Uchiyama K, Ebihara S, et al. Comparison of innervated and noninnervated free flaps in oral reconstruction. Plast Reconstr Surg 1999;104:1307–13

