Abstract
Cutaneous extramedullary plasmacytomas (EMPs) are rare plasma cell neoplasms of the skin occurring in 2–4% of patients with multiple myeloma (MM). We describe a man diagnosed with IgA lambda MM (Stage III) after rapidly enlarging cutaneous nodules developed in the surgical site of recently excised skin malignancies. Cutaneous EMP must be considered for expanding cutaneous nodules at sites of surgery or trauma.
Introduction
Extramedullary plasmacytoma (EMP) is a soft tissue tumour consisting of neoplastic proliferation of monoclonal plasma cells, commonly located in the submucosa of upper respiratory tract [Citation1]. Rarely, these plasma cell tumours infiltrate the skin. Cutaneous EMP (cEMP) are classified into two distinct entities. Primary cEMP arise devoid of systemic plasma cell involvement, with a typical indolent clinical course. Secondary cEMP, associated with poorer prognosis, represents dermal metastases from systemic progression of multiple myeloma (MM). The large tumour cell burden and extensive disease progression reflected by these cutaneous tumours is rarely the presenting feature of MM [Citation2]. We describe a unique case of a 77-year-old male diagnosed with IgA lambda MM (Stage III) subsequent to the development of cEMP on a full-thickness skin graft (FTSG) after excision of squamous cell carcinoma (SCC) on forehead. Furthermore, cEMPs were identified at the graft donor site on the neck and the surgical incision site for a malignant melanoma in the forearm. Cutaneous infiltration of MM is rare, yet in light of its poor prognosis, recognition and understanding of these dermal metastases is essential.
Case report
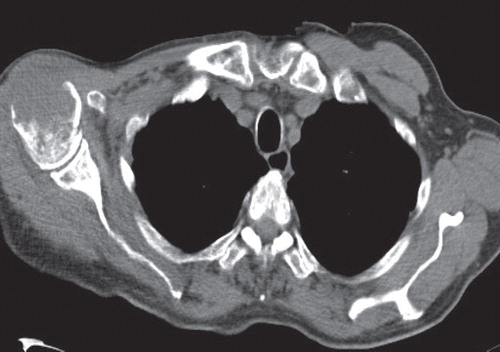
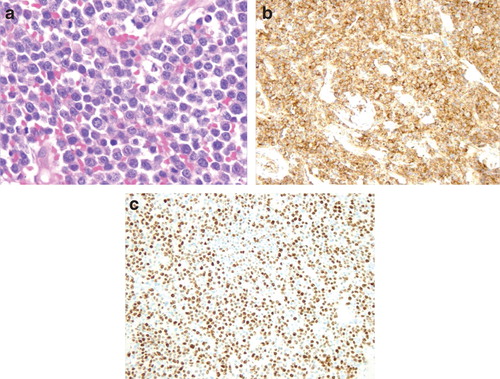
A 77-year-old male presented for a routine follow-up appointment two weeks after a wider excision of malignant melanoma (pT1a) from right forearm and excision of SCC from the forehead, reconstructed with a FTSG. Histological analysis confirmed adequate margins for both specimens. The FTSG had taken and with no evidence of any local or regional recurrence, a surveillance appointment was arranged for 3 months. However, 2 weeks later, he re-presented with multiple red dome-shaped cutaneous nodules on the FTSG located on the forehead (), surgical excision site on the right arm and the graft donor site. A punch biopsy identified infiltration by a cellular process with negative stains for S100 and MELAN A, excluding possible recurrence of melanoma. Seven days later, the nodules dramatically increased in size with associated ulceration (). Histology from a formal excision demonstrated complete replacement of the dermis and subcutaneous fat by sheets of immature plasma cells (). Immunohistochemistry was strongly positive for CD138, with a very high proliferation fraction >80% (). In the B-cell screening panel [Citation3], CD20, Pax5, CD79a and CD45 stained negative and tumour cells expressed CD56, cyclin D1 protein and EMA. Concordance of morphological and immunohistochemistry (CD138+/CD20−/CD45−) features confirmed a diagnosis of cEMP. Subsequent haematological investigations demonstrated a significant hypercalcaemia of 2.88 mmol/l (2.17–2.51 mmol/l), resulting in admission to hospital. This hypercalcaemia normalised after aggressive fluid resuscitation. The bone marrow biopsy demonstrated >60% of nucleated elements of plasma cells showing monotypic lambda expression on in situ hybridisation. Radiological imaging illustrated multiple osteolytic lesions throughout the skeletal system, particularly with considerable destruction of the right humeral head (). Serum protein electrophoresis confirmed monoclonal IgA gammopathy and plasma cell tumour markers demonstrated surface IgA with lambda light chain restricted (), resulting in a diagnosed of IgA lambda MM (Stage III). After a multidisciplinary discussion, the patient was commenced on a chemotherapy regime of VMP (bortezomib, melphalan, prednisone), which resulted in a significant reduction in size of all the cutaneous nodules. Targeted radiotherapy, 30 Gy in 10 fractions, was performed for the medullary plasmacytoma in the right humerus. Despite an initial good response to treatment, his condition deteriorated and he died 6 months later from hospital-acquired pneumonia.
Figure 1. (a) Cutaneous dome shaped nodules peripherally located on the full thickness skin graft, three weeks after excision of squamous cell carcinoma (b) 7 days later, rapid growth and ulceration of the lesions expanding beyond the graft site.
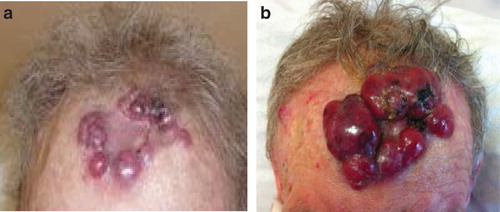
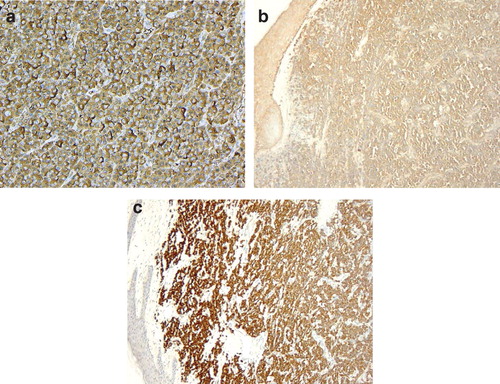
Figure 2. (a) Immature plasma cells, with large eccentric nuclei and scattered mitosis (H&E ×40). (b) Intense staining of CD138 (×10). (c) Positive staining for Ki67 (high proliferation index >80%) (×10).
Discussion
MM is an incurable haematological malignancy characterised by neoplastic proliferation of plasma cells producing monoclonal immunoglobulins. The systemic displacement of normal haematopoiesis is reflected by the range of presenting symptoms, including hypercalcemia, bone pain, anaemia and acute kidney injury [Citation4]. Dermatological manifestations of MM are exceptional. cEMP most frequently involve the face and extremities, yet unusual locations such as the testicles are described [Citation5]. We describe the first reported case of MM presenting as cEMP in a FTSG and other surgical sites, after an excision of skin malignancies.
Violaceous dome-shaped nodules, multiple or solitary, are the key clinical features of cEMP. The propensity to ulcerate reflects the potential for rapid expansive growth. These indistinct morphological characteristics creates a broad list of differentials, including; large B-cell lymphoma, poorly differentiated carcinoma, granulocytic sarcoma, metastatic melanoma and pyogenic granuloma. The ambiguous features of the plasma cells observed on hematoxylin and eosin (H & E) staining emphasises the importance of immunohistochemistry in establishing a diagnosis. The protein CD138 (syndecan-1) is very sensitive and specific marker of plasmacytic differentiation [Citation6]. The remarkably high proliferative index () of the plasma cells suggested the prospect of high grade B-cell lymphoma with extreme plasma cell differentiation; however, this was subsequently excluded following negative staining of the B-cell differentiation antigens (CD20, CD79a, CD45). With important clinical and prognostic differences, distinguishing between primary and secondary cEMP is paramount. Yet, this is not possible at a morphological and histopathological level. However, advancements in immunohistochemistry have identified greater overexpression of Cyclin D1 and CD56 in secondary cEMP specimens [Citation7]. Both these were expressed in our patient’s specimen, supporting a potentially new diagnostic method.
Cutaneous EMP typically arise from a direct extension of medullary or soft tissue plasmacytomas, rarely resulting from haematological or lymphatic metastatic spread of progressive MM. The absence of contiguous plasmacytomas surrounding our patient’s surgical sites indicates possible metastatic spread. The mechanisms by which cutaneous plasmacytomas favour surgical incisions sites are not completely understood. Yet, malignant tumours arising in scar tissue are a well-documented phenomenon, common examples include Marjolin’s ulcers and tumour seeding from biopsy tract sites [Citation8]. Cutaneous plasmacytomas have been reported, in known MM patients, to occur at surgical incision sites after insertion of a central venous catheter [Citation9]. It is speculated that plasma cells expressing cytokine receptor CXCR4 interact with the inflammatory cascade released due to tissue injury, facilitating the migration of myeloma cell into the skin. Within the scar tissue, stromal cells potentially promoting plasma cell survival and proliferation, creating a reservoir of malignant plasma cells [Citation10].
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Kato N, Kimura K, Yasukawa K, Aikawa K. Metastatic cutaneous plasmacytoma: a case report associated with IgA lambda multiple myeloma and a review of the literature of metastatic cutaneous plasmacytomas associated with multiple myeloma and primary cutaneous plasmacytomas. J Dermatol 1999;26:587–94.
- Diwan AG, Gholap NN, Taneja SR. Extramedullary plasmacytoma associated with multiple myeloma. J Assoc Physicians India 2013;51:231–2.
- Rizzo K, Nassiri M. Diagnostic workup of small B cell lymphomas: a laboratory perspective. Lymphoma 2012;2012:15 pages.
- Jowitt SN, Jacobs A, Batman PA, Sapherson DA. Atypical progression of multiple myeloma with extensive extramedullary disease. J Clin Pathol 1994;47:269–71.
- White J, Chan YF. Solitary testicular plasmacytoma. Br J Urol 1995;75:107–8.
- O’Connell FP, Pinkus JL, Pinkus GS. CD138 (syndecan-1), a plasma cell marker immunohistochemical profile in hematopoietic and nonhematopoietic neoplasms. Am J Clin Pathol 2004;121:254–63.
- Kremer M, Ott G, Nathrath M, Specht K, Stecker K, Alexiou C, et al. Primary extramedullary plasmacytoma and multiple myeloma: phenotypic differences revealed by immunohistochemical analysis. J Pathol 2005;205:92–101.
- Cotter M, Enright H. Plasmacytoma occurring in scar tissue. Br J Haematol 2003;121:680.
- Gaba RC, Kenny JP, Gundavaram P, Katz JR, Escuadro LR, Gaitonde S. Subcutaneous plasmacytoma metastasis precipitated by tunnelled central venous catheter insertion. Case Rep Oncol 2011;4:315–22.
- Rosenblum MD, Bredeson CN, Chang CC, Rizzo JD. Subcutaneous plasmacytomas with tropism to sites of previous trauma in a multiple myeloma patient treated with an autologous bone marrow transplant. Am J Hematol 2003;72:274–7.